Multiple subcellular localizations and functions of protein kinase Cδ in liver cancer
Kohji Yamada,Kiyotsugu Yoshida
Kohji Yamada,Kiyotsugu Yoshida,Department of Biochemistry,The Jikei University School of Medicine,Tokyo 105-8461,Japan
Abstract Protein kinase Cδ (PKCδ) is a member of the PKC family,and its implications have been reported in various biological and cancerous processes,including cell proliferation,cell death,tumor suppression,and tumor progression.In liver cancer cells,accumulating reports show the bi-functional regulation of PKCδ in cell death and survival.PKCδ function is defined by various factors,such as phosphorylation,catalytic domain cleavage,and subcellular localization.PKCδ has multiple intracellular distribution patterns,ranging from the cytosol to the nucleus.We recently found a unique extracellular localization of PKCδ in liver cancer and its growth factor-like function in liver cancer cells.In this review,we first discuss the structural features of PKCδ and then focus on the functional diversity of PKCδ based on its subcellular localization,such as the nucleus,cell surface,and extracellular space.These findings improve our knowledge of PKCδ involvement in the progression of liver cancer.
Key Words:Protein kinase Cδ;Liver cancer;Subcellular localization;Tumor suppression;Tumor progression
lNTRODUCTlON
The protein kinase C (PKC) family of serine/threonine kinase proteins in mammals,comprising the classical PKC (cPKC),novel PKC (nPKC),and atypical PKC (aPKC)subfamilies,is one of the defining families of AGC kinases[1,2].To date,10 isoforms of PKC have been identified in humans,including four cPKCs (PKCα,-βI,-βII,and -γ),four nPKCs (PKCδ,-ε,-η,and -θ),and two aPKCs (PKCζ and -λ/ι)[2,3].PKC activation depends on the conformational activation of certain intracellular factors.Notably,PCK activation is regulated not only by binding to lipid factors,such as diacylglycerol(DAG) and phorbol esters,but also by protein phosphorylation[4].PKCδ is often phosphorylated at several Tyr residues by various types of stimulations,including DNA-damaging reagents and oxidative stress[5,6].Kinases that phosphorylate PKCδ at Tyr include the Src family of tyrosine kinases (e.g.,Src,Fyn,Lyn,and Lck) and c-Abl[6].
Among PKC families,PKCδ is a unique non-signal peptide-containing intracellular protein that has been reported to translocate to a diverse range of distributions,including the cytosol,nucleus,endoplasmic reticulum,Golgi,mitochondria,and plasma membrane,in response to different stimuli and cell types[8].For example,a nuclear localization signal (NLS) was identified in the catalytic domain of PKCδ,which is necessary for the transport of PKCδ across the nuclear pore.Nuclear localization of PKCδ is associated with pro-apoptotic functions.Phosphorylation also affects the subcellular localization of PKCδ and its activation.Our recent study revealed that cytosolic PKCδ translocates to the extracellular space and acts as a growth factor for liver cancer cells or tumors[9].In this review,we summarize studies reported to date regarding the intracellular function of PKCδ in cancerous phenotypes of liver cancer.We then focus on and discuss the relationship with subcellular localizations,which exist in extracellular and intracellular locations,and the functions of PKCδ.Increased knowledge on where PKCδ protein is localized and how it functions in living cells allows a more profound understanding of the functional diversity of PKCδ.
STRUCTURAL FEATURES OF PKCδ
PKCδ comprises an N-terminal regulatory domain and a C-terminal kinase core domain[5].The C-terminal catalytic domain of PKC is conserved between isoforms and includes ATP-and substrate-binding sites and a kinase core[10,11].The Nterminal regulatory domain is much less conserved and contains specific motifs for each isoform that are activated in response to unique signals.The regulatory modules in this N-terminal domain include the pseudosubstrate motif and C1 and C2 domains,which bind to Ca2+and DAG.The affinity of the C1 and C2 domains for Ca2+and DAG determines the cofactor requirements for the activation of specific PKC isoforms.cPKCs have functional C1 and C2 domains that bind to both Ca2+and DAG[12-14],whereas nPKCs have a functional C1 domain that binds to DAG alone and a nonfunctional C2 domain,rendering these kinases independent of Ca2+for activation[15].
Generally,upon PKC activation,growth factors and G protein-coupled receptors trigger the hydrolysis of membrane lipids by recruiting phospholipase C[16,17].Phospholipase C generates DAG and inositol-1,4,5-triphosphate through the hydrolysis of membrane phosphoinositol.In response to DAG,PKC is translocated to the lipid membraneviathe C1 domain,enabling interaction with its substrates and the phosphorylation response[18].
PKCδ confers distinct allosteric regulationviaprotein binding to the C1,C2,and V5 domains,tyrosine phosphorylation,and the removal of the regulatory domain by caspase cleavage (Figure 1).In particular,various reports have identified that a variety of tyrosine phosphorylation sites affect cellular functions.For example,many studies have demonstrated that tyrosine phosphorylation of PKCδ plays a critical role in cell death in response to apoptotic stimuli.Tyrosine residues important in the context of apoptosis include Tyr64,Tyr155,Tyr187,Tyr311,Tyr332,and Tyr512[18-20].Furthermore,tyrosine phosphorylation of Tyr64 (C2 domain) and Tyr155 (C1a domain) is crucial for the exposure of PKCδ to NLS by disrupting the association between C2 and catalytic domains to enable nuclear transport.

Figure 1 Schematic representation of protein kinase Cδ domains.The N-terminal regulatory domain is composed of 1 to 329 amino acids,nonfunctional C2,pseudosubstrate,and lipid binding C1 (a and b) domains.The C-terminal catalytic domain is composed of 330 to 676 amino acids,ATP-binding C3,and kinase C4 domains.The 329 amino acids at the V3 region allow the cleavage site by caspase-3 to be constitutively active.The V5 region includes nuclear localization signal necessary for the nuclear transport of protein kinase Cδ.NLS:Nuclear localization signal.

Figure 2 Extracellular protein kinase Cδ shows oncogenic property in liver cancer.Model of the proliferative regulation of extracellular protein kinase Cδ (PKCδ) in liver cancer cells.PKCδ is secreted from living cells and resides at the plasma membrane through its association with glypican 3,leading to an increase in insulin-like growth factor 1 receptor activation and enhancement of subsequent proliferative signaling to increase cell growth.PKCδ:Protein kinase Cδ;GPC3:Glypican 3;ERK:Extracellular signal-regulated kinase;IGF1R:Insulin-like growth factor 1 receptor.
FUNCTlONAL FEATURES OF lNTRACELLULAR PKCδ lN LlVER CANCER
Tumor suppressive function
Studies on PKCδ-/-mice have confirmed the pro-apoptotic role of this molecule in response to several stimuli,such as DNA damage.Although these mice developed normally and were fertile,increased B cell proliferation was observed[21].Smooth muscle cells derived from PKCδ-/-mouse aortas were also shown to be resistant to cell death in response to several stimuli.Hence,these studies with PKCδ-/-mice demonstrated that PKCδ is not required for cell proliferation during development.
PKC also binds to and is activated by tumor-promoting phorbol esters[4].Therefore,PKC is considered a tumor-promoting protein.However,it has been reported that persistent treatment with phorbol esters causes degradation or downregulation of PKC[3,22-24].In particular,PKCδ has been reported to enhance ubiquitin proteasomal degradation upon activation of PKCδ by lipids.Furthermore,accumulating evidence on PKCδ in cancer has shown that downregulation,rather than activation,of PKCδ is associated with tumor progression.Therefore,PKCδ is believed to act as a tumor suppressor because its downregulation facilitates tumor promotion and causes cell cycle arrest or induces apoptosis in response to various stimuli,such as H2O2,ceramide,tumor necrosis factor-α (TNF-α),ultraviolet radiation,cisplatin,and etoposide[22,25-27].In fact,thePKCδgene is deleted in many cancers[28].Ectopic expression of PKCδ has been shown to decrease the anchorage-independent growth of NIH3T3 cells and reverse the transformation of rat fibroblasts and colonic epithelial cells by Src.Low levels of PKCδ have been reported in colon cancer,and overexpression of PKCδ suppresses the neoplastic phenotype of colon cancer cells[25].A recent report suggested that PKCδ is lost in human squamous cell carcinoma due to transcriptional repression[29].PKCδ has also been reported to decrease cell migration in breast cancer cells,whereas knockout of the PKCδ gene increases cell migration in mouse embryonic fibroblasts.These studies strongly support the role of PKCδ in tumor suppression.
Multiple reports suggest that PKCδ is responsible for apoptotic signaling in liver cancer cells (Table 1).In sorafenib-resistant hepatocellular carcinoma (HCC) cells,PKCδ activation was shown to induce cellular apoptosisviap38 activation[30].Annexin A3 (ANXA3) interacts with PKCδ and thereafter suppresses PKCδ/p38-associated apoptosis and activates autophagy for cell survival.Thus,inhibition of ANXA3 by a monoclonal antibody is likely to impair cell survival and tumor growth.Although FTY720,a synthetic sphingosine immunosuppressor,has been known to have antitumor effects on HCC cells,PKCδ activation occurs in FYT720-treated HCC cells.FTY720 is thought to activate PKCδviathe generation of reactive oxygen species(ROS) and subsequent caspase 3-dependent cleavage to induce apoptosis.The relationship between intracellular activation of PKCδ and apoptosis in HCC cells has also been reported in the antitumor mechanism of an antagonist of FZD7,which is a membrane receptor overexpressed in HCC[31].These lines of evidence suggest that PKCδ activation is not favorable for malignant transformation in liver cancer and maybe inactivated in these cells.

Table 1 The relationship between subcellular localizations and functions of protein kinase Cδ in liver cancer
Tumor promotive function
Many studies have shown that PKCδ promotes the survival of multiple types of cancers,including non-small cell lung cancer,breast cancer,pancreatic cancer,chronic lymphocytic leukemia,and liver cancer.
PKCδ has been reported to induce signal survival.In fact,PKCδ promotes cell survivalviaseveral well-known pro-survival pathways,including NF-κB,Akt,and extracellular signal-regulated kinase (ERK).It has been reported that PKCδ inhibits apoptosis by inhibiting apoptosis protein-2 and FLICE-like inhibitory proteinviaNFκB[32].
Numerous publications have reported that PKCδ is actively involved in the promotion of liver cancer,including cell migration,invasion,and tumor stage(Table 1).For example,claudin-1,a member of the tetraspanin family,plays a critical role in the acquisition of invasive capacity in human liver cells,and c-Abl-PKCδ signaling is important for malignant progression induced by claudin-1[33].This c-Abl-PKCδ signaling pathway was shown to activate MMP-2,a key factor in cell migration and invasion.The cross talk between PKC and ROS may induce mitogen-activated protein kinase (MAPK) activation for cell migration and progression.Mandalet al[34]found that activation of PKCδ generated mitochondrial ROS triggers the oxidation of heat shock protein 60 (HSP60),a chaperone protein in the mitochondria,which induces the activation of ERK and c-Jun N-terminal kinase (JNK) in the cytosol,resulting in gene expression leading to migration in liver cancer.PKCδ and hypoxia have also been reported to be associated with cell migration in liver cancer.Hypoxiainducible factor-2α expression regulates CUB domain-containing protein 1,which stimulates the phosphorylation of PKCδ at Tyr311 to induce malignant migration in various cancer cells,such as liver cancer cells[35].Furthermore,the levels of HSP27 are inversely correlated with tumor stage,as per the tumor,node and metastasis classification,in patients with HCC.Takaiet al[36] showed that PKCδ activation regulates the phosphorylation of HSP27viap38 MAPK.
There is supportive evidence that PKCδ acts as a tumor promoter in many types of cancers.For example,the mRNA levels of PKCδ were higher in estrogen receptor(ER)-positive tumors than in ER-negative tumors,and an increase in PKCδ mRNA was associated with reduced overall survival[37].PKCδ knockdown decreased the survival of MCF-7 and MDA-MB-231 breast cancer cells[38].Overexpression of PKCδ was also observed in human ductal pancreatic carcinomas compared to its normal counterparts.PKCδ has been reported to be associated with melanoma cell metastasis[39].A recent study demonstrated that integrin αvβ3-mediated invasion of melanoma cells is mediatedviaPKCα and PKCδ.
SUBCELLULAR LOCALlZATlONS AND FUNCTlONS OF PKCδ
Cytosol and plasma membrane
PKCδ is translated on the ribosome in the cytosol and generates its inactive cytosolic form.Similar to other PKC families,in response to DAG,PKCδ is also translocated to the plasma membraneviathe C1 domain,which exerts a subsequent phosphorylation response.PKCδ activation is also required for Akt activation by Ras[40] (1-98).Activating mutations with Ras or PI3K increases PKCδ levels and induces Akt activation.PKCδ also induces ERK1/2 activation[41,42].Akt and ERK1/2 activation have been implicated in the PKCδ-mediated increase in anchorage-independent growth and resistance of pancreatic ductal cancer cells to apoptotic stimuli[43].Conversely,cytosolic PKCδ reportedly triggers apoptosis by activating p38 MAPK to inhibit Akt[8],indicating that PKCδ activation can behave as both a prosurvival and pro-apoptotic factor.Liver damage has been reported to induce inflammation and PKCδ translocation to the plasma membrane[44,45].PKCδ activation has been observed in the tissues of patients with non-alcoholic steatohepatitis and non-alcoholic fatty liver disease and in a mouse model of hepatic cirrhosis[46-49].
Nucleus
Importantly,PKCδ is a PKC isoform that has been identified as a substrate for caspase-3[50].Cleavage of PKCδ by caspase-3 separates the regulatory domain and catalytic fragment to allow constitutive activation of PKCδ even in the absence of any co-factors[22] and then translocates to the nucleus,where the catalytic fragment of PKCδ induces apoptosis[22].Others and we have shown that full-length or fragmented PKCδ is translocated to the nucleus by transiting the nuclear pore[6,51].Nuclear PKCδ interacts with and phosphorylates its substrates such as α-Abl,p53,p73,lamin B,Rad9,topoisomerase II,heterogeneous nuclear ribonucleoprotein K (hnRNP-K),and DNAdependent protein kinase[22,52-54].Moreover,nuclear PKCδ regulates the transcription of target genes in response to cellular stresses such as DNA damage,which is implicated in pro-apoptotic functions.
Upon oxidative stress,we previously showed that PKCδ associates with and activates IKKα in the nucleus[55].Although IKKα activates NF-κB by phosphorylating IκB in the cytoplasm,which leads to prosurvival signaling,PKCδ-mediated IKKα activation at the nucleus causes phosphorylation of p53 at Ser20;however,it does not affect NF-κB activation.
The tumor suppressor p53 is a master regulator of cellular processes,such as cell cycle arrest,DNA repair,or apoptosis[56,57].Several studies have suggested that p53 is located downstream of PKCδ.In response to genotoxic stress,PKCδ phosphorylates p53 at Ser46 to trigger p53-mediated apoptosis.
In the nucleus,PKCδ also regulates p53 expression by increasingp53transcription.We previously reported that PKCδ interacts with the death-promoting transcription factor Btf to induce Btf-mediatedp53gene transcription and apoptosis[58].In addition,TNF-α treatment induces translocation of PKCδ into the nucleus[59].PKCδ can bind to the NF-κB RelA subunit and subsequently induce the transactivation of p65/RelA[59].These findings demonstrate that NF-κB is involved in PKCδ-mediated TNF/TNFrelated apoptosis-inducing ligand (TRAIL) resistance.PKCδ inhibition or knockdown decreased NF-κB expression and sensitized MCF7 cells to TNF/TRAIL-induced cell death[60].
Mitochondria
Bax and Bak are pro-apoptotic factors,and the Bcl-2 family regulates mitochondrial membrane permeability to induce apoptosis[61].Upon exposure to ionizing radiation,Bax and Bak are activatedviathe c-Abl-PKCδ-p38 pathway to trigger mitochondrial cell death[30,62].Mcl-1,an anti-apoptotic Bcl-2 family member,is a direct target of PKCδ.The catalytic fragment of PKCδ phosphorylates Mcl-1 and degrades it,leading to cell death.During the early stages of hypoxic stress,PKCδ induces autophagyviaJNK-mediated phosphorylation of Bcl-2 to dissociate the Bcl-2/beclin-1 complex,and prolonged hypoxic stress induces PKCδ cleavage[63].
Cell surface
We recently showed that PKCδ is localized at the cell surface of liver cancer cell lines(Figure 2).Cell surface PKCδ was found to be anchored by other cell surface proteins,such as heparan sulfate proteoglycans (HSPGs).Some growth factors,such as fibroblast growth factors,vascular endothelial growth factor,and hepatocyte growth factor[64,65] have cationic amino acid clusters that can interact with heparan sulfate,which is composed of one or more unbranched anionic polysaccharide(s) known as glycosaminoglycans[65-67].The cationic amino acid clusters closely resemble the NLS of intracellular proteins[68].In fact,extracellular NLS-containing proteins,such as importin α1,huRNP-K,and PKCδ are detected at the cell surface of human cells[9,69,70].These extracellular NLS-containing proteins are more likely to be located at the cell surface by binding to HSPGs.
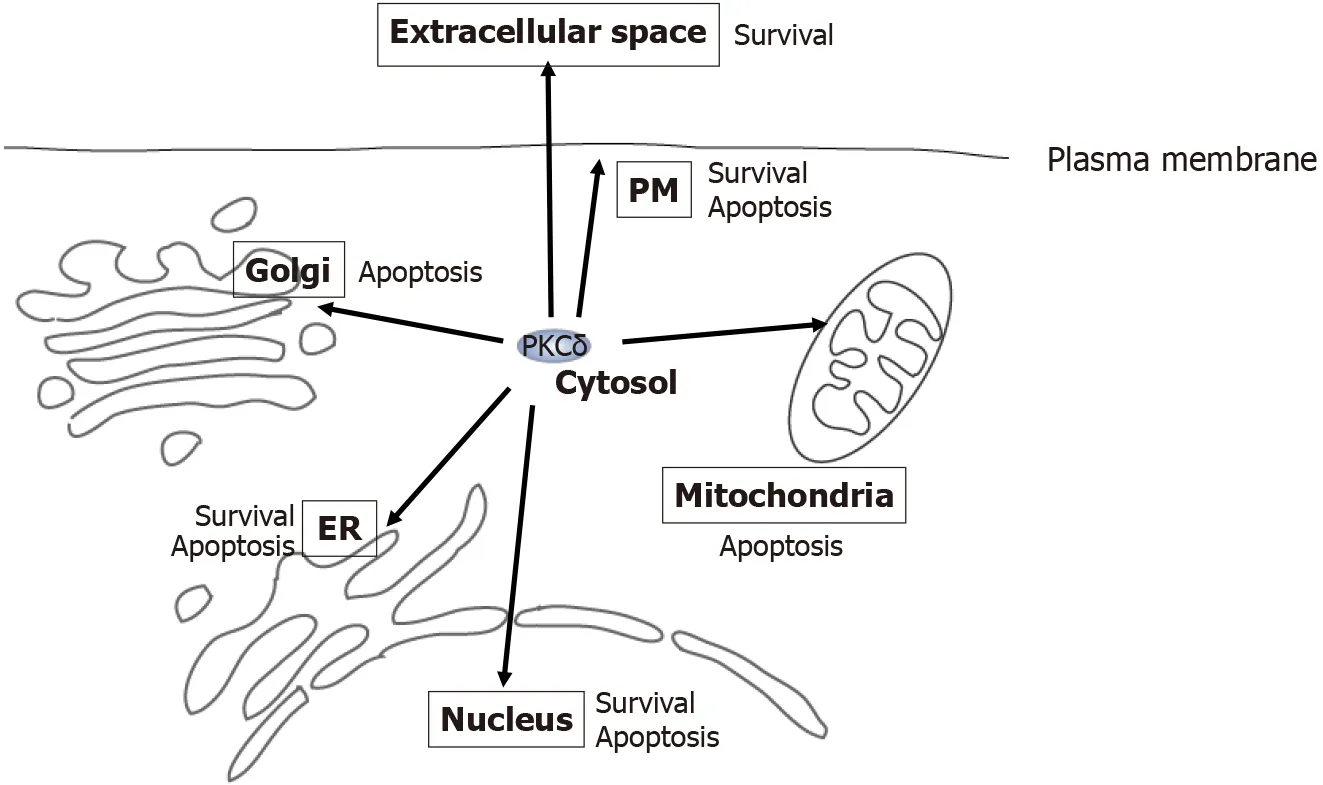
Figure 3 Multi-localization and functional diversity of protein kinase Cδ.Protein kinase Cδ (PKCδ) resides at various locations,including the cytosol,nucleus,estrogen receptor (ER),mitochondria,Golgi,extracellular space,and plasma membrane (inside and outside the cell).At each location,PKCδ acts as an apoptotic or survival factor in response to various stimuli,such as genotoxic stresses,phorbol ester,DNA damage,ER stress,tumor necrosis factor (TNF)-α,and TNF-related apoptosis-inducing ligand.PKCδ:Protein kinase Cδ;ER:Estrogen receptor;PM:Plasma membrane.
Furthermore,glypican3,a liver cancer-specific HSPG,was identified as a receptor for cell surface PKCδ[71].Both Chenget al[72,73] and we showed that GPC3 regulates the activation of insulin-like growth factor 1 receptor (IGF1R)[9].In fact,we found that extracellular PKCδ induces activation of IGF1Rviaassociation with GPC3 and its downstream signaling molecules,such as ERK1/2 and STAT3.Thus,these lines of evidence strongly suggest that cell surface PKCδ acts as a growth factor.In addition,we showed that anti-PKCδ monoclonal antibody (mAb) inhibits the proliferation and tumorigenesis of liver cancer cells,but not PKCδ-CRISPR knockout cells.Thus,cell surface PKCδ may be a potential therapeutic target for liver cancer.
Extracellular space
We also found that PKCδ is secreted into the extracellular space in liver cancer[9].Extracellular accumulation of PKCδ was detected in different liver cancer cell lines but not in hepatocytes,suggesting that PKCδ secretion may be specific to liver cancer cells.Interestingly,our proteomics study showed that PKC,rather than PKCδ,was not detected in the culture medium of liver cancer cell lines.This means that PKCδ is a unique isoform of the PKC superfamily that is secreted extracellularly.Furthermore,higher levels of PKCδ were detected in the serum of patients with liver cancer,but not in patients with chronic hepatitis,hepatic cirrhosis,or healthy donors.This increase in serum PKCδ levels was also noted in a limited number of AFP-and PIVKA-II-negative liver cancer patients.Based on these clinical data,we propose that serum PKCδ may be a novel biomarker for liver cancer.
Recently,we and other groups have reported the extracellular localization of proteins with no signal peptide-containing proteins,such as FGF1,FGF2,HMGB1,hnRNP-K,importin α1,and IL-1β[69,70,74-77].Secretion of these proteins is referred to as unconventional secretion[74,78].Since thePKCδgene does not encode a signal peptide,the extracellular secretion of PKCδ is also categorized as unconventional secretion.PKCδ has been shown to be full-length in the extracellular space and continues to be released from growing cells[9].Many studies have reported that IL-1β secretion often occurs in immune cells after induction of inflammatory stimulation[77,79,80].There are some differences in the secretion modes between immune and cancer cells.Unlike immune cells (IL-1β),liver cancer cells constitutively secrete importin α1 and PKCδ even under physiological culture conditions (using 10% FBS medium)[9,69].Conversely,some features were common between immune and liver cells,including the induction of unconventional secretion by ATP treatment[81] and independent of brefeldin A,an inhibitor in the“conventional”secretion pathway of signal peptide-containing proteins[82].
We found that PKCδ secretion was initiated in the cytosol.Phorbol ester treatment inhibited PKCδ secretion,and the NLS active mutant was not secreted into the extracellular space.In fact,secreted PKCδ showed a lower level of phosphorylation(Tyr311 and Thr505).These lines of evidence support the possibility that cytosolic PKCδ,as a starting point for extracellular localization,could contribute to tumor progression in liver cancer.
Other organelles
A previous study has shown that PKCδ is translocated to the ER in response to ER stress and interacts with ER-bound c-Abl[83].This PKCδ-c-Abl complex consequently moves to the mitochondria to trigger apoptosis[83].It has been reported that tyrosine phosphorylation of PKCδ is associated with this interaction with c-Abl[84].The chemical inhibitor rottlerin blocks the translocation of the PKCδ-c-Abl complex from the ER to the mitochondria,which confers protection against apoptosis[83].Another ER protein,p23 (Tmp21),interacts with PKCδ,which enables the retention of PKCδ in the ER[85].Translocation of PKCδ to the ER has also been reported in cells with Sindbis virus and in glioma cells treated with TRAIL,where PKCδ exerts an antiapoptotic effect.Furthermore,a small amount of PKCδ has been observed in the Golgi apparatus.Ceramide or IFN-γ stimulation has been shown to translocate PKCδ to the Golgi apparatus,which is associated with ceramide-induced apoptosis in HeLa cells.
CONCLUSlON
The apoptotic and survival functions of PKCδ are defined by cell and tissue types and their cellular conditions (Figure 3).In response to cellular stresses,PKCδ may be translocated to different organelles (including the cytosol and extracellular space),where PKCδ executes distinct functions in each location.Among the many types of tissues and cells,liver cancer cells have the most patterns of localization of PKCδ,including conventional intracellular and extracellular localization.Notably,extracellular PKCδ is involved in the tumorigenesis of liver cancer;therefore,it is a promising novel diagnostic and therapeutic target for liver cancer.Additional studies are required to elucidate further the various roles of PKCδ in liver cancer cells,which are dependent on the expression,subcellular distribution,and tumor microenvironment.
 World Journal of Gastroenterology2022年2期
World Journal of Gastroenterology2022年2期
- World Journal of Gastroenterology的其它文章
- Nanotheranostics:A powerful next-generation solution to tackle hepatocellular carcinoma
- Observational Study ldentification of functional tumor necrosis factor-alpha promoter variants associated with Helicobacter pylori infection in the Sudanese population:Computational approach
- Prospective Study Outreach onsite treatment with a simplified pangenotypic directacting anti-viral regimen for hepatitis C virus microelimination in a prison
- Therapeutic endoscopy for the treatment of post-bariatric surgery complications
- Update on the applications and limitations of alpha-fetoprotein for hepatocellular carcinoma
- Case Control Study Obesity is associated with decreased risk of microscopic colitis in women
