Chidamide combined with cyclophosphamide,doxorubicin,vincristine and prednisone in previously untreated patients with peripheral T-cell lymphoma
Lin Gui ,Junning Cao ,Dongmei Ji ,Huilai Zhang ,Qian Fan ,Jun Zhu ,Yuqin Song,Shiyu Jiang,Zhiqiang Ning,Jia Yu,Yuankai Shi
1Department of Medical Oncology,National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital,Chinese Academy of Medical Sciences &Peking Union Medical College,Beijing Key Laboratory of Clinical Study on Anticancer Molecular Targeted Drugs,Beijing 100021,China;2 Department of Medical Oncology,Fudan University Shanghai Cancer Center,Shanghai Medical College,Fudan University,Shanghai 200032,China;3Department of Lymphoma,Sino-US Center for Lymphoma and Leukemia Research,Tianjin Medical University Cancer Institute and Hospital,National Clinical Research Center of Cancer,Key Laboratory of Cancer Prevention and Therapy,Tianjin’s Clinical Research Center for Cancer,Tianjin 300060,China;4 Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing),Department of Lymphoma,Peking University Cancer Hospital &Institute,Beijing 100142,China;5 Shenzhen Chipscreen Biosciences,Co.LTD.,Shenzhen 518057,China
Abstract Objective:Chidamide is an oral histone deacetylase subtype-selective inhibitor approved for relapsed or refractory peripheral T-cell lymphoma (PTCL).This phase 1b study evaluated the safety,pharmacokinetics,and preliminary efficacy of chidamide in combination with cyclophosphamide,doxorubicin,vincristine and prednisone(CHOP) for treatment-naïve PTCL patients.Methods:This study was an open-label,multicenter trial composed of dose escalation and dose expansion.Patients received CHOP for six 21-d cycles and chidamide on d 1,4,8 and 11 in each cycle.Four dose levels of chidamide (20,25,30 and 35 mg) were evaluated.The primary objective was to evaluate the safety and tolerability of the combination regimen.Results:A total of 30 patients were evaluated in this study:15 in the dose-escalation part and 15 in the doseexpansion part.In the dose-escalation study,three patients were enrolled in the 35 mg chidamide cohort.One had dose-limiting toxicity with grade 3 vascular access complications,and one had grade 2 neutropenia with a sustained temperature >38 °C.Dose escalation was stopped at this chidamide dose level.The most common (≥10%) grade 3 or 4 adverse events (AEs) were leukopenia (90.0%),neutropenia (83.3%),vomiting (13.3%),thrombocytopenia(10.0%) and febrile neutropenia (10.0%).No significant changes in chidamide pharmacokinetic properties were observed before and after combination treatment.The objective response rate for the 28 patients evaluable for preliminary efficacy was 89.3% (25/28),with 16 (57.1%) achieving complete response or unconfirmed complete response.The estimated median progression-free survival was 14.0 months.In summary,we chose chidamide 30 mg as the recommended dose for phase 2.Conclusions:The addition of chidamide to standard CHOP chemotherapy was tolerable with promising preliminary efficacy in previously untreated PTCL patients,which supports further clinical studies with this combination regimen for the frontline treatment of PTCL.
Keywords:Chidamide;CHOP;PTCL;frontline treatment
Introduction
Peripheral T-cell lymphoma (PTCL) is a heterogeneous cohort of clinically aggressive diseases with poor prognosis.PTCL accounts for 25%-30% of all non-Hodgkin’s lymphoma (NHL) cases in China,which is higher than that in America and Europe (10%-15%) (1-4).Frontline therapies usually include cyclophosphamide,doxorubicin,vincristine and prednisone (CHOP) or CHOP-like regimens (3,4). However,efficacy results from anthracycline-containing regimens are not satisfactory,with low complete response rates (CRR) and poor progression-free survival (PFS) and overall survival (OS)(5),underscoring the high unmet medical need.
Chidamide (CS055/tucidinostat) is an oral histone deacetylase (HDAC) inhibitor with subtype specificity in the inhibition of HDAC 1,2,3 and 10 (6).As a genuine epigenetic modulator,chidamide has been shownin vitroandin vivoto induce apoptosis and growth arrest in cancer cells,reverse epithelial-mesenchymal transitions and drug resistance of cancer cells and enhance natural killer celland antigen-specific CD8+T lymphocyte-mediated antitumor activity (7-10).Based on results from multicenter pivotal phase 2 trials,chidamide was approved in China for relapsed or refractory PTCL (11,12).Real-world studies(RWSs) of chidamide in larger patient populations further demonstrated the favorable efficacy and potential survival benefit in relapsed or refractory settings (13).The main toxicity associated with chidamide treatment was acceptable hematological adverse events (AEs) (11,13).
In view of the unmet medical needs and overall promising efficacy with the well-tolerated safety profile of chidamide in patients with relapsed or refractory PTCL,we wanted to evaluate the safety,pharmacokinetics,and preliminary efficacy of chidamide in combination with CHOP for the frontline treatment of PTCL patients.
Materials and methods
Patients
Eligible patients were 18-65 years of age with histologically proven,previously untreated PTCL with the following subtypes:PTCL-not otherwise specified (PTCLNOS),angioimmunoblastic T-cell lymphoma (AITL),ALK-positive or ALK-negative anaplastic large cell lymphoma (ALCL),or other subtypes that investigators considered to be appropriate.Patients had no bone marrow involvement with neutrophil count ≥2.0×109/L,platelet count ≥100×109/L,and hemoglobin ≥110 g/L and had an Eastern Cooperative Oncology Cohort (ECOG)performance status of 0 or 1.The main exclusion criteria were meningeal or central nervous system (CNS)involvement of the disease,human immunodeficiency virus(HIV) or active hepatitis B virus (HBV) or hepatitis C virus(HCV) infection,inadequate hepatic or renal laboratory values [serum total bilirubin >1.5 the upper normal limit(ULN),alanine aminotransferase (ALT)/aspartate aminotransferase (AST) >2.5 ULN or >5 ULN for liver metastasis,and serum creatine >1.5 ULN],Q-T interval corrected (QTc) >450 ms,history of significant cardiovascular disease,active infection or persistent fever during 14 d before study entry,or mental disorders that could interfere with treatment compliance.
Written informed consent was obtained from every patient upon entry for screening and enrollment.The ethics committee of each participating hospital approved the study,which was conducted in accordance with the principles of Good Clinical Practice,the Declaration of Helsinki and the regulations of China.This trial was registered at ClinicalTrials.gov (NCT02809573).
Study design and treatment
This was an open-label,multicenter phase 1b study to evaluate the safety and tolerability,pharmacokinetics,and preliminary efficacy of chidamide in combination with the CHOP regimen in previously untreated PTCL patients.Patients received 6 cycles of CHOP at 3-week intervals[intravenous cyclophosphamide 750 mg/m2,doxorubicin 50 mg/m2,and vincristine 1.4 mg/m2(maximum 2 mg) on d 1,and oral prednisone 100 mg qd on d 1-5] in combination with various doses of oral chidamide on d 1,4,8 and 11 of each cycle.Patients who achieved a CR or unconfirmed CR(CR/CRu) at the end of combination treatment received maintenance treatment with chidamide alone on d 1,4,8 and 11 every 21 d for a maximum of 24 months.
The study was composed of a dose-escalation part and a dose-expansion part.In the dose-escalation study,a fourday lead-in period with a single dose of chidamide was applied before the first cycle of combination treatment started,and blood samples were collected for pharmacokinetic analysis.Blood samples were also collected before and after the first combination treatment at various time points up to 72 h for pharmacokinetic analysis.With the fixed standard CHOP regimen,a 3+3 design of dose escalation for chidamide with a starting dose of 20 mg was employed.Dose-limiting toxicities (DLTs)were evaluated in the first treatment cycle.If one DLT occurred out of three patients,another three patients were further evaluated at the same dose.If two DLTs occurred in a maximum of six patients,dose escalation was stopped.DLTs were defined according to the Common Terminology Criteria for Adverse Events (CTCAE)version 4.02 as grade 4 neutropenia lasting >5 d,febrile neutropenia,grade 4 thrombocytopenia or grade 3 thrombocytopenia with bleeding,grade 4 anemia,grade 3 or worse nonhematological toxicity or toxicities resulting in 2 weeks or more delay of the second cycle of combination treatment.A dose decrease in chidamide or CHOP was not permitted in the first combination treatment cycle.Prophylactic recombinant human granulocyte colonystimulating factor (rhG-CSF) was not permitted in this study.However,after the last dose of chidamide of each treatment cycle,if the white blood cell count (WBC) was<2×109/L or the absolute neutrophil count (ANC) was<1×109/L,rhG-CSF (filgrastim) at a dose of 5 μg/kg was applied until recovery of WBCs or ANCs.Medications for prophylaxis of tumor lysis and antiemetics were allowed.Once the maximum tolerated dose (MTD) was determined,in the expansion phase,we aimed to increase the cohorts of patients receiving the dose of MTD to a total of 9 patients.Since a dose of 5 mg below the MTD and a dose of 10 mg below the MTD may be used in clinical treatment when severe toxicities occur at the MTD,in the expansion phase,we also increased the cohorts of patients receiving a dose of 5 mg below the MTD and a dose of 10 mg below the MTD to a total of 9 patients.
Assessments
The incidence,nature,severity,and relatedness of AEs were graded according to the NCI-CTCAE version 4.02.Tumor assessment was carried out by the investigator using response criteria for NHL at screening,every 6 weeks for combination treatment and every 12 weeks for maintenance treatment (14).The best response of CR/CRu or partial response (PR) had to be confirmed after 4-6 weeks.Patients were withdrawn from the study if they developed progressive disease or unacceptable toxicity. Survival follow-up was performed every 6 months.
A pharmacokinetic study of chidamide was conducted in the dose-escalation part.Blood samples were collected at pre-dose and 1,2,4,8,12,24,48 and 72 h after the first dose in the run-in period and during the first cycle of combination treatment.The plasma concentration of chidamide was assayed according to a validated highperformance liquid chromatography coupled with tandem mass spectrometry (HPLC-MS/MS) method.Individual pharmacokinetic parameters were estimated following noncompartmental methods using Phoenix WinNonlin Version 7.0 (Pharsight Corporation Mountain View,CA,USA) and SAS version 9.4 (SAS Institute Inc.,Cary,NC,USA).
Statistical analysis
All analyses were descriptive,and formal hypotheses were not tested.Safety analysis included all patients who had received at least one dose of the study drug and for whom at least one safety case report form had been completed.Pharmacokinetic analysis included all patients who had received at least one dose of the study drug in the doseescalation study and for whom at least one blood sample had been tested.Efficacy analysis included all patients who had received at least one dose of the study drug and were evaluable for response.
Continuous variables were summarized with the sample size,mean,median and range.Categorical variables were summarized with the counts and percentages of patients in the corresponding categories.The CR rate (CRR) and overall response rate (ORR) were estimated using 95%exact binominal confidence intervals.PFS (from d 1 of treatment to progression or death from any cause),OS(from d 1 of treatment to death),and the duration of response (DOR,from the date of first documentation of a response to the date of first documented evidence of progression,relapse,or death from any cause) were evaluated using the Kaplan-Meier method.Patients lost to follow-up were censored at their last tumor assessment date.All statistical analyses were performed using SAS version 9.4.
Results
Patients
Between November 15,2016,and August 29,2018,30 treatment-naïve PTCL patients were enrolled in the study.The patient characteristics are shown inTable 1.The median age was 52.5 (range,42.0-58.0) years,and 19(63.3%) patients were males.The most common subtypes were PTCL-NOS (n=12,40.0%) and angioimmunoblastic T-cell lymphoma (AITL) (n=8,26.7%).Four patients of ALK-positive systemic ALCL with an International Prognostic Index (IPI) score ≥2 were included in the study.In the total cohort,11 (36.7%) patients had extranodal involvement,and the majority of patients (19 of 30) had Ann Arbor stage III or IV disease.

Table 1 Patient baseline and disease characteristics
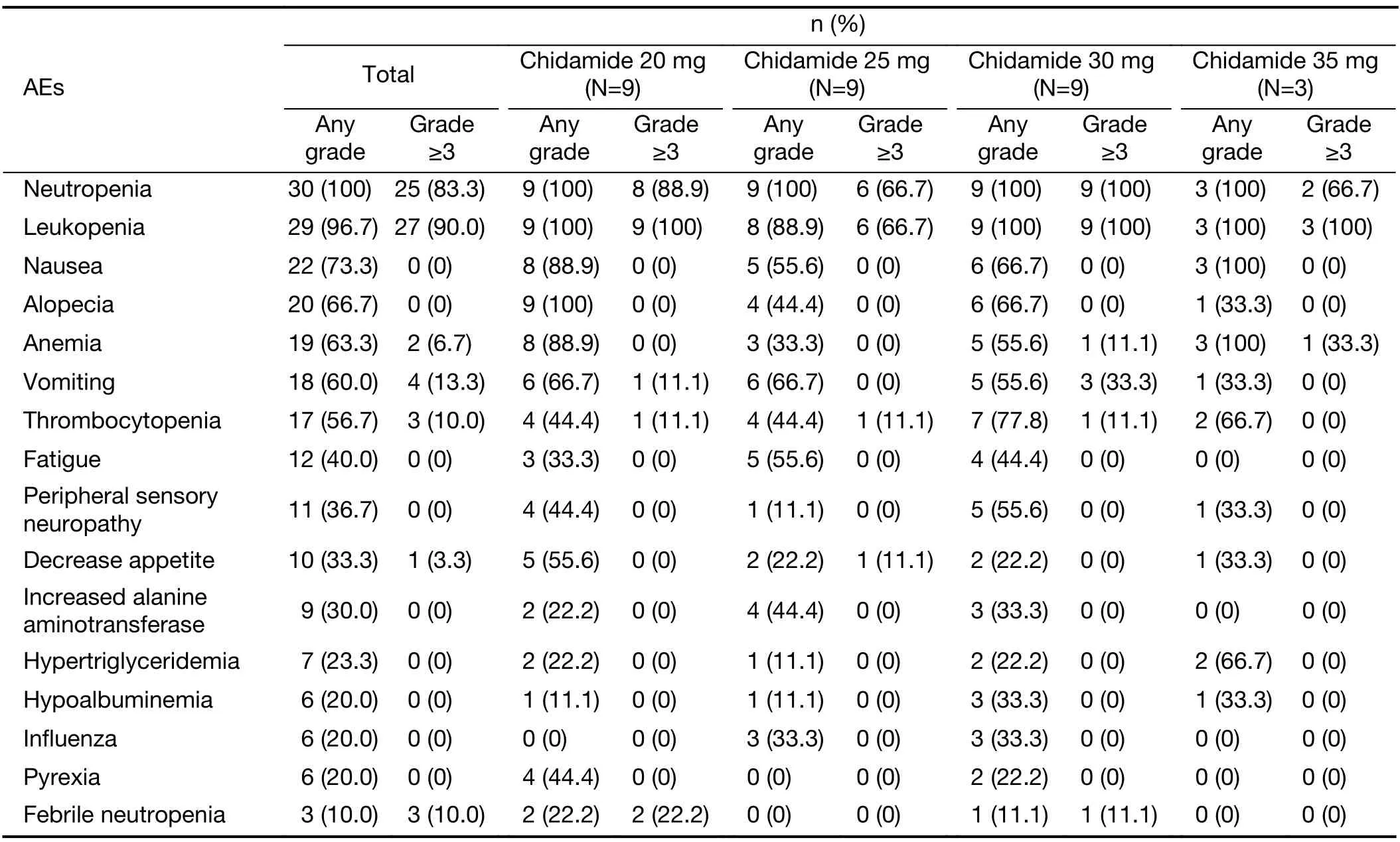
Table 2 AEs of any grade occurring in ≥20% of patients and grade ≥3 AEs occurring in ≥10% of patients in combination treatment
Dose escalation and expansion
During dose escalation,two patients experienced DLT in the first cycle of chidamide plus CHOP.One patient out of the first three enrolled in the chidamide 20 mg cohort had grade 3 febrile neutropenia on d 13 that met the DLT definition in the protocol.Another three patients were further evaluated in this dose cohort,and no DLTs were observed.No DLT occurred in the chidamide 25 mg and 30 mg cohorts.One DLT with grade 3 vascular access complications was reported in the chidamide 35 mg cohort.Additionally,in this dose cohort,one patient experienced grade 2 neutropenia (ANC 1.05 G/L) with sustained temperature >38 °C (Tmax38.5 °C) and received rhG-CSF treatment on d 11 at the emergency clinic outside the study center. After an overall evaluation of tolerability characteristics among cohorts,the dose-escalation committee decided to stop chidamide escalation at 35 mg.A total of 15 patients were treated in the dose-escalation part.Chidamide 30 mg on d 1,4,8 and 11 plus CHOP was considered the candidate dosage for the subsequent phase 2 study in treatment-naïve PTCL.
In the expansion phase,another 6 patients were treated in the chidamide 30 mg cohort,and 6 patients and 3patients were treated in the chidamide 25 mg and 20 mg dose cohorts,respectively.In total,9 patients were enrolled in the 30 mg,25 mg,and 20 mg dose cohorts in this study(Figure 1).
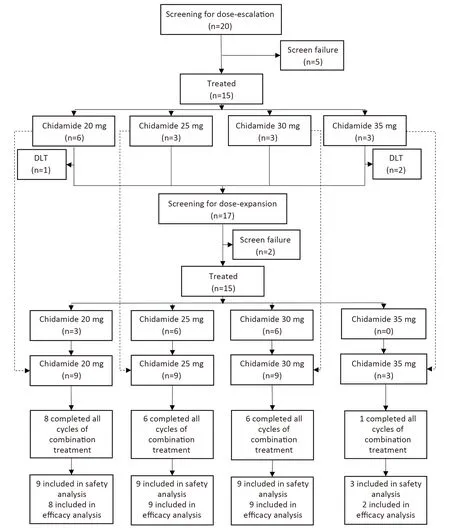
Figure 1 Study profile.
Treatment
Twenty-one (70.0%) patients completed all six cycles of combination treatment.Discontinuation of combination treatment prematurely occurred due to toxicity (4 patients),disease progression (4 patients),and withdrawal of consent(1 patient). Sixteen patients with CR/CRu entered maintenance treatment.
Safety
Table 2summarizes the AEs of any grade occurring in≥20% of patients and grade 3-4 AEs occurring in ≥10% of patients in any cycle of combination treatment.All patients had a certain degree of hematological toxicity,and the most common grade 3 or 4 hematological AEs were leukopenia(90.0%),neutropenia (83.3%),thrombocytopenia (10.0%),and febrile neutropenia (10.0%).Twelve of 15 patients in the dose-escalation study and 7 of 15 patients in the doseexpansion study were given rhG-CSF after the last dose of chidamide was completed in the first treatment cycle for ≥grade 3 AEs of a decrease in WBCs or ANCs.Vomiting(13.3%) was the most common grade 3-4 nonhematological AE.Other nonhematological AEs were almost all grade 1-2,including alopecia,fatigue,peripheral sensory neuropathy,and gastrointestinal disorders (nausea and decreased appetite).Eleven (36.7%) patients had at least one chidamide dose reduction due to AEs in the combination phase.No grade 5 AEs occurred.
A total of 12 treatment-related SAEs were observed in 6(20.0%) of 30 patients during combination treatment:one,two,two and one patient in the 20 mg,25 mg,30 mg and 35 mg cohorts,respectively.These SAEs included neutropenia [3 (10.0%)],leukopenia [2 (6.7%)],interstitial pneumonia [2 (6.7%)],thrombocytopenia [1 (3.3%)],febrile neutropenia [1 (3.3%)],pneumonia [1 (3.3%)],hyponatremia [1 (3.3%)],and vascular access complications[1 (3.3%)].Two SAEs (one case of interstitial pneumonia and one case of pyrexia) were reported in one (6.3%) of 16 patients during chidamide maintenance treatment.This patient was in the 20 mg cohort.Chidamide was paused,and when the AEs were resolved,treatment continued without dose adjustment.
Three patients who prematurely interrupted study treatment due to DLTs were described above.Treatment was permanently discontinued in another patient in the dose-expansion cohort after four treatment cycles because of disabling peripheral sensorimotor neuropathy.Another patient had grade 3 increased bilirubin after three cycles of CHOP,and treatment was discontinued.
Pharmacokinetics
All 15 patients in the dose-escalation study were evaluated for pharmacokinetic properties of chidamide before and after the first dose of combined administration with CHOP.The mean plasma concentration-time profiles of chidamide for each dose of 20,25,30,or 35 mg were similar when administered alone or following concomitant administration with CHOP (Figure 2). Other pharmacokinetic parameters for chidamide,including the time to reach maximum concentration (Tmax),maximum plasma drug concentration (Cmax),area under the curve(AUC),and half-life (t1/2),were comparable before and after combination treatment (Table 3).On the other hand,over the dose range of 20-35 mg,the Cmaxand AUC of chidamide increased proportionately with increasing dose,demonstrating the linear and dose-dependent PK profile of chidamide in both monotherapy and combination treatment (Table 3).
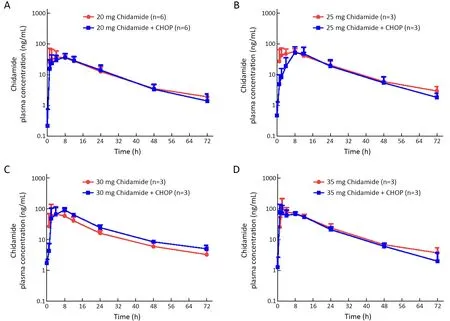
Figure 2 Mean plasma concentration-time curves of Chidamide at 20 mg (A);25 mg (B);30 mg (C);and 35 mg (D) before and after concomitant administration with CHOP.CHOP,cyclophosphamide,doxorubicin,vincristine,and prednisone.
Antitumor activity
Given that 1 patient in the 20 mg cohort and 1 patient in the 35 mg cohort discontinued the study before evaluation because of DLT,the remaining 28 patients (in the 20-35 mg dose cohorts) were considered evaluable for efficacy.A summary of the confirmed best overall response on the basis of investigator review is provided inTable 4.A total of 16 (57.1%) patients achieved the best response of CR/CRu;4 (50.0%),5 (55.6%),and 7 (77.8%) patients achieved the best response to 20 mg,25 mg and 30 mg,respectively;and 9 (32.1%) patients achieved PR.ORR (CR+PR) was 89.3%for 28 evaluable patients. Sixteen patients entered chidamide maintenance treatment.The median duration of follow-up was 17.4 (10.5,21.2) months at the time of data cutoff (February 5,2020). The median time for maintenance treatment was 279.0 (4,748) d,with no treatment discontinuation due to AEs.In the total cohort,the estimated median PFS was 14.0 months,and the median OS and DOR were not achieved.PFS and OS at 12 months were 59.3% and 100%,respectively.
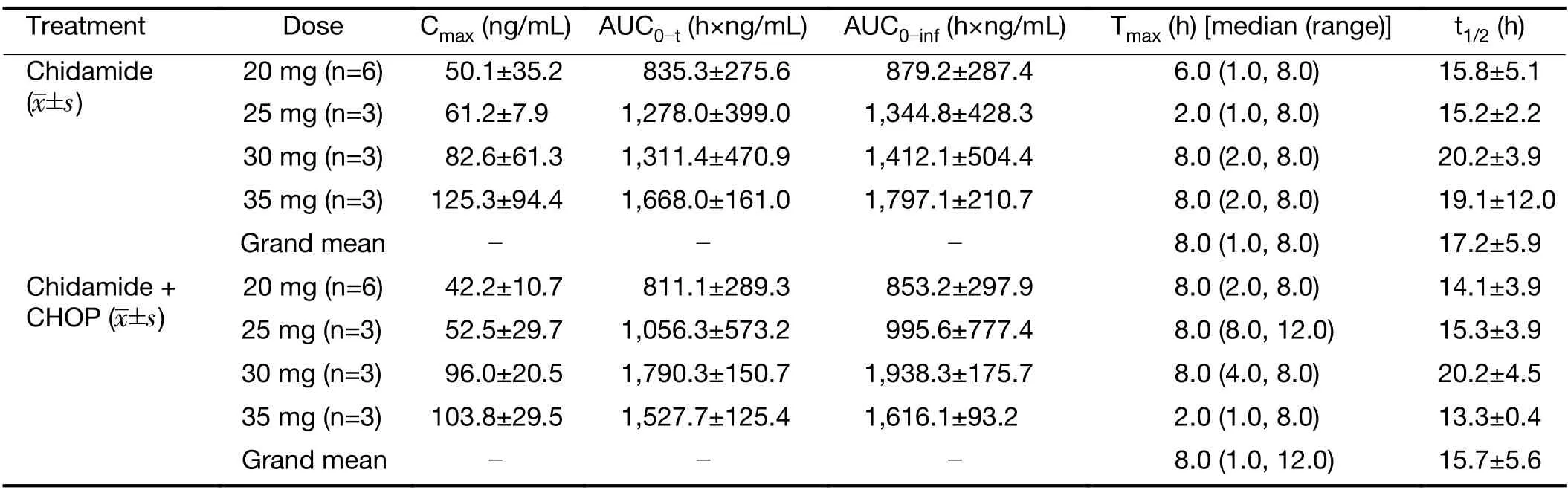
Table 3 Pharmacokinetic parameters of chidamide
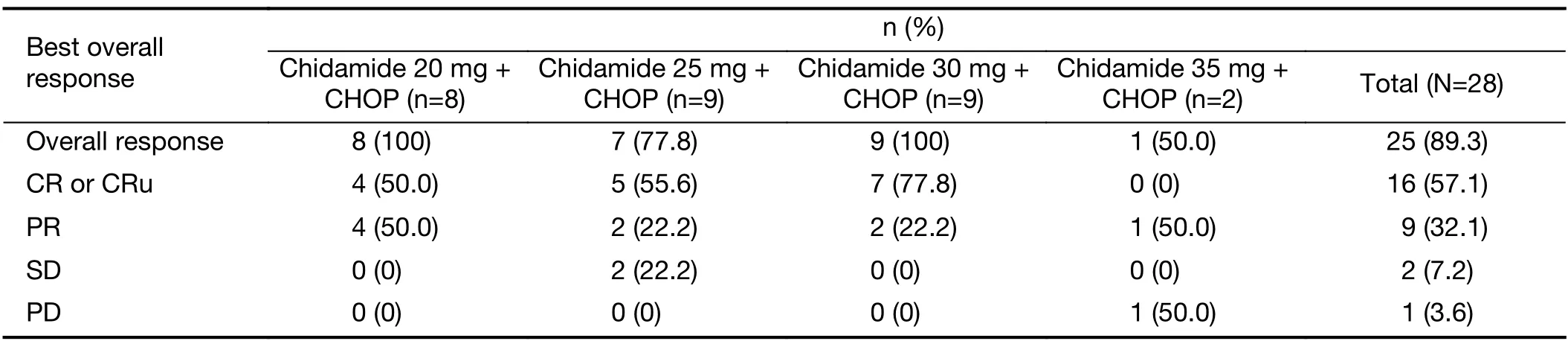
Table 4 Best ORR for evaluable subjects
Discussion
Accumulating evidence suggests that altered epigenetic modifications could contribute to the initiation and development of different types of malignances,including T-cell lymphomas (15,16).As epigenetic modulators,several HDAC inhibitors,including romidepsin,belinostat and chidamide,have been demonstrated to have singleagent activity in patients with relapsed or refractory PTCL and are widely used in the treatment of this patient population (17-19).CHOP or CHOP-like regimens are the historical standard and the most widely used frontline approach for PTCL (20-22).However,apart from ALKpositive ALCL,the remission and survival benefits of these anthracycline-containing regimens are limited in most PTCL subtypes (23).Despite efforts in frontline therapy with intensified approaches,such as the addition of etoposide to CHOP and consolidation with stem cell transplantation,durable remission and survival outcomes are still not satisfactory (23).A breakthrough has recently been achieved in which brentuximab vedotin in combination with CHP (CHOP without vincristine)demonstrated a significant improvement in PFS and OS over CHOP in patients with previously untreated systemic ALCL or other CD30-expressing PTCLs,including AITL and PTCL-NOS (24).Although this regimen approved by the US Food and Drug Administration (FDA) is for only CD30-expressing PTCLs,which may limit its wide application in many other PTCL subtypes,it represents a major advance for the frontline treatment of PTCL patients with targeted agents in combination with CHOP or CHOP-like chemotherapies.
Study results from real-world application of chidamide in 383 patients with relapsed or refractory PTCL showed an ORR of 39% and 51% in monotherapy and in combination chemotherapy,respectively,with well accepted safety and tolerability (13).Hematotoxicity is the most encountered side effect of chidamide (11,13).Based on its overall efficacy and safety profile in relapsed or refractory PTCL patients,we sought to examine the potential of this oral subtype-specific HDAC inhibitor in combination with CHOP in frontline treatment.In the standard 3-week CHOP regimen,hematotoxicity occurred mostly on d 10-14 of each treatment cycle (25).To reduce the potential overlapping hematotoxicity in the combination treatment for treatment-naïve PTCL patients,we used a chidamide dosing regimen of twice per week for 2 consecutive weeks in a 3-week cycle with the CHOP regimen,rather than the successive weekly dosing used in monotherapy for relapsed or refractory PTCL patients.Furthermore,rhG-CSF was allowed to be used after the last dose of chidamide in the first treatment cycle when the grade 3 or above AEs of a decrease in WBCs or ANCs occurred.The results of this phase 1b study showed that the addition of chidamide to the standard CHOP regimen had an acceptable safety profile in previously untreated PTCL patients.Hematological toxicity and gastrointestinal disorders were the most common adverse reactions associated with the combination treatment,which were anticipated and manageable.Generally,we did not observe a significant difference in the frequency and severity of AEs among the chidamide dose cohorts,each with 9 patients.In addition,77.8% (7/9) of patients in the 30 mg dose group achieved CR/CRu,which was higher in number than the CR rates achieved in the 20 mg or 25 mg dose groups.The recommended phase 2 dose (RP2D) for chidamide combined with CHOP was determined to be 30 mg twice a week for two weeks.
The results of the chidamide pharmacokinetic analysis indicate no significant changes in the chidamide pharmacokinetic properties before and after combination treatment.The pharmacokinetic properties of chidamide in combination with CHOP displayed similar pharmacokinetic properties to those obtained from phase 1 studies of chidamide alone and chidamide in combination with paclitaxel and carboplatin (26,27).These results suggested a lack of significant drug-drug interactions between the chidamide and CHOP regimens during coadministration.
Because of the rare and highly heterogeneous nature of the disease,there have been fewer prospective randomized clinical studies of PTCL with the CHOP regimen as frontline treatment.The efficacy reported,mostly from phase 2 studies and retrospective analyses,varies greatly.For instance,CRRs are reported within a wide range from 29% to 43% among different studies (5,21,28).Recently,HDAC inhibitors have been integrated with upfront chemotherapy of PTCLs.Romidepsin was combined with CHOP in a phase 1b/2 study including 35 untreated PTCL patients (29).The ORR was 68%,with a CR of 51%.Twenty-nine (78%) of these patients had grade 3-4 thrombocytopenia.The final analysis of phase 3 study comparing romidepsin plus CHOP with CHOP revealed that the PFS,ORR and OS appeared to be similar between the two groups (30).The high rates of emergent AEs with the addition of romidepsin hampered the ability to adequately administer 6 cycles of CHOP and reduced the patient’s benefit from the combination.Belinostat was administered in combination with CHOP in a phase 1 study of treatment-naïve PTCL patients (31).In this study,patients received primary prophylaxis with rhG-CSF support.At the time of the report,eighteen of 23 patients(78%) had completed all 6 cycles of treatment,and no grade ≥3 thrombocytopenia was observed.The ORR was 89% (16/18),with a CR of 72%.Chidamide was combined with CHOEP (CHOP with etoposide) in a phase 1b/2 study (32).The RP2D for chidamide was determined to be 20 mg twice a week for 3 weeks.A total of 113 patients were enrolled in the phase 2 study,and the ORR was 60.2%,with a CR of 40.7%.The median PFS was 10.7 months,with a 1-year PFS rate of 49.9%.Thirty-five(31%) of the patients had grade ≥3 thrombocytopenia in this study.The current study combining chidamide with CHOP showed potentially more promising efficacy results and better safety features.Clinical activity was demonstrated with an ORR of 89.3%,a CR/CRu rate of 57.1%,and a median PFS of 14.0 months.Grade ≥3 thrombocytopenia was observed in 3 (10.0%) patients,which was lower than that seen in most of other studies.
Conclusions
The results of this phase 1b study showed that the addition of chidamide to the standard CHOP regimen may be safely administered in previously untreated PTCL patients.Hematological toxicity and gastrointestinal disorders were associated mostly with the combination treatment,which were manageable with standard supportive care.Promising preliminary antitumor activity of the combination regimen was observed.Based on the overall results from this study,the combination of CHOP for six cycles with chidamide 30 mg twice per week for two consecutive weeks in each cycle is recommended as a treatment regimen for the planned clinical studies in frontline treatment of PTCL.
Acknowledgements
The study was supported by the sponsor Shenzhen Chipscreen Biosciences Co.,LTD.and by grants from the China National Major Project for New Drug Innovation(No.2017ZX09304015) and the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences(CIFMS) (No.2016-I2M-1-001).
Footnote
Conflicts of Interest:ZN and JY are employees of Shenzhen Chipscreen Biosciences Co.,LTD.All the remaining authors have no conflicts of interest to declare.
 Chinese Journal of Cancer Research2021年5期
Chinese Journal of Cancer Research2021年5期
- Chinese Journal of Cancer Research的其它文章
- Lung cancer risk in never-smokers:An overview of environmental and genetic factors
- Better prognostic determination of cT3 rectal cancer through measurement of distance to mesorectal fascia:A multicenter study
- A radiomics prognostic scoring system for predicting progression-free survival in patients with stage IV non-small cell lung cancer treated with platinum-based chemotherapy
- Single patient classifier as a prognostic biomarker in pT1N1 gastric cancer:Results from two large Korean cohorts
- Coexisting opportunities and challenges:In which scenarios can minimal/measurable residual disease play a role in advancednon-small cell lung cancer?
- Integrating pathomics with radiomics and genomics for cancer prognosis:A brief review
