Coexisting opportunities and challenges:In which scenarios can minimal/measurable residual disease play a role in advancednon-small cell lung cancer?
Hanfei Guo ,Wenqian Li ,Bin Wang ,Neifei Chen ,Lei Qian ,Jiuwei Cui
1Cancer Center,the First Hospital of Jilin University,Changchun 130021,China;2 Department of Neurosurgery,the First Hospital of Jilin University,Changchun 130021,China
Abstract Curative therapy was not previously available for patients with advanced non-small cell lung cancer (NSCLC);thus,the concept of minimal/measurable (or molecular) residual disease (MRD) was not applicable to these patients.However,advances in targeted and immunotherapy have revolutionized the treatment landscape for patients with advanced NSCLC,with emerging evidence of long-term survival and even the hope of complete remission (CR) by imaging examination.The latest research shows that patients with oligometastatic lung cancer can benefit from local treatment.After removing the lesions,the choice of follow-up therapy and monitoring of the lesions could remain uncertain.MRD plays a role in identifying early-stage NSCLC patients with high risks of recurrence and determining adjuvant therapy after radical treatment.In recent years,evidence has been accumulating regarding the use of circulating cell-free tumor DNA (ctDNA) to assess MRD in solid tumors.This study discussed the possible applications of ctDNA-based MRD monitoring in advanced NSCLC and described the current challenges and unresolved problems in the application of MRD in advanced NSCLC.
Keywords:Minimal residual disease;non-small cell lung cancer;circulating tumor DNA
Introduction
Lung cancer is a leading cause of cancer-related deaths worldwide (1).Approximately 75% of patients with nonsmall cell lung cancer (NSCLC) are diagnosed after the disease has reached an advanced and unresectable stage (2),at which point surgical resection is not a feasible option and the prognosis is very poor (3).Thus,there is an unmet medical need to develop strategies to improve the cure rate of patients with advanced lung cancer.
Minimal/measurable residual disease (MRD,also known as molecular residual disease),refers to the small number of cancer cells remaining in the body after cancer treatment that do not respond or are resistant to treatment,which cannot be detected by traditional imaging [including positron emission tomography/computed tomography(PET/CT)] or laboratory methods [microscopic observation of cells and/or tracking of abnormal serum protein markers (tumor biomarker) in the blood].MRD in solid tumors is now referred to as measurable residual disease in the National Comprehensive Cancer Network(NCCN) guidelines (4).While these residual cancer cells may be small in number and do not cause any signs or symptoms at that time,they represent the continued existence of cancer and the possibility of clinical progression (5).In addition to describing the clinical significance of the ability to evaluate tumors,MRD can also describe the sensitivity of detection techniques and the understanding of tumors at the molecular level.Therefore,the blood-based assessment of MRD is desirable.Recent advances in fluid biopsies have allowed the ultrasensitive detection of circulating tumor cells (CTCs) and circulating cell-free tumor DNA (ctDNA) derived from residual or occult micrometastatic lesions in patients with solid tumors.Data from several clinical studies have reported that CTCs and ctDNA following curative treatment strongly predict recurrence in multiple tumor types (6-8).In patients with distant metastasis,high levels of CTCs and ctDNA are also associated with a poor prognosis (9).
The shift from monitoring dominant metastatic lesions to monitoring MRD may change the way we manage patients with solid tumors.For patients with early-stage lung cancer,MRD has been widely used to evaluate the risk of recurrence and inform the choice of systemic adjuvant therapy after radical local treatment (RLT) (8,10).MRD is mainly used in advanced lung cancer to assess disease-free status (no evidence of disease,NED),meaning that no sign of residual tumor is found by using the existing examination methods after treatment,indicating that tumors that can be found at this stage have been“completely removed”from the patient’s body.For example,1) advanced lung cancer patients with an effect evaluation of complete remission (CR) after chemotherapy;2) oligometastatic disease (OMD) after surgery;and 3) no evidence of active disease based on existing imaging techniques.
Advanced lung cancer patients with CR by imaging examination
At present,the detection of disease recurrence mainly depends on traditional imaging examinations,including CT and PET.Although technological advances have greatly improved imaging performance in recent decades,there remain several limitations (11).The most crucial disadvantage of these imaging techniques is that they detect only space-occupying lesions but not MRDs.In recent years,the development of tyrosine kinase inhibitors (TKIs)for patients harboring certain molecular aberrations and immunotherapy,such as immune checkpoint inhibitors(ICIs),have revolutionized the treatment of advanced NSCLC.Patients with advanced NSCLC have achieved CR rates of 1%-7% after targeted therapy or immunotherapy (12-32) (Table 1).Notably,the 5-year follow-up data showed CR rates of patients who completed long-term immunotherapy (35 cycles/2 years) as high as 15% and 10% in the KEYNOTE-010 and KEYNOTE-024 studies,respectively (23,29).However,among patients who have achieved CR,monitoring for disease recurrence is a major clinical problem when lesions on imaging modalities have completely resolved.Clinical studies have indicated that treatment with programmed death-ligand 1(PD-L1) blockade can produce durable responses in patients with NSCLC;however,the optimal treatment duration and the availability and duration of“drug holidays”remain unknown.Drug resistance is a major challenge for patients treated with targeted therapies.However,the monitoring of drug resistance when there are no visible lesions in imaging remains a challenge.Therefore,we urgently need a less invasive but more sensitive tool to accurately detect MRD to improve patient survival rates and quality of life.
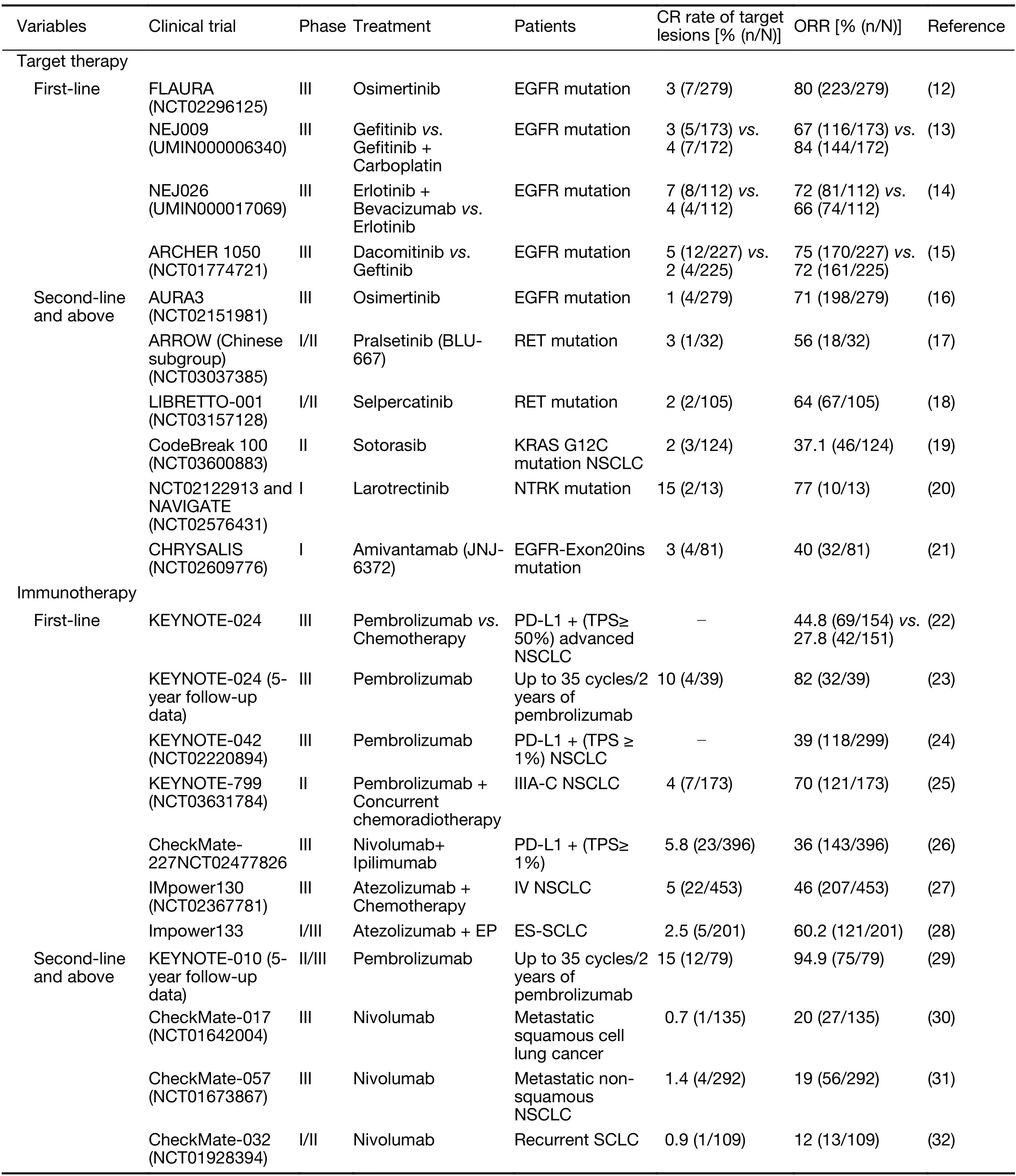
Table 1 CR rates of patients with advanced NSCLC in major clinical trials
Although research is lacking on MRD in patients with advanced lung cancer who have achieved CR,ctDNAbased MRD detection following curative-intent treatment has been widely proven to be a predictor of progressionfree survival (PFS) in patients with advanced lung cancer.
A prospective study showed that ctDNA analysis of longterm responders (PFS≥12 months) to PD-L1 blockade may differentiate those who will achieve ongoing benefit from those at risk of eventual progression.The best overall responses (BORs) according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria were CR (11 patients),partial response (PR,15 patients),and stable disease (SD,5 patients).At the surveillance time point,27 of 31 patients had undetectable ctDNA,25 (93%) of whom remained progression-free in the subsequent follow-up(range:4.76-24.21 months).In contrast,all four patients with detectable ctDNA eventually progressed [Fisher P<0.0001;positive predictive value=1;95% confidence interval (95% CI):0.51-1;negative predictive value=0.93;95% CI:0.80-0.99)] (33).Another prospective study showed that,among 65 patients with locally advanced NSCLC,freedom from progression 24 months after chemoradiation therapy (CRT) was much higher in the ctDNA MRD-negative group than that in the ctDNA MRD-positive group in both the consolidation ICI (87.5%vs.0%,P<0.0001) and no consolidation ICI (100%vs.0,P=0.0006) cohorts.Although the difference was not significant,these results suggested that patients with positive MRD may benefit from consolidation ICI(P=0.04),while patients with negative ctDNA after CRT may not (P=0.23).ctDNA MRD monitoring following curative-intent treatment strongly predicted recurrence,with a mean lead time of 4.1 months between ctDNA detection and radiographic progression (6).These results suggested that ctDNA analysis may be helpful for personalized treatment and recurrence risk monitoring in patients who have achieved a radiographic CR after initial treatment.
Advanced lung cancer patients with OMD
OMD refers to a state with a limited number of metastatic sites,a concept first proposed by Hellman and Weichselbaum in 1995.This state is considered an intermediate status between locally advanced and widely metastatic phases (34).The definition of OMD remains controversial;most retrospective studies defined OMD as a synchronous metastasis based on M1b staging of the eighth edition of The International Association for the Study of Lung Cancer (IASLC) Classification (within 6 months of diagnosis) or up to three metastatic cerebral metastases(35);however,some are defined as no more than five metastases (36).Stage M1b is defined as the presence of one metastasis in a single organ;the median overall survival(mOS) of patients with stage M1b disease (11.4 months) is similar to that of patients with one metastasis to the contralateral lung (stage M1a) (11.8 months),whereas mOS of patients with multiple lesions in one other organ in addition to the primary site (7.0 months) is similar to that for plurimetastatic patients (6.2 months) (37). The European Organization of Research and Treatment of Cancer (EORTC) consensus defines OMD as up to five metastases and three organs (38).In contrast,the Canadian consensus defines OMD as three or fewer to five or fewer metastases and up to six extracranial lesions (39).Analysis of patients with metastatic NSCLC at all sites showed that approximately 30%-50% of the advanced lung cancers were oligometastatic at the initial diagnosis (36,40,41).
Growing evidence has been recognized in the European Society for Medical Oncology (ESMO) and NCCN guidelines,which recommend consideration of RLT to all visible disease sites as an option for selected patients with OMD (42-44).
A phase II prospective trial in which 49 patients with stage IV NSCLC were randomized to LCT arm (local consolidative therapy with radiotherapy/surgery) or MT/O arm (maintenance therapy or observation) after first-line systemic therapy.The result showed that the LCT arm prolonged the median PFS (14.2 monthsvs.4.4 months,P=0.022) and OS (41.2 monthsvs.17.0 months,P=0.017) (35).
In general,adjuvant treatment can be used for MRD or micrometastatic lesions that may not be detected by traditional imaging methods;thus,the combination of RLT (surgery and radiotherapy) and adjuvant treatment can effectively reduce local recurrence and improve survival rates.A single-arm study of locally ablative therapy followed by sequential pembrolizumab adjuvant therapy in patients with oligometastatic lung cancer showed that ICI adjuvant therapy significantly improved the median PFS(19.1 months) compared to the historical median PFS (6.6 months) (P=0.005) (45).As the existing routine evaluation methods for solid tumors do not consider the evaluation of MRD,adjuvant therapy may be used“blindly”,and its success or failure is retrospectively evaluated after years of follow-up.Improved methods to detect MRD and/or micrometastatic disease will help to identify patients who might benefit from adjuvant therapies and those who may not (8).The research on this topic has mainly focused on early-stage NSCLC.Studies have shown that detection of ctDNA MRD after radical treatment can reliably identify patients with final recurrence,with a sensitivity of 93% and a specificity of 96%,which is superior to those for standard imaging monitoring (10).
The concept of OMD points to a special patient population in whom RLT may provide a better prognosis than that for patients with widely metastatic NSCLC.However,unlike patients with early-stage lung cancer who undergo RLT,patients with OMD may have a greater tendency for subsequent metastasis.This holds new promise for the treatment of patients with oligometastatic lung cancer and is also accompanied by significant challenges.For example,the sequence of systemic therapyvs.local therapy remains controversial (46,47).There are no studies on predictive markers to help determine patients with OMD who may benefit from postoperative adjuvant therapy.However,existing studies have provided evidence that the combination of RLT and systemic therapy is safe in patients with OMD (40,48) and can prolong survival(41).MRD monitoring can provide information as part of a more individualized clinical decision-making process and can help to improve patient quality of life (49).For patients with MRD,systemic treatment may be considered following RLT.For patients without detected MRD,a“drug holiday”may be possible,with continued monitoring of MRD (Figure 1).
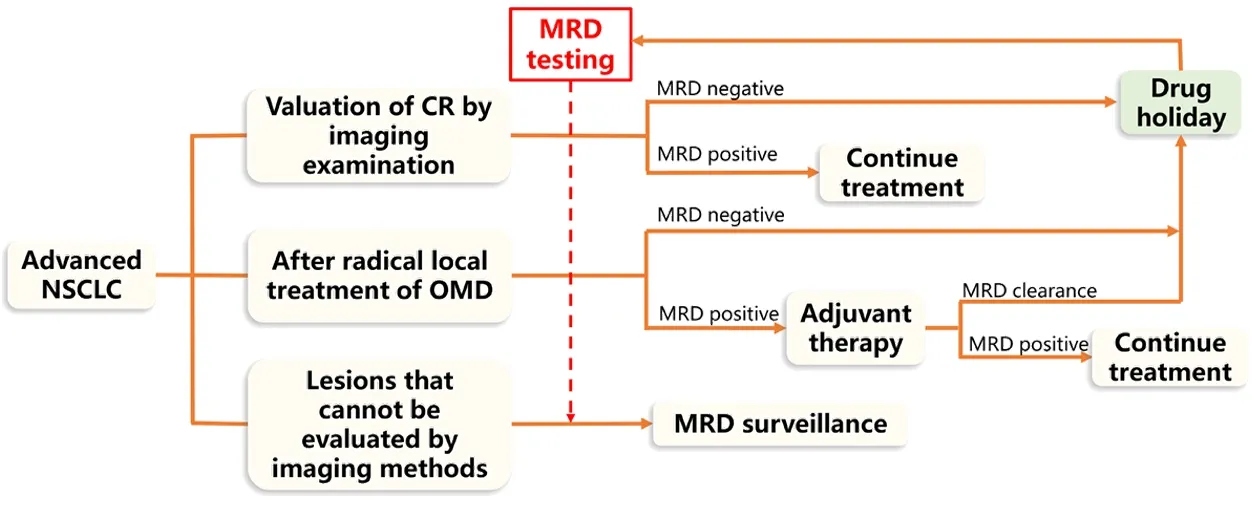
Figure 1 MRD as a potential tool for clinical decision-making in patients with advanced NSCLC.NSCLC,non-small-cell lung cancer;CR,complete response;OMD,oligometastatic disease;MRD,minimal/measurable (or molecular) residual disease.
Other lesions that cannot be evaluated by imaging
Imaging examinations are routinely used for clinical cancer staging and treatment response tracking.However,since gross disease is difficult to distinguish from inflammation or fibrosis changes induced by therapy (especially radiotherapy and immunotherapy) (50),it can be challenging to monitor the disease in following clinical scenarios such as cavities,fibrosis,and scars.Second,pseudoprogression may occur after immunotherapy (51).Third,while lesions remain stable,PET-CT may show no metabolic activity after treatment.Although the target lesions are still visible on imaging,the local response is considered complete in these cases,as these lesions may not be active (52).
The incidence of pseudoprogression in NSCLC is about 3%-5% (53,54).In these cases,the amount of ctDNA would decrease or remain stable,whereas the levels would increase in true progression,with a sensitivity of 90%-100% (55).ctDNA MRD can be used as a supplementary method in cases in which it is difficult to distinguish inflammatory changes or fibrosis from true progression using imaging methods.
Challenges and future prospects
Although MRD can be used to evaluate lesions beyond current imaging methods,which may change the treatment patterns of advanced lung cancer,some unresolved problems remain.
First,there is a lack of forward-looking experiments.While data published to date indicate that ctDNA MRD is a reliable prognostic biomarker,there is a lack of data supporting ctDNA as a predictive biomarker in advanced NSCLC.Some prospective cohort studies showed that ctDNA analysis of patients with NSCLC who achieved CR,PR,or SD ≥1 year from PD-L1 blockade may differentiate those who will achieve ongoing benefit from those at risk of eventual progression (33) and that consolidation ICI therapy improves outcomes for NSCLC patients with MRD detected after CRT (6),which provides preliminary evidence to support the predictive power of ctDNA testing.There is also a lack of studies on MRD in patients with advanced NSCLC.However,several clinical trials are currently underway to verify the role of ctDNAguided MRD assessment in patients with early-stage NSCLC (NCT04585477,NCT04642469,NCT04367311,NCT03774758,and NCT04585490).
Second,there remain limitations in detection technology,including that for MRD,the application of next-generation sequencing (NGS),changes in ctDNA level,lack of a standardized platform,and so on.MRD detection and monitoring based on bone marrow samples is a well-established procedure for the management of hematological malignancies (56).However,it is challenging in patients with solid tumors,as the sampling of tissue that might contain disseminated tumor cells (DTCs) (usually not located in the bone marrow fluid),such as posttreatment lung lobar specimens,is too invasive.Recent advances in detecting MRD with CTCs and ctDNA have identified alternative strategies to identify patients at high risk of relapse who instead require adjuvant therapy(8,10,57).ctDNA is more commonly used in clinical research to monitor the MRD of solid tumors,as it is easier to separate,more stable,and higher in proportion in the bloodstream,with higher sensitivity than that for CTCs(6,58).The current laboratory techniques and ctDNAanalysis include sequencing,polymerase chain reaction(PCR),and NGS (59).Increasing observable events(variables) (60) and combined methylation analysis methods can improve the sensitivity of MRD detection (61).
In summary,although the definition of MRD for solid cancer is imperfect,the ctDNA detection criteria are uncertain,and there remain many problems to be solved in MRD itself,it is a feasible scientific hypothesis to guide the systemic treatment of locally advanced or advanced lung cancer,as shown inTable 1.For patients who cannot undergo surgery but achieve radiographic CR after treatment (chemoradiation,target treatment,and/or immune treatment),MRD surveillance can help to determine the need for maintenance therapy and select the population that will benefit from consolidation therapy with ICI.The use of adjuvant therapy guided by MRD monitoring after local therapy for OMD can reduce the treatment burden for patients without detectable MRD and allow patients to enjoy“drug holidays”.Regarding the challenges caused by cavity,fibrosis,scar formation,and false progression caused by emerging treatment forms such as immunotherapy to traditional imaging evaluation,the detection of ctDNA MRD can also be used to help determine the prognosis and formulate further treatment strategies.Monitoring ctDNA MRD mutations is currently used to detect treatment response,emergence of resistance,and metastatic progression (62).Therefore,research is needed on MRD-based treatment strategies in patients with locally advanced/advanced lung cancer to provide accurate consolidation treatment plans and extend the duration of CR.
The emergence of new therapies provides realistic hope for the effective treatment,or even cure,of patients with advanced lung cancer.Whether disease assessment through MRD can change our understanding of advanced lung cancer and if it is possible to determine the prognosis and precise intervention for advanced lung cancer based on MRD detection to change the treatment strategy for advanced lung cancer remain to be determined.We look forward to the design of relevant clinical studies to answer these questions.
Acknowledgements
This study was supported by Jilin Scientific and Technological Development Program (CN) (No.2019 0303146SF).
Footnote
Conflicts of Interest:The authors have no conflicts of interest to declare.
 Chinese Journal of Cancer Research2021年5期
Chinese Journal of Cancer Research2021年5期
- Chinese Journal of Cancer Research的其它文章
- Lung cancer risk in never-smokers:An overview of environmental and genetic factors
- Chidamide combined with cyclophosphamide,doxorubicin,vincristine and prednisone in previously untreated patients with peripheral T-cell lymphoma
- Better prognostic determination of cT3 rectal cancer through measurement of distance to mesorectal fascia:A multicenter study
- A radiomics prognostic scoring system for predicting progression-free survival in patients with stage IV non-small cell lung cancer treated with platinum-based chemotherapy
- Single patient classifier as a prognostic biomarker in pT1N1 gastric cancer:Results from two large Korean cohorts
- Integrating pathomics with radiomics and genomics for cancer prognosis:A brief review
