Better prognostic determination of cT3 rectal cancer through measurement of distance to mesorectal fascia:A multicenter study
Xiaoyan Zhang ,Qiaoyuan Lu ,Xiangjie Guo ,Wuteng Cao ,Hongmei Zhang ,Tao Yu ,Xiaoting Li,Zhen Guan,Xueping Li,Ruijia Sun,Yingshi Sun
1Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing),Department of Radiology,Peking University Cancer Hospital &Institute,Beijing 100142,China;2 Department of Forensic Medicine,Shanxi Medical University,Taiyuan 030001,China;3Department of Radiology,the Sixth Affiliated Hospital,Sun Yat-sen University,Guangzhou 510655,China;4Department of Radiology,National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital,Chinese Academy of Medical Sciences and Peking Union Medical College,Beijing 100021,China;5Department of Medical Imaging,Cancer Hospital of China Medical University,Shenyang 110042,China
Abstract Objective:To forward the magnetic resonance imaging (MRI) based distance between the deepest tumor invasion and mesorectal fascia (DMRF),and to explore its prognosis differentiation value in cT3 stage rectal cancer with comparison of cT3 substage.Methods:This was a retrospective,multicenter cohort study including cT3 rectal cancer patients undergoing neoadjuvant chemoradiotherapy followed by radical surgery from January 2013 to September 2014.DMRF and cT3 substage were evaluated from baseline MRI.The cutoff of DMRF was determined by disease progression.Multivariate cox regression was used to test the prognostic values of baseline variables.Results:A total of 804 patients were included,of which 226 (28.1%) developed progression.A DMRF cutoff of 7 mm was chosen.DMRF category,the clock position of the deepest position of tumor invasion (CDTI) and extramural venous invasion (EMVI) were independent predictors for disease progression,and hazard ratios (HRs)were 0.26 [95% confidence interval (95% CI),0.13-0.56],1.88 (95% CI,1.33-2.65) and 1.57 (95% CI,1.13-2.18),respectively.cT3 substage was not a predictor for disease progression.Conclusions:The measurement of DMRF value on baseline MRI can better distinguish cT3 rectal cancer prognosis rather than cT3 substage,and was recommended in clinical evaluation.
Keywords:Rectal cancer;T3 stage;substage;distance to mesorectal fascia;magnetic resonance imaging
Introduction
China is facing the increasing incidence and mortality of colorectal cancer,accompanied by a heavy socioeconomic burden (1-3).The 5-year survival rate of patients with cT3 rectal cancer is different,from 30% to 80%,which suggests that further stratification is needed before the next treatment (4).It is a key challenge for individualized medicine to select high-risk cT3 patients who can really benefit from neoadjuvant chemoradiotherapy (NCRT) and low-risk T3 patients who do not need NCRT for direct surgery.
Many studies have proposed that extramural depth(EMD) of tumor invasion should be used for T3 stage stratification (5-11),because EMD has been proved to be an independent predictor of local recurrence of T3 stage rectal cancer (5-7,12-14).At present,the most commonly used criteria include Union for International Cancer Control (UICC) (T3a,EMD≤1 mm;T3b,1<EMD≤5 mm;T3c,5<EMD≤15 mm;T3d,EMD>15 mm) and Radiological Society of North America (RSNA) standards(T3a,EMD≤5 mm;T3b,5<EMD≤10 mm;T3c,EMD>10 mm) (15-17).
As well as we know,the EMD cutoff value is derived from pathological research,but there are obvious differences betweenin vitroandin vivoimages,and the measurement discrepancy can be up to 7 mm (18),which leads to excessive T3 substage on baseline magnetic resonance imaging (MRI) evaluation (19) and poor reproducibility of EMD measurement (20) especially for T3a (EMD≤1 mm) in UICC standard (21);The data of UICC and RSNA criteria are from western population which BMI is relatively high,for Chinese people with relatively low BMI,it is unknown how effective T3 substage is based on EMD measurement.In our clinical practice,we also found that the intestinal wall tumor located often cannot be accurately judged,so the measurement of the invasion distance outside the tumor intestinal wall only relies on supposition;T3 substage based on EMD can be affected by the tumor’s location.If the tumor located in the lower rectum or in the anterior wall of the rectum,the surrounding mesorectum is definitely thin,and as long as the tumor does not invade adjacent organs,its T3 substage will always less than T3c/d.Are there any other simple and reproducible indicators that can be used to stratify T3 rectal cancer patients? In our daily clinical work,we found that the distance between the deepest tumor invasion site and the mesorectal fascia (DMRF) was easy to measure,and was not limited by the mesorectum thickness.Therefore,we designed this multicenter retrospective study to determine whether DMRF can be used to stratify T3 patients,and to determine the feasibility of DMRF in practice by comparing with T3 substage.
Materials and methods
Study design and participants
It was a retrospective,multicenter,cohort study.The protocol was approved by the Institutional Review Board of Peking University Cancer Hospital &Institute,and patient informed consent was waived.
Biopsy-proven rectal adenocarcinoma patients with cT3 stage determined by pre-treatment MRI,who underwent NCRT followed by total mesorectal excision (TME) were consecutively included from four centers (The Sixth Affiliated Hospital,Sun Yat-Sen University;Cancer Hospital,Chinese Academy of Medical Sciences and Peking Union Medical College;Liaoning Province Cancer Hospital &Institute;Peking University Cancer Hospital &Institute.) during the period from January 2013 to September 2014.Patient were excluded if conditions as:1)history or concurrent of other malignancies;2) failure to complete NCRT;3) insufficient MR image quality for measurements;4) mucinous adenocarcinoma,or lack of pathological results after TME;or 5) lost to follow-up after surgery.
Finally,804 patients were included in analysis (Figure 1,Table 1),with an average age of 55.4±10.9 years.There were 537 males and 267 females,and 552 patients (from The Sixth Affiliated Hospital,Sun Yat-Sen University;Cancer Hospital,Chinese Academy of Medical Sciences and Peking Union Medical College;Liaoning Province Cancer Hospital &Institute) and 252 patients (Peking University Cancer Hospital &Institute) were assigned into training cohort and independent validation cohort,respectively.
MRI analysis
All patients received pre-treatment MR scanning,which was performed within one week before NCRT.Axial,axial oblique,sagittal T2-weighted images,axial T1-weighted images and diffusion-weighted images were included in the examination on 1.5T or 3.0T MR.In order to reduce intestinal peristalsis,the patient was given 20 mg anisodamine intramuscularly 30 min before the scan without any bowel preparation.
MRI scanner and scanning parameters of each unit can be seen in the attachment (Supplementary Table S1).All MRI images were retrieved from the picture archiving and communication system for further image indices evaluation and measurement.We measured parameters on MRI,including DMRF (Figure 2),the distance from the inferior part of the tumor to the anal verge (DTA),the clock position of the deepest position of tumor invasion located at the intestine wall (CDTI),EMD of tumor invasion,and different time clock positions (Supplementary Figure S1).The subjective MRI evaluation indices include tumor stages (mrT stages),lymph node metastasis (mrN status),extramural venous invasion (mrEMVI) status (Supplementary Table S2).
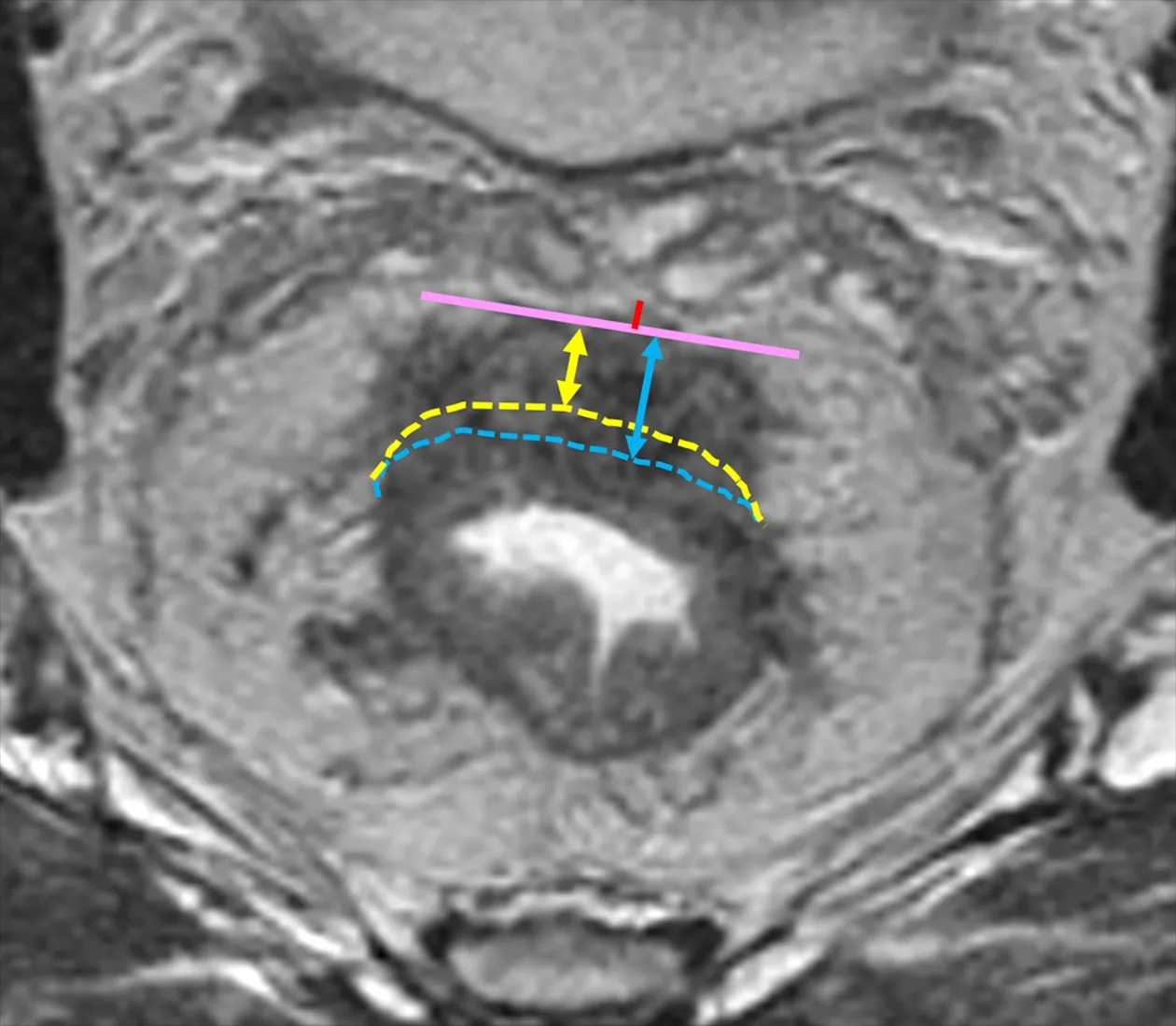
Figure 2 MRI measurement of distance between the deepest tumor invasion and mesorectal fascia (DMRF) and extramural depths (EMD) of tumor invasion.Pink solid line represents the deepest tumor invasion.Yellow and blue dotted lines represent the imaginary line of the muscularis propria layer,and thus yellow and blue double arrowheads represent different EMDs for same tumor.The length of red line represents the DMRF value.

Table 1 Patient and tumor characteristics
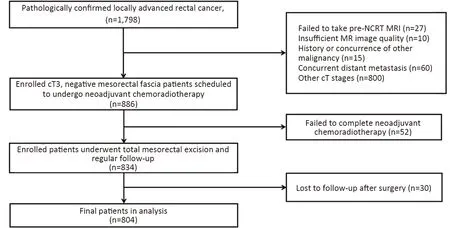
Figure 1 Flow chart of patient enrollment.NCRT,neoadjuvant chemoradiotherapy;MRI,magnetic resonance imaging.
Two experienced radiologists (XYZ and HMZ,with over 10 years’ experience in pelvic tumors imaging)independently conducted measurements.For classification variables,a third experienced radiologist (YSS,with over 20 years’ experience in pelvic tumors imaging) was introduced for arbitration;for continuous variables,the means were used for following analysis.
Inter-observer reproducibility was evaluated for the measurements of DMRF and EMD.Intra-class coefficient(ICC) was used for assessing inter-rater agreement,0-0.20,0.21-0.40,0.41-0.60,0.61-0.80 and 0.81-1.00 indicated poor,fair,moderate,substantial or excellent agreement,respectively.Excellent agreement was obtained for the measurements of DMRF (ICC=0.94) and substantial agreement was obtained for the measurements of EMD(ICC=0.77).
Treatment protocol
All participants received NACT.Three NACT regimens were used in 4 centers.Regimen 1:Radiation therapy regimen: 45-50.4 Gy/25-28 fractions/5-5.5 weeks;Concurrent chemotherapy regimen:Capecitabine 800 mg/m2orally twice per day.Regimen 2:Radiation therapy regimen: 50 Gy/25 fractions/5 weeks; Concurrent chemotherapy regimen:Capecitabine 800 mg/m2orally twice per day.Regimen 3:Radiation therapy regimen:45-50.4 Gy/22-28 fractions/5-6 weeks; Concurrent chemotherapy regimen:Capecitabine 825 mg/m2orally twice per day or 5 Fluorouracil 225 mg/m2intervenous drop daily.All participants received R0 resection after NACT.Pathology tumor staging was evaluated according to the American Joint Committee on Cancer system.
Follow-up
Participants were followed up after the operation at every 3 months for 2 years,then every 6 months for 5 years,finally once a year since the 6th year.The follow-up includes physical examination,routine blood test and the imaging examinations consisting of the chest,abdomen and pelvis.The date of surgery,last follow-up,death and cause of death,distant or local recurrence and site of recurrence were recorded.Patients were defined as suffering disease progression if death,distant metastasis or local recurrence occurred. Patients without disease progression were censored at the last follow-up.
Statistical analysis
Continuous variables were described as,categorical variables were described as numbers and percentages.Chisquare test or student’sttest was used for comparisons of categorical and continuous variables between the training cohort and the independent validation cohort.Receiver operating characteristic curve was conducted for selecting the cutoff for DMRF using the maximum Youden’s method.Univariate and multivariate cox regression was used to test the prognostic values of baseline variables,hazard ratios (HRs) with 95% confidence interval (95% CI)were obtained.The time-dependent receiver operating characteristic (ROC) curves for censored survival data were performed,and the area under the curve (AUC) was calculated for evaluating the capability of multivariate cox model in predicting the 3-year’s disease progression.The model was also presented as a nomogram.Kaplan-Meier method was used to compare survival curves between DMRF groups or T3 substages. Calculations were performed using the IBM SPSS (Version 22.0;IBM Corp.,New York,USA) and R package 3.6.2 (R Foundation for Statistical Computing,Vienna,Austria). A two-sided P<0.05 was considered as statistically significant.
Results
Patients
Patient and tumor characteristics are listed inTable 1.All patient and tumor characteristics were comparable between training and validation cohort.Median follow-up duration after surgery were 47 (95% CI,45-49) months and 47(95% CI,44-50) months for training and validation cohort,respectively.In the training cohort,159 (28.8%) patients suffered disease progression,including 82 (14.9%) patients suffering death,125 (22.6%) patients suffering distant metastasis and 16 (2.9%) patients suffering local recurrence.In the independent validation cohort,67(26.6%) patients suffered disease progression,including 37(14.7%) patients suffering death,59 (23.4%) patients suffering distant metastasis and 8 (3.2%) patients suffering local recurrence.
Survival analysis of baseline variables according to disease progression in training cohort
A cutoff of 7 mm was selected by ROC analysis for DMRF according to disease progression.In univariate cox analysis,DMRF category was significantly associated with disease progression (HR=0.26;95% CI,0.12-0.54;P<0.001),as well as baseline MRI EMVI (HR=1.50;95% CI,1.09-2.08;P=0.015) and CDTI (HR=1.94;95% CI,1.38-2.74;P<0.001).In contrast,cT3 stage was not found significantly related with disease progression (P=0.055) (Table 2,Figure 3).
In multivariate cox analysis,DMRF category,CDTI and baseline MRI EMVI were independent predictors for disease progression,and HRs were 0.26 (95% CI,0.13-0.56),1.88 (95% CI,1.33-2.65) and 1.57 (95% CI,1.13-2.18),respectively (Table 2).The AUC yielded by the time-dependent ROC was 0.623 (95% CI,0.502-0.744) for predicting 3-year’s disease progression.The nomogram for the multivariate cox model is inFigure 4.
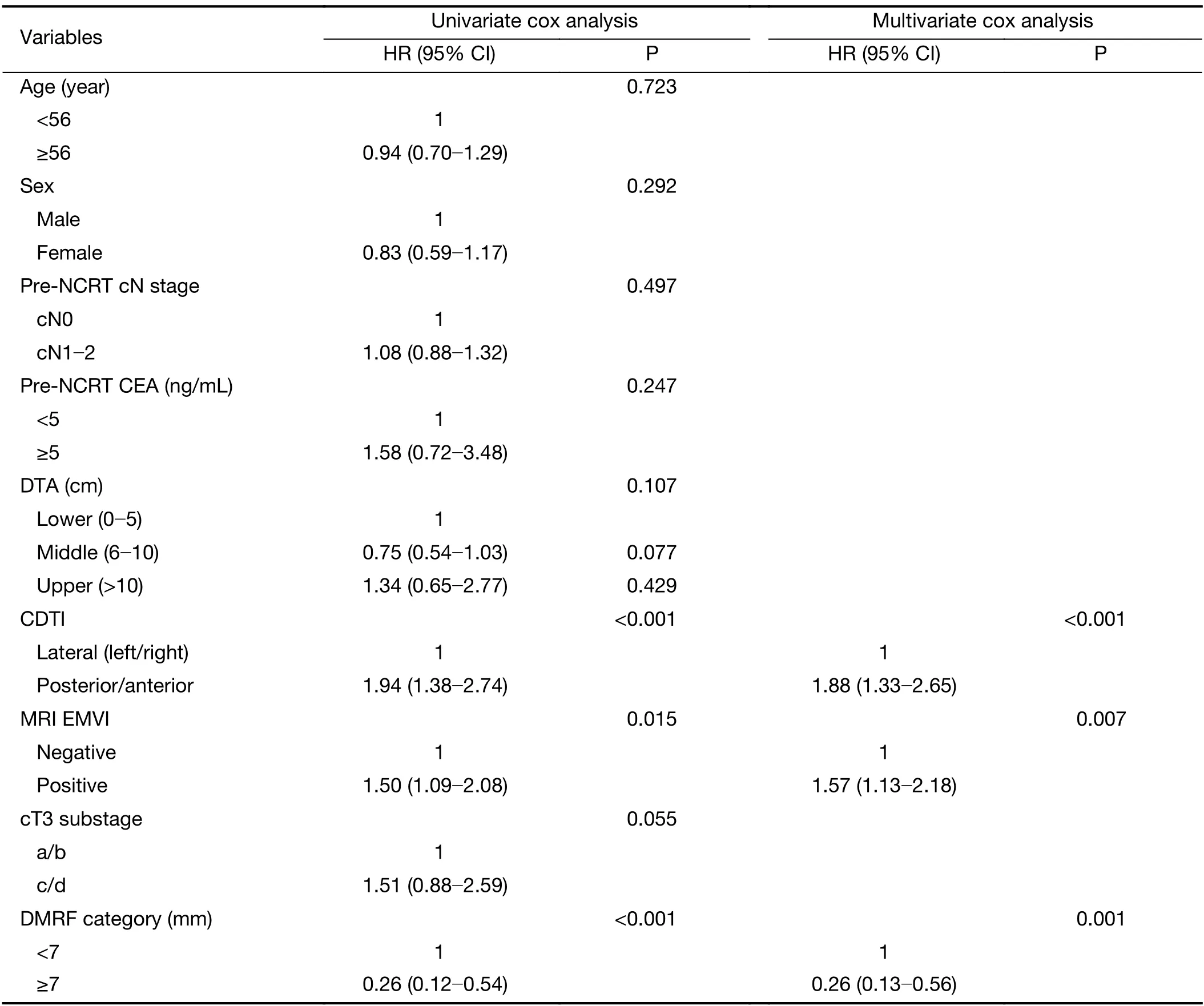
Table 2 Survival analysis of baseline variables according to disease progression in training cohort
Validation of DMRF category in independent validation cohort
In the independent validation cohort,DMRF category can also distinguish disease progression groups (P=0.020)(Figure 3C);the AUC yielded by the time-dependent ROC was 0.734 (95% CI,0.676-0.791) for predicting 3-year’s disease progression (Figure 4).
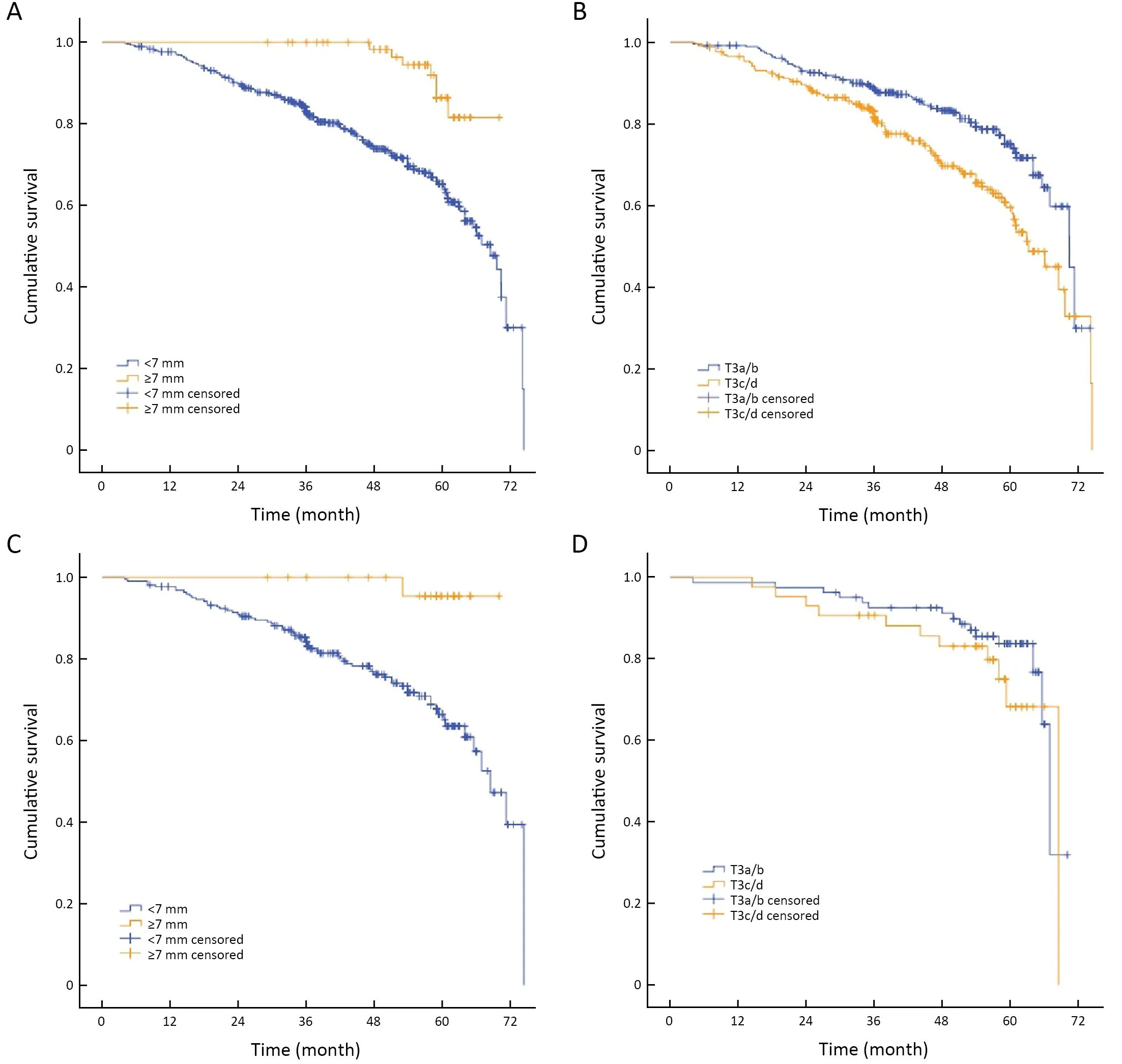
Figure 3 Kaplan-Meier curves for pre-NCRT DMRF category and cT3 stage according to disease progression in training cohort and independent validation cohort.(A) DMRF (<7 mm,≥7 mm) according to disease progression in training cohort (P<0.001);(B) cT3 substage(T3a/b,T3c/d) according to disease progression in training cohort (P=0.055);(C) DMRF (<7 mm,≥7 mm) according to disease progression in independent validation cohort (P=0.020);and (D) cT3 substage (T3a/b,T3c/d) according to disease progression in independent validation cohort (P=0.279).NCRT,neoadjuvant chemoradiotherapy;DMRF,distance between the deepest tumor invasion and mesorectal fascia.
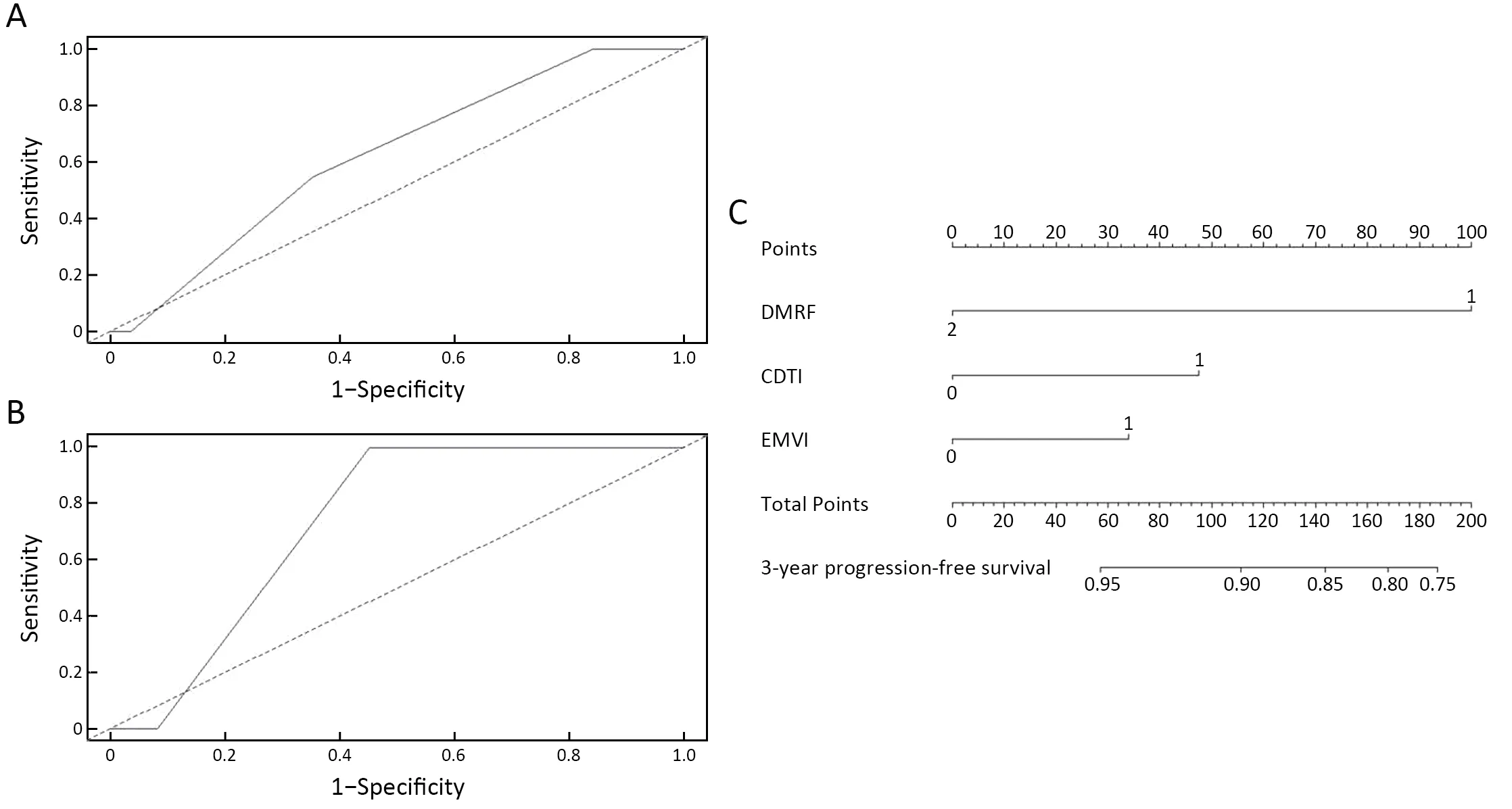
Figure 4 Time-dependent ROC curves of multivariate cox model in training cohort (A) and independent validation cohort (B),and nomogram for multivariate cox model (C).ROC,receiver operating characteristic;DMRF,distance between the deepest tumor invasion and mesorectal fascia;CDTI,clock position of the deepest position of tumor invasion located at the intestine wall;EMVI,extramural venous invasion.
Discussion
Our results showed that DMRF measurement can stratify cT3 rectal cancer well compared with EMD,which is easy to measure and has good consistency.The further the distance between the tumor and MRF,the better the prognosis (cutoff=7 mm).We noticed that cT3 stage rectal cancer patients had a wide range of 5-year survival rate,but still receive uniform NCRT (4,22-25),which leads to the need for prognostic stratification for these patients.Although many studies proposed that EMD of tumorinvasion should be used for T3 stage stratification,in our study,we did not observe a significant association between EMD-based T3 substage and prognosis.It indicates that the existing EMD-based substage may not accurately reflect T3 stage rectal cancer prognosis.One possible reason may be that EMD is limited by the tumor’s location(high or low rectal cancer),actually by the mesorectum thickness.A clinical MRI study of 183 Chinese reported that the mean mesorectum thickness was found to be<12 mm,suggesting that the existing EMD-based T3 substage including 15 mm may not suitable for Chinese population because of relatively thin mesorectum thickness(26).In this study,55.6% (160/288) of T3a/b tumors were located at the lower rectum,while only 46.2% (122/264) of T3c/d tumors were located at the lower rectum.
Another reason may be that the inter-observer agreement for EMD-based T3 substage is not satisfactory due to the difficulty in measurements.The result was similar with the study from Dr.Amandeep Pooni and his colleagues (20).In their study,Kappa score for EMD were 0.37 (95% CI,0.16-0.82),and inter-rater reliability was the highest for distance to mesorectal fascia.
Our study showed that cT3 rectal cancer patients could be divided into high risk and low risk based on different DMRF value (7 mm).The DMRF value is relatively easy to measure,because T2WI can show the deepest depth of tumor invasion (same signal with tumor on T2WI) and MRF (low signal on T2WI) very clearly.The consistency of DMRF measurement is better than that of EMD.
Thus,we consider DMRF may be a more reliable,simple,and specific criterion for systematic risk and survival stratification.It should be considered as an important MRI criterion for preoperative treatment decision making in the real-world setting (20).In this study,we did not analyze the association between MRF status and prognosis,considering the close relationship between MRF status and DMRF.In terms of measurement principle,positive MRF status is only a special DMRF state(≤1 mm).The advantages of this study are as follows:1)The applicability of EMD-based T3 substage criteria was verified for the first time by using this multi-center dataset in China,we did not observe a significant association between EMD-based T3 substage and prognosis;and 2)Excellent agreement was obtained for DMRF dichotomy.The accuracy and reliability of DMRF was much better than EMD-based T3 substage.The prognostic value of DMRF with its cutoff was validated in a multicenter,independent cohort,which serves as a strong evidence for its extrapolation capability.
In multivariate cox analysis,CDTI (the clock position of the deepest position of tumor invasion) and baseline MRI EMVI were independent predictors for disease progression,except DMRF category.The nomogram for the multivariate cox model was constructed for clinic use.This study also had several limitations:First,the sample was retrospective,although from multicenter dataset.Second,the results of this study showed the potential value of DMRF in T3 rectal cancer stratification.Our results showed that DMRF,CDTI,and EMVI were related to prognosis,but the efficiency of the 3-year-PFS model was still not satisfactory only by using these three factors.We still need to explore other possible influencing factors,such as molecular and/or genetic predictors,and to incorporate DMRF with these factors for stratification well in rectal cancer patients.
Conclusions
This was the first study to define DMRF’s value,which can stratify cT3 rectal cancers with different prognosis.DMRF,as a good predictor for prognosis and of excellent interobserver reproducibility,is thus recommended to be measured on baseline MRI and considered for managing individualized treatment strategy for T3 rectal cancer patients.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 82071881,91959116,81971584);National Key R&D Program of China (No.2019YFC0117705);Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (No.ZYLX201803);Beijing Hospitals Authority Ascent Plan (No.DFL20191103);Capital’s Funds for Health Improvement and Research (No.2020-1-2151);and Beijing Natural Science Foundation (No.Z200015,Z180001).
Footnote
Conflicts of Interest:The authors have no conflicts of interest to declare.
 Chinese Journal of Cancer Research2021年5期
Chinese Journal of Cancer Research2021年5期
- Chinese Journal of Cancer Research的其它文章
- Lung cancer risk in never-smokers:An overview of environmental and genetic factors
- Chidamide combined with cyclophosphamide,doxorubicin,vincristine and prednisone in previously untreated patients with peripheral T-cell lymphoma
- A radiomics prognostic scoring system for predicting progression-free survival in patients with stage IV non-small cell lung cancer treated with platinum-based chemotherapy
- Single patient classifier as a prognostic biomarker in pT1N1 gastric cancer:Results from two large Korean cohorts
- Coexisting opportunities and challenges:In which scenarios can minimal/measurable residual disease play a role in advancednon-small cell lung cancer?
- Integrating pathomics with radiomics and genomics for cancer prognosis:A brief review
