Single patient classifier as a prognostic biomarker in pT1N1 gastric cancer:Results from two large Korean cohorts
Yoon Young Choi ,Eunji Jang ,Hyunki Kim ,Kyoung-Mee Kim ,Sung Hoon Noh ,Tae Sung Sohn ,Yong-Min Huh,Ji Yeong An,Jae-Ho Cheong,7,8
1Department of Surgery,CHA Ilsan Medical Center,CHA University School of Medicine,Goyang-si 1205,Korea;2Department of Surgery,Yonsei University Health System,Seoul 03722,Korea;3Department of Radiology,Yonsei University Health System,Seoul 03722,Korea;4Department of Pathology,Yonsei University Health System,Seoul 03722,Korea;5 Department of Pathology and Translational Genomics,Samsung Medical Center Sungkyunkwan University School of Medicine,Seoul 06351,Korea;6 Department of Surgery,Samsung Medical Center,Sungkyunkwan University School of Medicine,Seoul 06351,Korea;7YUHS-KRIBB Medical Convergence Research Institute,Seoul 03722,Korea;8Brain Korea 21 PLUS Project for Medical Science,Yonsei University College of Medicine,Seoul 03722,Korea
Abstract Objective:Benefits of adjuvant treatment in pT1N1 gastric cancer (GC) remain controversial.Additionally,an effective biomarker for early GC is the need of the hour.The prognostic and predictive roles of single patient classifier (SPC) were validated in stage II/III GC.In this study,we aimed to elucidate the role of SPC as a biomarker for pT1N1 GC.Methods:The present retrospective biomarker study (NCT03485105) enrolled patients treated for pT1N1 GC between 1996 and 2012 from two large hospitals (the Y cohort and S cohort).For SPC,mRNA expression of four classifier genes (GZMB,WARS,SFRP4 and CDX1) were evaluated by real-time reverse transcription-polymerase chain reaction assay.The SPC was revised targeting pT1 stages and the prognosis was stratified as high-and lowrisk group by the expression of SFRP4,a representative epithelial-mesenchymal transition marker.Results:SPC was evaluated in 875 patients (n=391 and 484 in the Y and S cohorts,respectively).Among 864 patients whose SPC result was available,41 (4.7%) patients experience GC recurrence.According to revised SPC,254 (29.4%) patients were classified as high risk [123 (31.5%) and 131 (27.1%) in the Y and S cohorts,respectively].The high risk was related to frequent recurrence in both Y and S cohort (log-rank P=0.023,P<0.001,respectively),while there was no difference by GZMB and WARS expression.Multivariable analyses of the overall-cohort confirmed the high risk of revised SPC as a significant prognostic factor [hazard ratio (HR):4.402 (2.293-8.449),P<0.001] of GC.A significant difference was not detected by SPC in the prognosis of patients in the presence and absence of adjuvant treatment (log-rank P=0.670).Conclusions:The present study revealed the revised SPC as a prognostic biomarker of pT1N1 GC and suggested the use of the revised SPC for early-stage GC as like stage II/III.
Keywords:Gastric cancer;biomarker;prognosis;chemotherapy
Introduction
Gastric cancer (GC) is one of the most frequent and lethal cancers worldwide (1,2).Surgical resection remains the fundamental treatment strategy for GC.The need for additional chemotherapy is determined by staging,which reflects the tumor burden in patients.While surgery is the sole therapy for regional cancer,systemic cancer is treated with surgery and chemotherapy (3,4).The pT1N1 stage of cancer,which is limited to the mucosal/submucosal layer of the stomach,with one or two metastatic lymph nodes(LNs),accounts for around 5% of GCs (5,6).This substage is regarded as regional cancer in the East,based on its classification as stage I (7,8),while it is considered as systemic cancer in the West,based on the involvement of metastatic lymph node(s) (9,10).Guidelines for therapy,therefore differ between the East and West,and the decision for including adjuvant therapy is left to the clinicians (7-10).
Advancing technology and several efforts have made it possible to understand GC at the molecular level (11-15).Few scientific results that provided promising evidences have been translated to real world applications (14,16-19).Single patient classifier (SPC,nProfiler® 1 Stomach Cancer Assay) is a readily applicable clinicalex vivodevice that predicts prognosis and response to adjuvant chemotherapy in stage II/III GC (14,20,21).SPC is a fourgene-based real-time reverse transcription-polymerase chain reaction (RT-PCR) assay that classifies patients into low-,intermediate-,and high-risk groups based on prognosis.In addition,SPC is applied to classify patients into responsive and non-responsive groups to predict the extent of benefit that could be derived from chemotherapy.In situations wherein a similar biology of GC exists in stage I and II/III,SPC aids in the prediction of prognosis and response to chemotherapy in pT1N1,similar to stage II/III GC.Thus,in the present study,we enrolled two large patient cohorts from Korea targeting pT1N1 GC (22,23),to evaluate the applicability of SPC as a biomarker for identifying this specific substage of GC.
Materials and methods
Study cohorts and data collection
Clinical data and formalin-fixed paraffin-embedded (FFPE)tissues were obtained from patients diagnosed with GC between January 1990 and December 2012 at the Severance Hospital (Y cohort) and the Samsung Medical Center (S cohort).The inclusion criteria were as follows:1)surgical R0 resection;2) pathological stage T1 (mucosa/submucosa) and N1 (one or two metastatic LNs);3) D1+or higher LN dissection;4) more than 15 retrieved LNs;and 5) availability of FFPE tissues.Patients who underwent neoadjuvant chemotherapy or radiotherapy prior to gastric surgery or who passed away within 30 d of surgery were excluded. The cohorts were previously a part of retrospective studies related to pT1N1 GC (22,23).Clinicopathological features,including sex,age,period of operation (1996-2006 and 2007-2012),histological type(differentiated,undifferentiated,and lymphoepitheliomalike cell),Lauren classification (intestinal,diffuse,and others),depth of tumor invasion (mucosa and submucosa),number of retrieved and metastatic LNs,tumor size (<2,2-5,and >5 cm),location (upper/middle and lower body),adjuvant treatment (chemotherapy or chemoradiation therapy),microsatellite instability (MSI),and SPC status,were documented.In addition,patient survival and tumor recurrence were evaluated.This study was approved by the Institutional Review Boards of Severance Hospital (4-2017-0914) and Samsung Medical Center (2017-05-101),and the need for informed consent was waived.This retrospective biomarker study was registered at clinicaltrial.gov(NCT03485105).
SPC
SPC is anex vivodevice that validates biomarkers by evaluation of genomic applications in practice and prevention (EGAPP):1) analytic validity;2) clinical validity;and 3) clinical utility (24).RNA was extracted from tumor-enriched tissue cores measuring 3 mm obtained from two pieces of FFPE samples using the MasterPure complete DNA and RNA purification kit (Epicentre Technologies,Madison,WI,USA) as per the manufacturer’s instructions. SPC is a commercial International Organization for Standardization (ISO)-13485 certified,Good Manufacturing Practice grade kit(nProfiler® 1 Stomach Cancer Assay,Novomics Co.,Ltd.,Seoul,Korea) based on four identified classifier genes(GZMB,WARS,SFRP4,andCDX1) and five reference genes(ACTB,ATP5E,HPRT1,GPX1,andUBB).For prognosis,the low-risk (highly immune) group is defined based on the positive expression of bothGZMBandWARS.Among the others (not low-risk group),based on the expression ofSFRP4[epithelial-mesenchymal transition (EMT)-related classifier gene],the patients were classified into intermediate-risk (SFRP4negative) and high-risk (SFRP4-positive) groups.Responsive and non-responsive groups were defined based on the expression ofCDX1;positivity forCDX1indicated response to chemotherapy.The lowrisk group was classified as the non-responsive group,regardless ofCDX1expression.The definition of positivity for each gene was based on the relative difference quantitation cycle (dCq) values compared to the five reference genes.The original cut-off dCq values for stage II/III GC are -5.18 forGZMB,-2.14 forWARS,-3.63 forSFRP4,and -2.69 forCDX1(14).Analytical validation was confirmed for stage II/III GC and the clinical utility was validated by multiple cohorts,including a randomized controlled trial cohort (14,20,21).Both retrospective and prospective validation multi-center studies for stage II/III GC are ongoing (NCT04600518,NCT04487717).
Revised SPC for pT1N1 GC
Because the distribution of expression inSFRP4in pT1N1 was different to stage II/III GC,and the expression ofGZMBandWARSwas not related to the prognosis in pT1N1 GC,we revised the SPC algorithm.The revised SPC classifies pT1N1 GC into two groups;SFRP4-positive(high-risk) and negative (low-risk),regardless of theGZMBandWARSexpression levels with cut-off value of dCq ≥-4.66.The details are inSupplementary materials.
MSI status
DNA was extracted from the 3 mm core of FFPE samples to evaluate the MSI status.Five quasimonomorphic mononucleotide markers (BAT-25,BAT-26,NR-21,NR-24,and MONO-27) were evaluated using PCR.Instability in two or more markers was classified as MSI-high (MSIH),while lack of instability was defined as microsatellite stable (MSS) (25,26).
Statistical analysis
Categorical data are described as numbers with percentages that were analyzed using the Chi-square or Fisher’s exact test.Continuous variables are described as means with standard deviations analyzed using an independentt-test or the Mann-Whitney test,as appropriate.For survival analyses,GC-specific recurrence was analyzed.Presence of cancer in the remanent stomach was not considered as recurrence.Survival graphs were generated using the Kaplan-Meier method.Prognoses were compared using the log-rank test and Cox proportional hazards model and are described based on the hazard ratio (HR) and its 95%confidence interval (95% CI).Institution was used as an adjustment variable in univariate and multivariate analyses.For multivariable analyses,variables with a P-value less than 0.05 and possible clinical confounding factors related to the prognosis were selected. In addition,multicollinearity was considered.The revised cut-off value forSFRP4expression was determined using a step-Miner analysis that is used to identify the threshold to convert continuous gene expression value into Boolean values(High/Low) (27).The equivUMP package was used for an equivalence test with an equivalence limit of 0.5.Because the null hypothesis of this test is the values between two groups are different,statistical significance represents the values are equivalence.A P-value less than 0.05 was considered statistically significant.Statistical analyses were performed using R software (Version 3.6.1;R Foundation for Statistical Computing,Vienna,Austria),and“survival”package was mainly used for survival analysis.
Results
Populations
Table 1andSupplementary Table S1summarize the baseline characteristics of the entire population and the cohorts.A total of 875 patients (391 and 484 in the Y and S cohort,respectively) were enrolled in this study.FFPE samples were not available for patients treated between the years 1990 and 1995. Comparison of demographics and clinicopathologic characteristics between the two cohorts showed significant differences,particularly related to adjuvant treatment:11.5% (45/391) of patients were treated with chemotherapy following surgery in the Y cohort,while 56.0% (271/484) of patients received either adjuvant chemotherapy or chemoradiation therapy in the S cohort.Therefore,institution was used as an adjustment variable for survival (Cox) analyses.MSI and SPC statuses were available for 838 and 864 patients,respectively.
Characteristics and prognosis by revised SPC algorithm
A significant association was seen between the expression of revised SPC and clinicopathological characteristics:high risk frequently indicated a male patient (P=0.008),a smaller tumor size (P<0.001),lower body location (P=0.015),and submucosal invasion (P<0.001,Table 2).In contrast,there was no significant association between the revised SPC and histology or Lauren classification.The high risk of revisedSPC was associated with significantGZMBandWARSpositivity (P<0.001 and P=0.004,respectively) and reducedCDX1positivity (P<0.001),while MSI status was not(P=0.813,Supplementary Table S2).
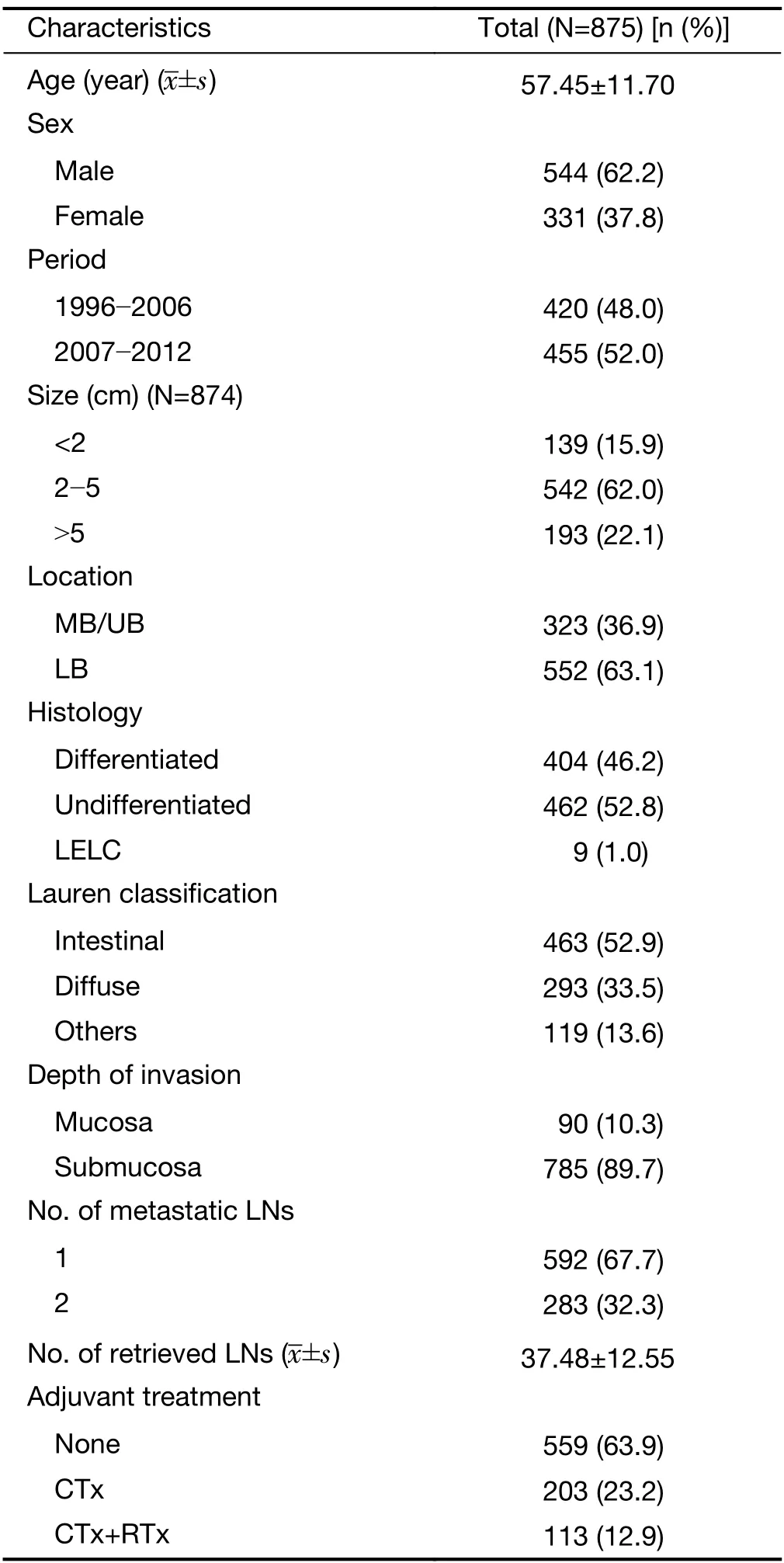
Table 1 Baseline characteristics of enrolled patients
The prognosis of pT1N1 GC that was stratified by the revised SPC was significantly different in the Y cohort:5-year GC-specific recurrence-free probability of the low-and high-risk groups were 96.5% (95% CI:94.3-98.8) and 90.0% (95% CI:84.8-95.5) in the Y cohort,98.9% (95%CI:97.7-100) and 90.6% (95% CI:85.3-96.1) in the S cohort,and 97.8% (95% CI:96.6-99.0) and 90.3% (95%CI:86.6-94.2) in the overall cohort,respectively (P=0.023,<0.001,and <0.001,respectively,Figure 1).The institutionadjusted HR of the high-risk compared to low-risk group was 4.274 (95% CI:3.950-8.701,P<0.001).The influence of multiple factors associated with prognosis and the revised SPC was adjusted in pT1N1 GC by selecting significant variables in univariate Cox analyses(Supplementary Table S3) and factors that were related to prognosis,such as sex,depth of invasion,number of metastatic LNs,and adjuvant treatment.Considering multicollinearity,the Lauren classification was selected,as its P-value was lower than that of histology.In multivariable analyses,the revised SPC was an independent prognostic factor,with an HR of 4.402 (95% CI:2.293-8.449,Table 3).The most frequent recurrent pattern of pT1N1 GC was hematogenous recurrence (46.3%,19/41,Table 4),unlike peritoneal recurrence in advanced GC (3).The type of recurrence did not differ according to the revised SPC.
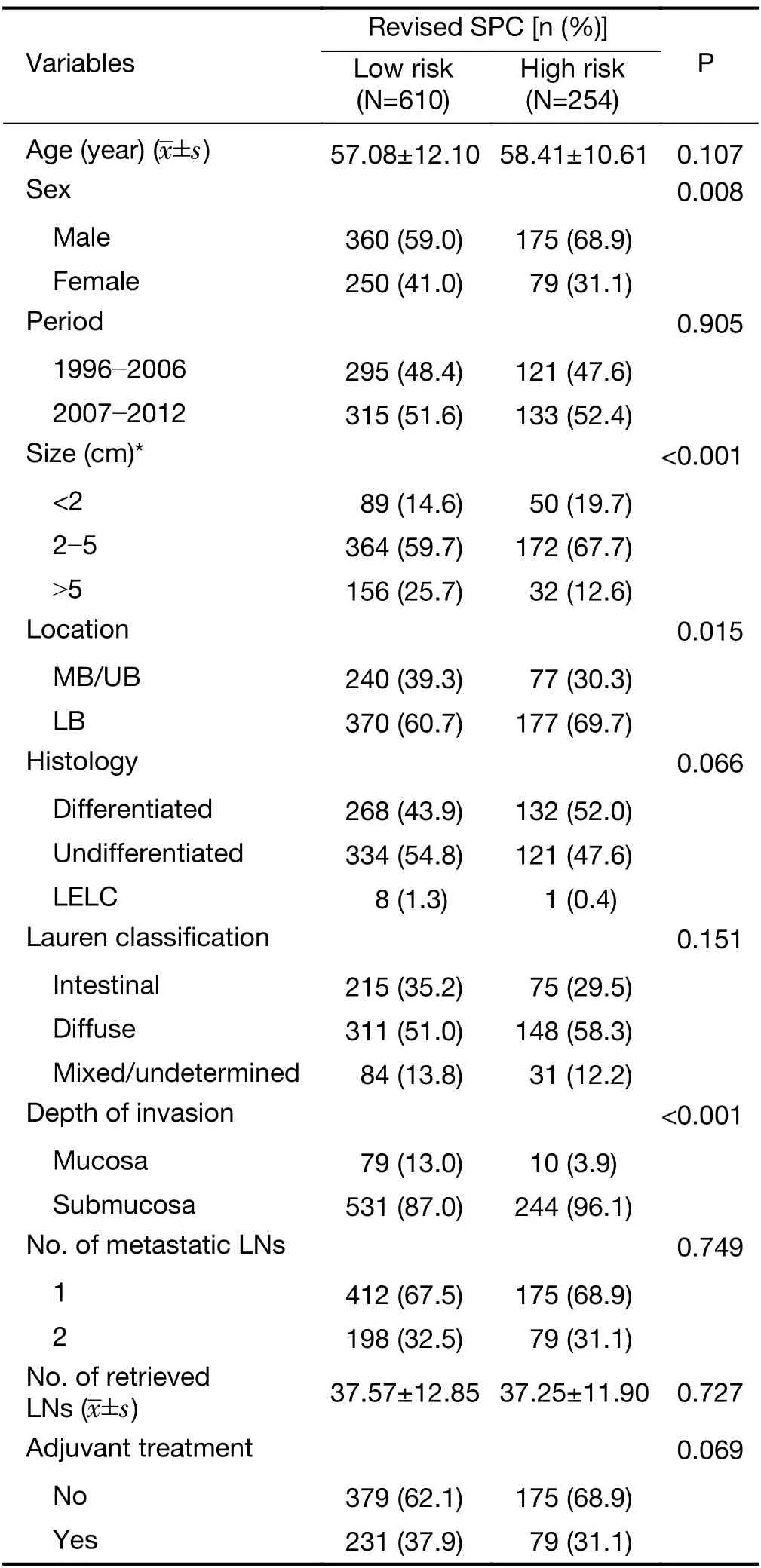
Table 2 Association between expression of revised SPC and clinical features
Benefit from adjuvant treatment by SPC
Presence or absence of adjuvant therapy did not influence the prognosis of pT1N1 GC,which was in accordance with the previously published data (adjusted HR:1.626,95% CI:0.782-3.378,Supplementary Table S3) (17,22).This result indicates an absence of beneficial effect of adjuvant treatment in pT1N1 GC.Fifty-four percent of patients(465 out of 864) wereCDX1-positive,and 23 of theCDX1-positive patients wereGZMBandWARS-positive;therefore,442 patients were classified into the responsive group(chemotherapy sensitive),and the remaining 422 patients were classified into the non-responsive group(chemotherapy resistance) by SPC prediction. The prognosis was similar among the responsive and nonresponsive groups in the presence and absence of adjuvant treatment (P=0.670,Figure 2A),probably due to the lack of any beneficial effect of adjuvant treatment in this cohort.Taken together,our results indicate that SPC was not effective in predicting the response to chemotherapy in pT1N1 GC.In multivariable analyses,adjuvant treatment was associated with poor prognosis compared to surgery alone,and the difference was statistically significant [HR:2.600 (95% CI:1.154-5.861),P=0.021,Table 3].The benefits of adjuvant treatment did not differ with the revised SPC (P<0.001;Figure 2B).

Figure 2 Kaplan-Meier curves of pT1N1 gastric cancer recurrence according to adjuvant treatment.(A) SPC prediction for chemotherapy(P=0.670);(B) revised SPC in overall cohort (P<0.001).Resistant,resistant to chemotherapy;Sensitive,sensitive to chemotherapy;adj Tx,adjuvant treatment;SA,surgery alone;SPC,single patient classifier.
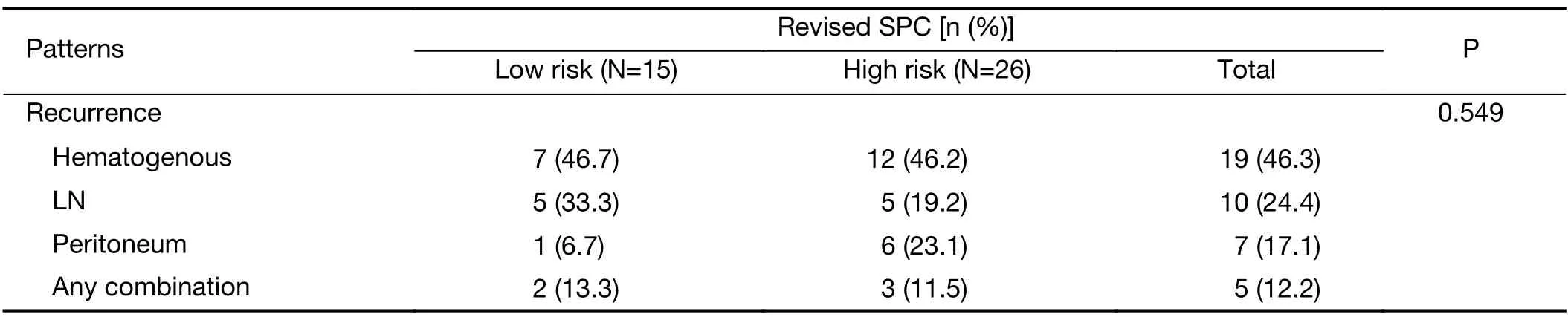
Table 4 Patterns of recurrence of pT1N1 gastric cancer expressing revised SPC
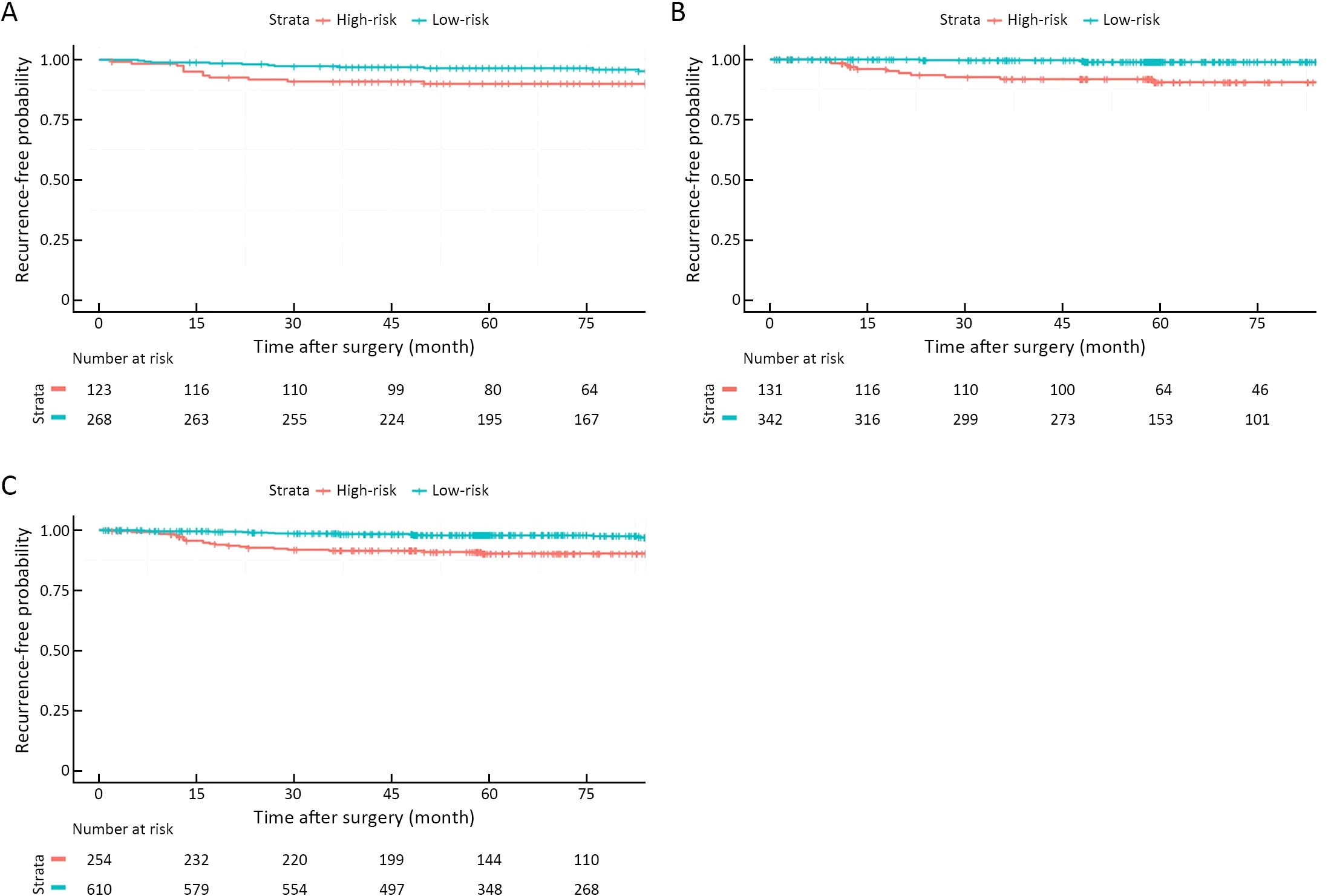
Figure 1 Kaplan-Meier curves of pT1N1 gastric cancer recurrence according to revised expression of SFRP4 in (A) Y cohort (training cohort) (P=0.023);(B) S cohort (validation cohort) (P<0.001);and (C) overall cohort (P<0.001).
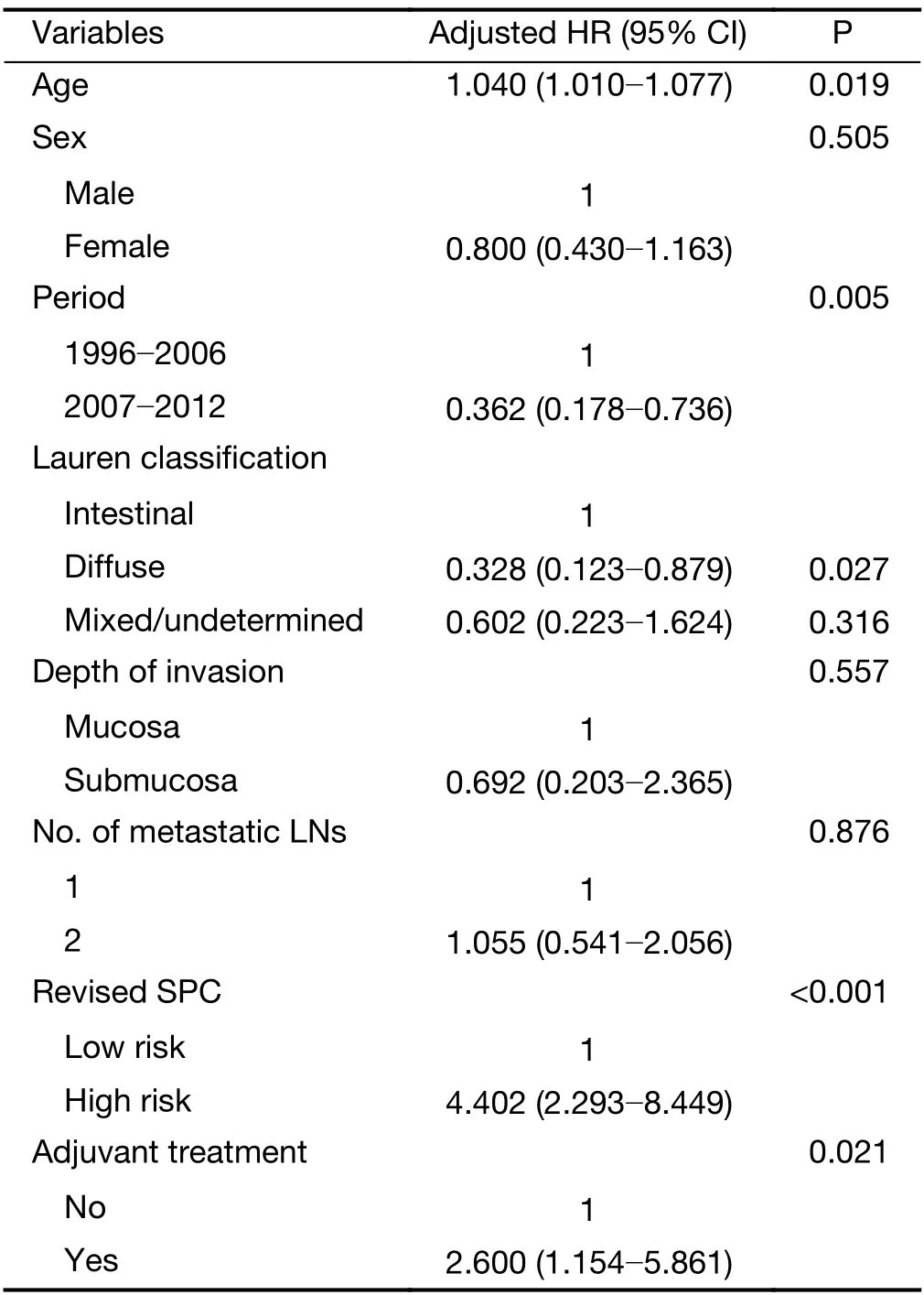
Table 3 Multivariable Cox proportional hazard model for recurrence of pT1N1 gastric cancer
Discussion
Based on the current scientific knowledge related to the prognosis of resectable GC (11-14,17,20),tumors of the highly immune subtypes,including MSI-H,Epstein-Barr virus (EBV)-positive,and low risk of SPC types,have better prognosis compared to those of the EMT-related subtypes that are associated with poor prognosis.In the present study,the highly immune,which isGZMB-andWARS-positive subtypes of SPC did not differentiate between the prognoses of pT1N1 GC,similar to the MSI in other cohorts of early GC (28).Prognosis of stage I GC is already promising (<5% of tumor recurrence),therefore the space for better survival will be very small.Conversely,enhanced expression ofSFRP4(high risk of the revised SPC),an EMT marker,is associated with poor prognosis of pT1N1,similar to stage II/III GC.This suggests that EMT mainly explains tumor recurrence in overall resectable GC,regardless of early and advanced stages.
To prove the role of biomarkers in predicting drug response,the interaction between drug treatment and the biomarker should be statistically significant (29).We speculated that SPC might play an important role in predicting the beneficial effects of chemotherapy in pT1N1 as like stage II/III GC,at least in the high-risk group;however,such a role was not observed in this study.In addition,there was no benefit of adjuvant treatment;instead,this treatment seemed to be harmful in the present cohort.Therefore,evaluating biomarkers for predicting the chemotherapy response might be insignificant in a cohort.The role of adjuvant treatment in stage I GC remains uncertain.Hence,predictive biomarker studies in this subgroup might not be promising.
EMT is a representative molecular characteristic related to cancer metastasis,poor prognosis,and refractory behavior to conventional chemotherapy in both gastric and colorectal cancers (11,12,14,30,31).In GC,the subtypewith EMT is genomically stable,with fewer somatic mutations,and is resistant to the beneficial effects of targeted therapy or immune checkpoint inhibitors and is thus clinically challenging to treat (11-14).Development of novel therapies targeting the EMT subtype of GC is the need of the hour.Few preclinical studies have shown the possibility of cancer metabolism-related therapy targeting this refractory subtype of GC (32,33).These EMTtargeted treatment strategies might prove to be helpful forSFRP4-positive GC,although the beneficial effects need to be validated at the clinical level.
These results could be translated for clinic use:1) for low-risk (SFRP4-negative) pT1N1 with a very low likelihood of tumor recurrence (<2.5%) and a possible stage modification from the current IB-IA;2) for high-risk(SFRP4-positive) pT1N1 with a 10 % tumor recurrence risk and lack of beneficial effects from adjuvant treatment,accordingly,the follow-up schedule (short-term as stage II)could be suitably revised.In addition,this study showed the possibility of using the revised SPC even for early-stage GC with a relatively good prognosis;therefore,expanding this study to other substages of stage I GCs (pT1N0,pT2N0) is worthy of investigation.We are evaluating the revised SPC by focusing on patients diagnosed with stage I GC (except pT1N1) associated with tumor recurrence.The results that will be obtained from the ongoing study would indicate whether the revised SPC could be applicable in patients with overall resectable GC,including stage I GC.
The role of revised SPC as a prognostic marker for pT1N1 GC was evaluated and validated in two independent cohorts with a large number of patients,considering the rarity of this specific substage of GC.As there has been no randomized controlled trial covering this specific stage of GC,the results of the present study provide are novel;however,they need to be validated in other cohorts,especially in different countries,covering various ethnicities.Since the number of events and tumor recurrence is small,type II errors need to be considered,especially for subgroup analysis.Despite the pre-planned biomarker study design,the retrospective nature of the cohort and various adjuvant treatment strategies in the cohort are other possible limitations of this study.
Conclusions
The present results indicated the low risk of revised SPC,lowSFRP4expression,as a better prognostic biomarker for pT1N1 GC.Accordingly,it can be said that the revised SPC is effective for the stratification of prognoses of patients with pT1N1 GC and for precision medicine strategies.
Acknowledgements
This study was supported by the Alumni Association of the Department of Surgery at the Yonsei University Health System (No.2017-01).We appreciate Ms.Heyseon Kim for their support to experiment of single patient classifier for this study.
Footnote
Conflicts of Interest:Jae-Ho Cheong,Yong-Min Huh,and Sung Hoon Noh are the founders and shareholders of Novomics Co.,Ltd.All the remaining authors have no conflicts of interest to declare.
 Chinese Journal of Cancer Research2021年5期
Chinese Journal of Cancer Research2021年5期
- Chinese Journal of Cancer Research的其它文章
- Lung cancer risk in never-smokers:An overview of environmental and genetic factors
- Chidamide combined with cyclophosphamide,doxorubicin,vincristine and prednisone in previously untreated patients with peripheral T-cell lymphoma
- Better prognostic determination of cT3 rectal cancer through measurement of distance to mesorectal fascia:A multicenter study
- A radiomics prognostic scoring system for predicting progression-free survival in patients with stage IV non-small cell lung cancer treated with platinum-based chemotherapy
- Coexisting opportunities and challenges:In which scenarios can minimal/measurable residual disease play a role in advancednon-small cell lung cancer?
- Integrating pathomics with radiomics and genomics for cancer prognosis:A brief review
