Mosaic trisomy 12 diagnosed in a female patient: clinical features,genetic analysis, and review of the literature
Daniela Hainz · Marcus Krüger · Daniela Reber · Karl Mehnert · Theresa Brunet · Gabriele Lederer ·Sabine Langer-Freitag · Julia Hoefele
Mosaic trisomy 12 is a rare genetic condition with a highly variable phenotype. Clinical features associated with this condition include developmental delay, intellectual disability, dysmorphic facial features, short stature, pigmentary dysplasia, complex congenital heart defects and hypotonia(Table 1). To date, 20 patients have been described in which mosaic trisomy 12 was observed in both extraembryonic and neonatal/infant tissues. Chen et al. reported two cases without phenotypic abnormalities [ 1]. Of the 20 previously reported cases, 4 resulted in neonatal or infant death. Those findings support the hypothesis that mosaic trisomy 12 manifests across a wide spectrum of phenotypes and that predicting the degree of abnormalities is quite difficult [ 2- 4]. In several cases, trisomy 12 mosaicism was detected prenatally in amnion fluid, but not postnatally [ 5- 8]. Even if the mosaicism is not confirmed in one tissue, it may still be present in other tissues of the child.
The described patient is the second child of a 37-year-old mother. The healthy parents already had a healthy son. Upon prenatal screening, amniocentesis was performed owing to fetal abnormalities including muscular ventricular septal defect, aberrant right subclavian artery and unbalanced ventricles with hypertrophy of the right ventricle detected by ultrasound examination at gestational week 21. Chromosome analysis revealed in 18/55 metaphases (33%) mosaicism for trisomy 12 in cultured amniocytes. Fluorescence in situ hybridization (FISH) analysis on 58 uncultured amniocytes found 24 cells with trisomy 12 consistent with 41%mosaicism for trisomy 12. An array-CGH analysis (arraycomparative genomic hybridization) of fetal DNA displayed an additional chromosome 12 in 30-40% of all analyzed cells (Fig. 1). The parents decided to continue the pregnancy.Ultrasound in gestational week 24 confirmed the abnormalities listed above and additionally showed an increased vascular resistance of the left uterine artery. In gestational week 28, the development of polyhydramnios was discovered. The following ultrasound examinations showed no deterioration of the previously stated findings.
After birth, cytogenetic analysis of neonatal lymphocytes revealed a 46,XX karyotype. Metaphase and interphase FISH analysis on 10 metaphases and 200 interphase cells did not give evidence for trisomy 12. Following these results,interphase FISH analysis on uncultured urinary cells was conducted at five weeks of age and revealed 28% (28/100 cells) mosaicism for trisomy 12. Thereafter, interphase FISH on lymphocytes was repeated showing three signals in 7/200 cells consistent with 3.5% mosaicism for trisomy 12 (Fig. 2). Repetitive chromosome analysis confirmed the normal karyotype of the neonatal blood sample. The varying results described above are shown in Table 2.
The female baby was delivered spontaneously at 38 weeks of gestation with a birth weight of 3820 g (91th percentile),length of 52 cm (72th percentile), and head circumference of 38 cm (> 99th percentile). She showed multiple dysmorphic features, such as the prominent forehead, broad flat nasal bridge, low-set ears, prominent cheeks, flat profile, single transverse palmar crease on both sides, camptodactyly of the fifth finger on both sides, clinodactyly of the fourth finger on both sides, overlapping toes, deep plantar crease on both sides, short neck, and anteriorly placed anus (Fig. 3). The ophthalmologic examination displayed a missing upper eyelid crease and coloboma of the right eye as well as blepharophimosis, ptosis, epicanthus, and tapetoretinal abnormalities on both sides. Echocardiography demonstrated a ventricular septal defect, an atrial septal defect, a patent ductus arteriosus botalli, an aberrant right subclavian artery, and a bicuspid aortic valve. At six weeks of age, the child underwent an interventional occlusion of the patent ductus. A tracheobronchoscopy that was performed, after weaning from the respirator failed and after recurrent pneumonias at 10 weeks of age revealed severe tracheobronchomalacia. Additionally,the girl had unexplained episodes of hypoglycemia. Further diagnostics showed a transient hypopituitarism with a decreased level of cortisol. Magnetic resonance tomography(MRI) at 4 months of age detected mild cerebral atrophy with enlarged inner and outer cerebrospinal fluid spaces as well as the delay of myelination in the cerebrum. Owing to feeding problems, a percutaneous endoscopic gastrostomy tube (PEG) was inserted at an age of almost 5 months. The girl stayed in the hospital for about 5 months and was discharged with a non-invasive respiratory support and PEG.She was breastfed and ventilated during the day only with continuous positive airway pressure. At night, the girl was fed over the tube and was ventilated with a pressure-controlled ventilation. Five months later, she did not need respiratory support during the day anymore. By 13 months of age,the girl still had pressure-controlled ventilation overnight.She was breastfed, ate baby foods, and was given only water via the PEG. Her weight was 8100 g (8th percentile); her length was 71 cm (3rd percentile); and her head circumference was 46.5 cm (66th percentile). The brainstem evoked response audiometry displayed a normal signal in the left ear and could not be analyzed in the right ear due to a narrow ear canal. The pupillary light reflex showed no abnormalities,and she was able to fixate briefly on an object and people.She presented with an unremarkable smooth pursuit eye movement but with a persistent nystagmus. Coloboma of the right eye and tapetoretinal abnormalities were still present.Closure of the atrial septal defect is scheduled. Concerning the terms of development, she reached the age-appropriate milestones with delay. At 9 months of age the girl was able to roll to the sides and from front to back, but not from back to front and could lift and uphold her head for a few minutes. At 13 months, she could raise her chest supported by arms when placed in the prone position for a short period of time. She was still not able to roll from back to front. She transferred objects from hand to hand and sometimes used a pincer grip but had general muscular hypotonia. She was babbling but not using any words. She received physical and occupational therapy. According to the new classification of genetic mosaicism the mosaicism status of the individual can be classified as follows: A3B2C1D4aE1F3 [ 9].
The phenotype of trisomy 12 mosaicism as reported in the literature is variable and, therefore, recognition is quite difficult. Consistent abnormalities that have been found in at least three of the up to now reported patients are dysmorphic(cranio-) facial features, developmental delay, intellectual disability, pigmentary dysplasia, congenital heart defects,muscular hypotonia, microcephaly, short neck, and short stature (Table 1). Of these shared features, our patient had dysmorphic facial anomalies, a complex congenital heart defect, developmental delay, and short neck. Parallels may be drawn to Leschot et al. and Hu et al. (case 4), whose patients also had unexplained hypoglycemia [ 10, 11]. Further diagnostics in our patient showed a transient hypopituitarism with a decreased production of cortisol. Another similarity can be discovered with the patient reported by DeLozier-Blanchet et al. who had camptodactyly as well as with the patient described by Al-Hertani et al. who also suffered from tracheomalacia [ 4, 12]. However, the latter was induced by a vascular ring, in contrast to our patient. Hu et al. also described an anteriorly placed anus in one patient(case 1), cerebral atrophy as well as enlargement of the lateral ventricles (case 2), and a coloboma of one eye (case 4)[ 11]. These features occurred in our patient as well. Varying dysmorphic (cranio-) facial features appear to be the most common finding occurring in 16 of the 21 cases followed by congenital heart defects detected in nine cases (Table 1).
Furthermore, using the Face2Gene application might be an additional possibility to help support the prenatal diagnosis of mosaic trisomy 12. The Face2Gene application is a facial analysis technology using a software called DeepGestalt. It provides valuable assistance for recognizing genetic syndromes by analyzing facial images of patients[ 13]. Because phenotype descriptions are rather subjective and the phenotype of trisomy 12 mosaicism is quite variable,which complicates its definition, using an automated facial analysis might lead to a faster diagnosis.
Providing a prognosis for children with mosaic trisomy 12 might be challenging because there seems to be no association between the degree of mosaicism and the severity of the phenotype. Von Koskull et al. reported a girl with 25% mosaicism for trisomy 12 in skin fibroblasts with a complex congenital heart defect who died at five weeks of age after an attempted surgical heart procedure [ 14]. Bischoff et al. described a rather low incidence of trisomic cells(5%) in spleen tissue in their patient who had an unfavorable diagnosis of Potter sequence. The patient died immediately after birth [ 15]. The same percentage of trisomic cells was found in a female patient described by Chen et al. with no phenotypic abnormalities at all [ 1]. Boulard et al. reported a 15-year old girl with 80% mosaicism for trisomy 12 in ovarian fibroblasts. She presented with pituitary stalk interruption, polycystic ovary syndrome, strabismus, conductive hearing loss, atrial septal defect, and delayed growth but normal cognitive development [ 16]. In the four cases with a fatal outcome, the detected counts for mosaic trisomy 12 ranged from 5 to 26%. The percentage of trisomic cells in the other 17 patients varied from 3.5% to100% (Table 1). Theseobservations give no evidence for a correlation between a lower mosaic trisomy 12 level and a less severe outcome,nor a higher mosaic trisomy 12 level and a more severe outcome. Table 1 also illustrates that no clear association exists between the manifestation of clinical features and the type of tissue trisomy 12 cells.

Table 1 Reported cases of patients diagnosed postnatally with mosaic trisomy 12

Table 1 (continued)

Table 1 (continued)

Table 1 (continued)

Table 1 (continued)
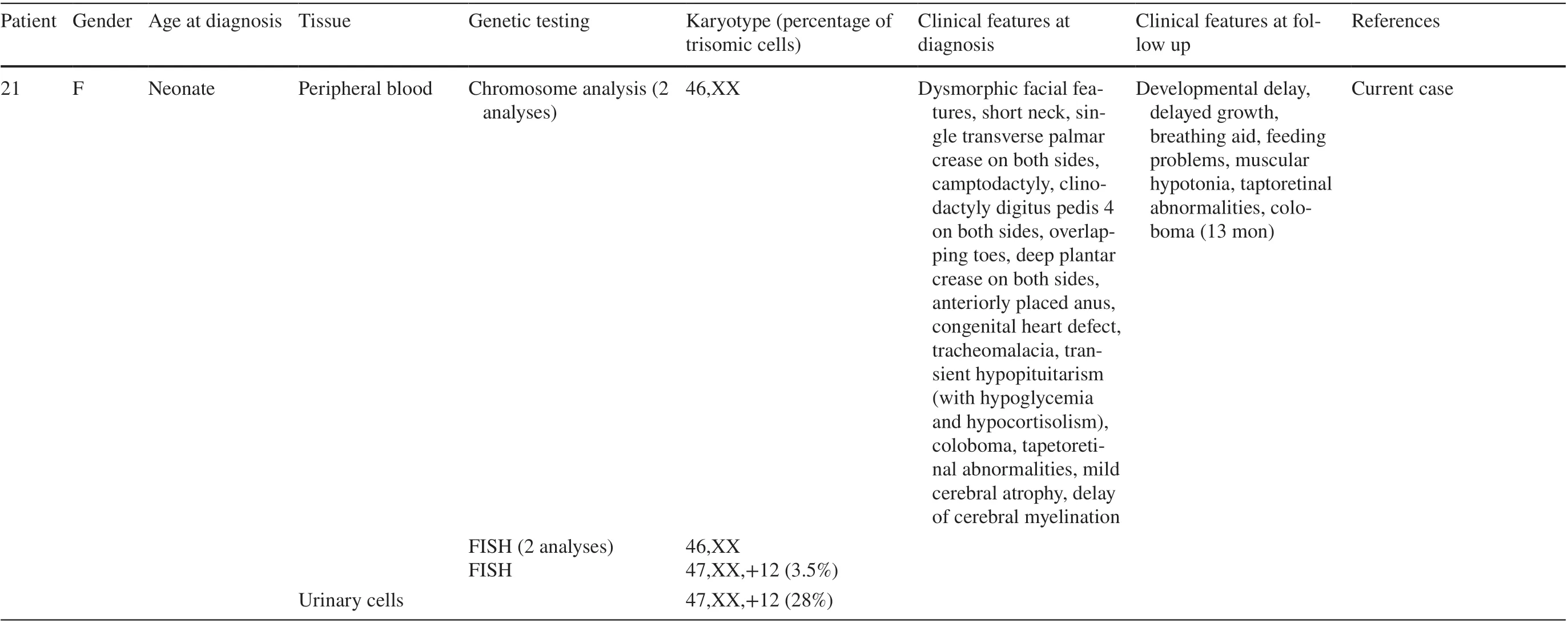
Table 1 (continued)
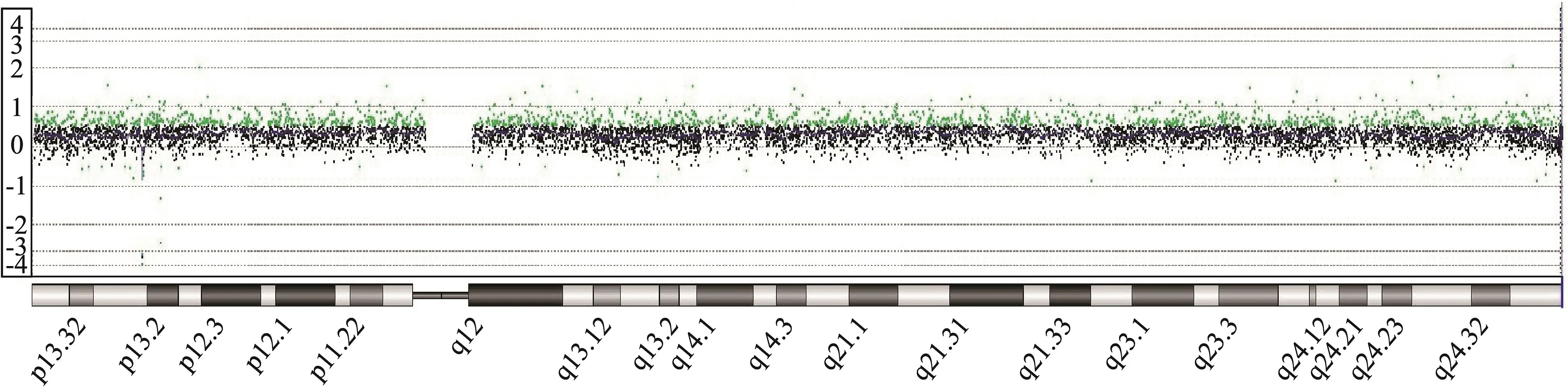
Fig. 1 Detailed view profile (array-CGH) of chromosome 12 showing the trisomy 12 mosaicism. X-axis, chromosome 12 ideogram from p (left side) to q arm (right side); Y-axis, intensity

Fig. 2 Fluorescence in situ hybridization images of metaphase ( a) and interphase ( b)of chromosome 12p subtelomeres (spectrum orange: 12q;spectrum green: 12p; both from Abbott) of lymphocytes showing a regular karyotype ( a) and trisomy 12 ( b)
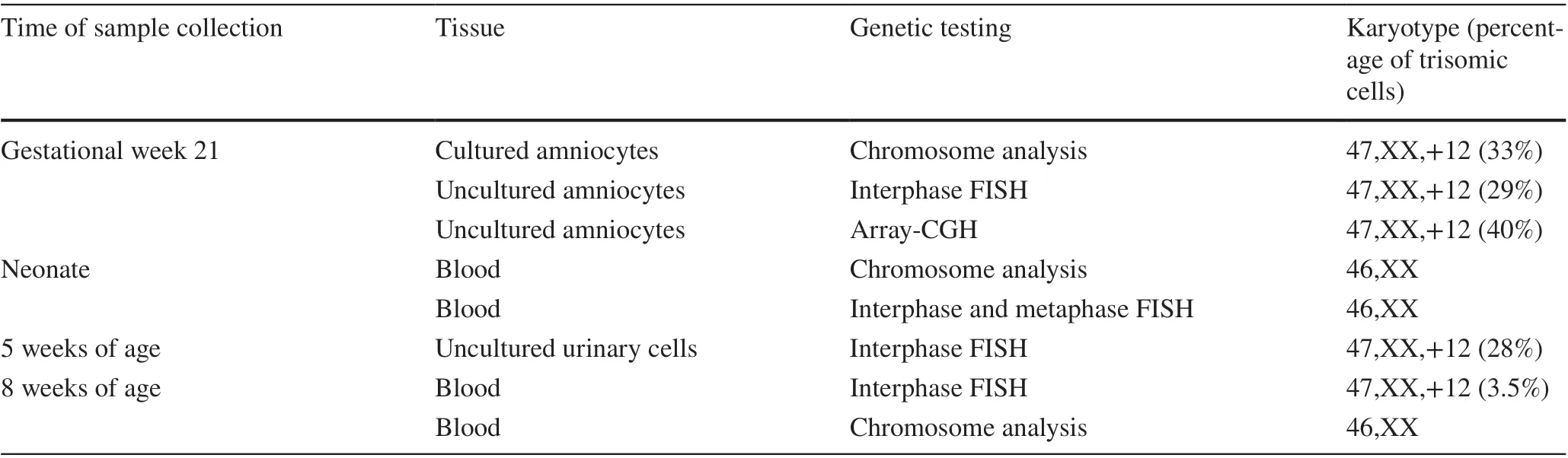
Table 2 Cytogenetic and molecular genetic findings
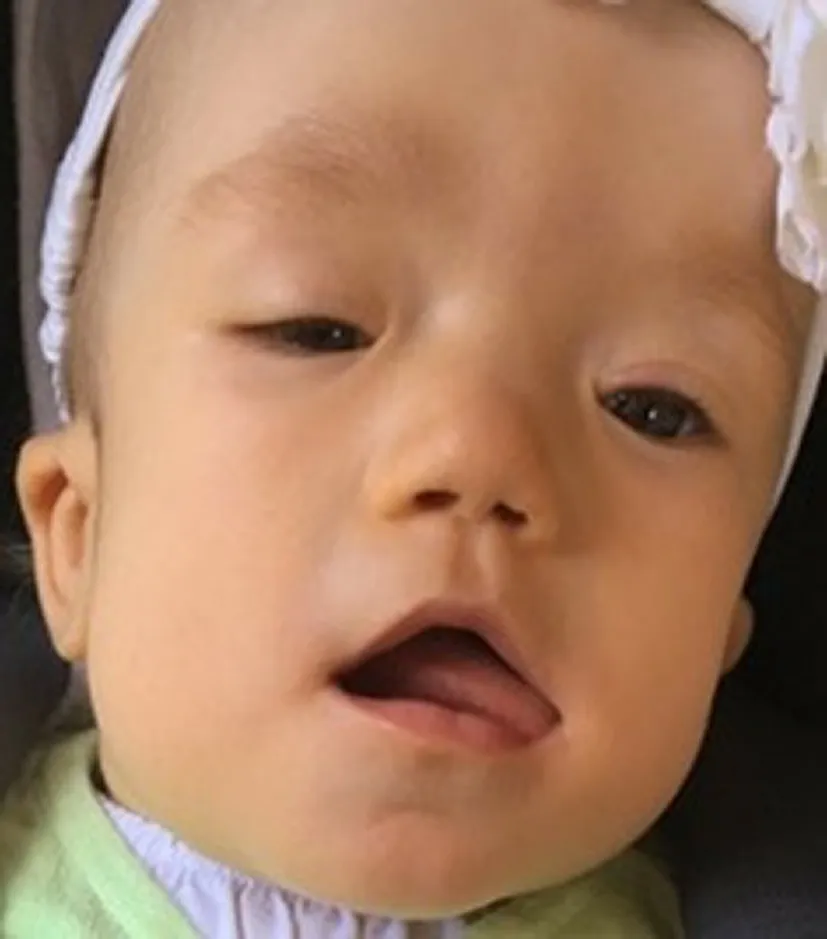
Fig. 3 Facial dysmorphic features include prominent forehead, ptosis,epicanthus, left missing upper eyelid crease, broad flat nasal bridge,low-set ears and prominent cheeks. Parents gave consent for publishing the photograph of the case
Moreover, there have been observations regarding differing results in repeated amniocentesis complicating genetic counseling already prenatally [ 1, 17]. Inconsistent trisomy 12 mosaicism levels have been reported by various authors. For examples, Spiro et al. reported 7.5%versus 48%, Chen et al. reported 16.7% vs. 39.1% in 2013 and 17.2% vs. 40% in 2017 [ 1, 17]. These findings make a reliable prognosis almost impossible. Additionally, as Aughton et al. and Al-Hertani et al. already have stated,there seems to be a female preponderance with mosaic trisomy 12 [ 4, 18]. Table 1 shows that the male:female sex ratio of patients diagnosed postnatally is now 4:17 supporting the statement above. The reason for this phenomenon is thus far unknown.
Cytogenetic studies in the blood are an efficient method for the detection of mosaics, especially if an adequate number of cells are analyzed. However, this method may not be sufficient or decisive if there are mosaics restricted to tissues. The present case confirms again that genetic analysis of the blood can be an unreliable indicator of the child’s karyotype after prenatal detection of mosaic trisomy 12. In our patient, trisomic cells were detected in only one of four blood analyses and ultimately with a rather low incidence of 3.5% compared to the prenatal results. This might be due to the fact that the amniotic fluid contains cells of all three germ layers (endoderm, mesoderm,ectoderm), and analysis of blood lymphocytes only represents cells of the mesoderm. Hu et al. reported similar results with mosaic trisomy 12 detected in only one of four blood analyses as well (case 1) [ 11]. These results are consistent with several other cases in which trisomic cells were not found in the blood at all but were found in different tissues. In our patient, the presence of mosaic trisomy 12 could be confirmed for the first time in urinary cells after birth. Out of the 21 cases, trisomic cells were detected in peripheral or cord blood in 41% analyses(14/34), in skin cells in 59% analyses (10/17), in urinary cells in 83% analyses (5/6) and in 100% analyses in ovarian cells(1/1), spleen cells (1/1), and buccal cells (1/1). In addition,this review of the literature suggests that identifying mosaic trisomy 12 is not always achieved using chromosome analysis. Chromosome analysis was performed 42 times but only detected trisomic cells 17 times. Array-CGH identified trisomy 12 mosaicism in four out of five conducted analyses, FISH in eleven out of 13 analyses. Thus, chromosome analysis led in 40% to a correct diagnosis, whereas FISH detected trisomic cells in 85% and array-CGH in 80%.In conclusion, sole analysis of peripheral or cord blood,as well as using only chromosome analysis for diagnostic testing, might fail to reveal mosaic trisomy 12. Once again,it is evident that classical cytogenetic techniques are still the gold standard for the detection of low-level mosaicism and chromosomal rearrangements. Chromosomal microarray analysis cannot detect this kind of chromosomal aberration owing to its limitation (> 20% mosaic cell lines). However,in cases in which it is found prenatally or if a constellation of abnormalities including dysmorphic facial features, congenital heart defects, pigmentary dysplasia, hypotonia, and developmental delay is detected postnatally, several tissues should be analyzed for identification of mosaic trisomy 12. In addition, it should be taken into account that FISH and array-CGH allow the examination of more cells than chromosome analysis along with a higher chance to detect the mosaic trisomy 12. Therefore, if mosaic trisomy 12 is not revealed by chromosome analysis, FISH or array-CGH should be done as well. It should even be considered to perform FISH or array-CGH first.
AcknowledgementsThe authors would like to thank the family for their participation. This work was supported by the German Research Foundation (DFG) and the Technical University of Munich (TUM) in the framework of the Open Access Publishing Program.
Authors contributionsKM, TB, GL, SL and JH performed a genetic analysis in this patient. MK and DR treated the patient. DH, TH, GB,SLF and JH organized the manuscript. All authors approved the final version.
FundingOpen Access funding enabled and organized by Projekt DEAL.
Compliance with ethical standards
Ethical approvalThis study was approved by the Ethics Committee of the Technical University of Munich, Munich, Germany (#5360/12S).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Consent for publicationWritten informed consent was obtained from both parents for genetic testing and for publication of this case.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http:// creat iveco mmons. org/ licen ses/ by/4. 0/.
 World Journal of Pediatrics2021年4期
World Journal of Pediatrics2021年4期
- World Journal of Pediatrics的其它文章
- Increased asprosin is associated with non-alcoholic fatty liver disease in children with obesity
- Increasing prevalence and influencing factors of childhood asthma:a cross-sectional study in Shanghai, China
- Responsible genes in children with primary vesicoureteral reflux:findings from the Chinese Children Genetic Kidney Disease Database
- Pediatric interfacility transport effects on mortality and length of stay
- Impact of probiotics supplement on the gut microbiota in neonates with antibiotic exposure:an open-label single-center randomized parallel controlled study
- Characteristics of immune and inflammatory responses among different age groups of pediatric patients with COVID-19 in China
