Increasing prevalence and influencing factors of childhood asthma:a cross-sectional study in Shanghai, China
Ya-Bin Hu · Yi-Ting Chen · Shi-Jian Liu · Fan Jiang · Mei-Qin Wu · Chong-Huai Yan · Jian-Guo Tan ·Guang-Jun Yu · Yi Hu · Yong Yin · Jia-Jie Qu · Sheng-Hui Li · Shi-Lu Tong,10,11,12
Abstract
Keywords Asthma · Children · Prevalence · Risk/protective factors
Introduction
Asthma, one of the most common chronic diseases, affects more than 300 million individuals worldwide, especially in children [ 1, 2]. The prevalence of asthma has increased dramatically during the past three decades along with economic development and urbanization in many regions of the world[ 3, 4]. With the largest population in the world, China has undergone unprecedented changes in socioeconomic development and urbanization. There has been massive migration of people from rural areas to cities for a better life. These changes have been associated with a rapid increase in the prevalence of asthma leading to an enormous burden on the health care system. In 2000 a national survey including 43 cities from 31 provinces showed that the overall prevalence of asthma among children aged 0-14 years was 2.0%, with the highest prevalence in Shanghai (4.5%) [ 5]. In 2005 a study covering 8 major cities in China reported the average asthma prevalence of 3.3% among children aged 6-13 years,also with the highest rate in Shanghai (7.2%) [ 6]. A third national survey, conducted in 43 cities from 27 provinces during September 2009-August 2010, found that the prevalence of asthma in children of 0-14 years was 3.0%, again with the highest prevalence in Shanghai (7.6%) [ 7]. The prevalence of childhood asthma increased significantly in Shanghai from 1990 to 2011 [ 8].
The rapidly increased prevalence of childhood asthma is likely to be related to changes of lifestyle and environmental exposures during pregnancy or in early life. Accumulating evidence suggests that exposure to household renovation and mold/dampness during pregnancy and early childhood was associated with an increased risk for childhood asthma[ 9- 11]. In addition, being male, younger age, having allergy history and exposure to passive smoking were also associated with elevated risk for childhood asthma [ 6, 12- 16].Since the last nationwide survey was conducted almost a decade ago in China, the current prevalence of childhood asthma is unclear. Therefore, we conducted a populationbased cross-sectional study among pre-school children aged 3-7 years in Shanghai, the largest metropolitan city in China, to reveal the latest prevalence of childhood asthma and to explore possible risk/protective factors.
Methods
Study participants
Shanghai is located in the Yangtze River estuary, which has over 27 million residents in 2021 [ 17]. A city-wide,cross-sectional survey was conducted during April-June 2019 across Shanghai. To obtain a representative sample, a multi-stage and multi-strata sampling approach was used. Three urban districts (Xuhui, Putuo, and Yangpu)and five suburban/rural districts (Pudong, Minhang, Jinshan, Qingpu, and Chongming) were randomly selected from 16 districts of Shanghai (7 urban districts and 9 suburban/rural districts). Then, a total of 31 kindergartens were randomly selected. We first explained the purpose and content of the project to the principals and teachers in detail at a study launch meeting before the survey began.Then, teachers conveyed these messages to parents through parents’ meetings at schools. An invitation letter was sent to all children’s parents, and a written informed consent was obtained from parents before filling out the questionnaire if they agreed to allow their children to take part.Each child’s mother completed the questionnaire. Overall, 6389 pre-school children were invited to participate in this survey, and 6237 (response rate: 97.6%) completed the questionnaires. After the exclusion of some children with inappropriate ages (e.g., < 3 years), 6163 (96.5%)pre-school children aged 3-7 years were finally included in this study.
The ethical application and the consent procedure of this study were approved by the Ethics Committees of Shanghai Jiao Tong University School of Medicine and Shanghai Children’s Medical Center.
Questionnaire
The questionnaire included questions on childhood asthma,general characteristics of children and parents, socioeconomic status (SES), allergy history of children and their family members, exposure during pregnancy, children’s lifestyle habits, and home environmental exposures in early life.
Assessment of asthma
The core question of The International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire was applied to assess asthma status: whether the child has ever been diagnosed with asthma by a doctor [ 18]. We conducted two questionnaire surveys (2 weeks apart) with 290 preschool children in the same kindergarten, and then compared and analyzed the data to calculate theCronbach’s Alphacoefficient and the intra-class correlation coefficient (ICC).TheCronbach’s alphacoefficient of the ISAAC allergic questionnaire in our sampled children was 0.91. The ICC of retest reliability was 0.94. Validity presented by Kaiser-Meyer-Olkin (KMO) was also 0.94. These results show the high validity and reliability of the questionnaire for the preschool children [ 19].
Assessment ofindependent variables
The questions about general characteristics of children and parents, SES (having the ownership of residence), allergy history of children and their family members, exposure during pregnancy, children’s lifestyle habits, and home environmental exposures in early life were similar to those used for the Swedish Dampness in Buildings and health (DBH)study [ 20] and the China, Children, Homes, Health (CCHH)study [ 21]. All questions were translated into Mandarin with appropriate cultural contents.
Statistical analysis
Statistical descriptions were made utilizing the frequency and percentage for categorical variables, and using the mean and standard deviation (SD) for continuous variables. Pearson’s Chi-squared (χ 2 ) test was used to assess the difference in the prevalence of asthma between different groups. We first implemented univariate logistic regression to calculate the unadjusted odds ratios (OR) and 95% confidence interval(CI), and then developed multivariable logistic regression models to calculate adjusted OR (AOR) and 95% CI. We also conducted the cross-validation analysis for the multivariable logistic regression model, where 6163 observations were randomly divided into a training set (80%) and a test set(20%). A fitting model was constructed based on the training set, and then the predicted prevalence of asthma was validated using the test set. The prediction accuracy was 0.849.Besides, we calculated the area under the curve of the final model based on the total samples, where the C-index was 0.703 (95% CI: 0.684, 0.722). We also used the bootstrap method of sampling 1000 times to draw the calibration curve and found that the final model fitted the data reasonably well(Fig. S1). All statistical analyses were conducted using R(version 3.6.3; R Core Team).Pvalue < 0.05 (two-sided)was set as the level of statistical significance.
Results
Characteristics of the sample
Table 1 depicts the summary statistics of characteristics among 6163 kindergarten children, and the overall prevalence of childhood asthma was 14.6%. This study included 3254 boys and 2909 girls, with the asthma prevalence of 16.2% and 12.9% (P< 0.001), respectively. Mean age was 5.2 years (SD = 0.9). The age-specific prevalence was 14.3%,15.6%, 15.6%, 13.8% and 14.0% for 3, 4, 5, 6 and 7 years,respectively (Fig. 1).
Time trends in the prevalence of childhood asthma
Figure 2 shows a gradual and consistent increase in the prevalence of asthma among the children aged 3-7 years in Shanghai during 1990-2019. We extracted some results from previous surveys: Huang et al. [ 8] and National cooperation group on childhood asthma [ 5]. The prevalence increased dramatically from 2.1% in 1990 to 14.6% in 2019. In 1990, 2000, 2011 and 2019, the prevalence was higher for male (2.8%, 6.5%,11.9% and 16.2%, respectively) than female (1.3%, 3.2%,8.4% and 12.9%, respectively, allP< 0.05). Among the children aged 3-7 years, the prevalence of asthma elevated from 1990 to 2019 in every age group (Fig. 1). We also compared the total and sex-specific prevalence of childhood asthma at every age group from 1990 to 2019. The prevalence of asthma in male was higher than that in female at every age group, but in many cases the 95% CIs corresponding to male and female prevalence overlapped in some age groups due to the small samples in some age groups (Fig. 1).
Prevalence of childhood asthma between different groups
Of the 6163 children, there were 443 (7.2%) preterm births,with the highest prevalence of childhood asthma (19.4%)(Table 1). Children born in autumn had the highest prevalence of asthma (15.7%), with the lowest prevalence in winter (12.8%). Children born by cesarean section had the higher prevalence of asthma compared to natural vaginal delivery, with the highest prevalence (18.3%) by elective cesarean section without indications. The prevalence of childhood asthma was higher in the group having the ownership of residence (16.4%) compared with those renting a house (9.5%). The prevalence of childhood asthma (16.9%)was higher in the group with miscarriage than those with no miscarriage (13.9%). The prevalence of asthma was higher in the group with allergy history (child: 24.8%, family: 23.6%, respectively) than those without allergy history(child: 10.9%, family: 11.3%, respectively).
The prevalence of asthma in the group spending less than 30 min/day on outdoor activities/sports (18.1%) was higher compared to those spending more time outdoors. The prevalence of asthma in the group having indoor plants (12.8%)was lower than those without them (16.5%). The more frequent cleaning rooms and curtains, the lower the prevalence of childhood asthma, with the lowest value for cleaning rooms daily (12.6%) and washing curtains monthly (7.8%).
Potential risk/protective factors for childhood asthma
Table 2 presents the results of logistic regression models about risk/protective factors for childhood asthma. Beingmale was associated with increased AOR (1.25, 95% CI 1.08, 1.45) of asthma. Children’s age was associated with decreased AOR (0.88, 95% CI 0.81, 0.96) of asthma. Children born in spring or in autumn were associated with increased AOR (1.25, 95% CI 1.01, 1.56 and 1.36, 95% CI 1.11, 1.67, respectively) of asthma compared with those born in winter. Preterm delivery was associated with elevated AOR (1.40, 95% CI 1.07, 1.81) of asthma. Elective cesarean section without indication was associated with higher AOR (1.45, 95% CI 1.18, 1.79) of asthma.Compared with holding the ownership of residence, renting a house was associated with attenuated AOR (0.70,95% CI 0.57, 0.86) of childhood asthma. Miscarriage was associated with increased AOR (1.20, 95% CI 1.01,1.41) of childhood asthma. Having an allergy history was also associated with elevated AOR (child: 2.25, 95% CI 1.93, 2.63; family: 1.85, 95% CI 1.58, 2.16) of childhood asthma. Children exposed to passive smoking, latex paint on bedroom and dust were associated with elevated AOR of asthma.
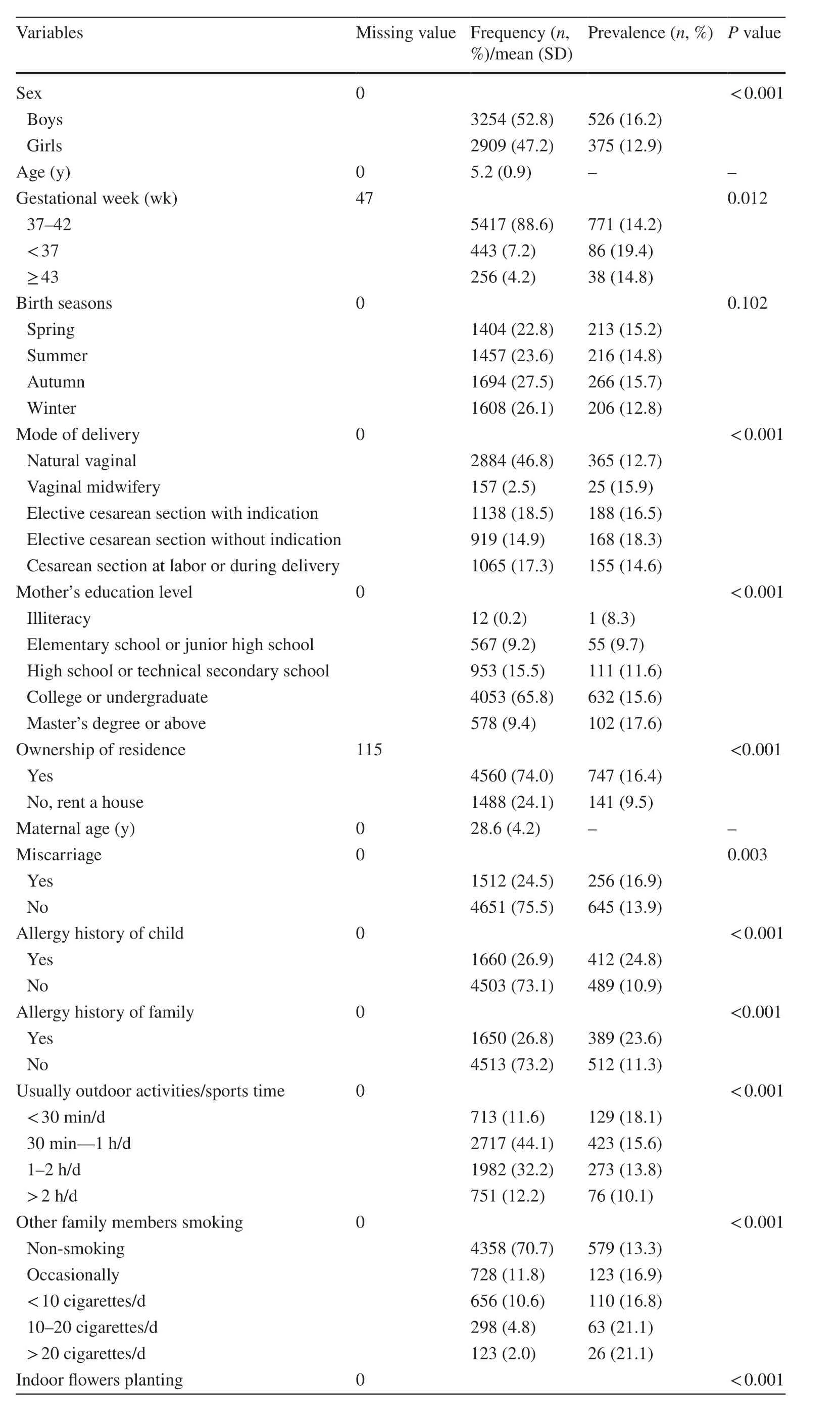
Table 1 Summary statistics of 6163 kindergarten children and the prevalence of childhood asthma by group

Table 1 (continued)
Children spending more time on outdoor activities/sports were associated with decreased AOR (0.54, 95%CI 0.39, 0.74 for > 2 h/day) of asthma compared to those spending less time outdoors. Having indoor plants was associated with reduced AOR (0.80, 95% CI 0.69, 0.93)of childhood asthma. Compared to cleaning rooms daily,cleaning rooms 1-3 times/week or 1-3 times/month was associated with increased AOR (1.36, 95% CI 1.14, 1.62 and 1.50, 95% CI 1.13, 2.01, respectively) of childhood asthma.
Tables S1 and S2 are shown to make our data available for future analyses of trends in asthma prevalence.

Fig. 1 Prevalence of childhood asthma at different age groups in Shanghai during 1990 to 2019. Previous data were collected from two nationwide cross-sectional surveys (1990 and 2000) [ 5] and a big multicenter survey (2011) [ 8]
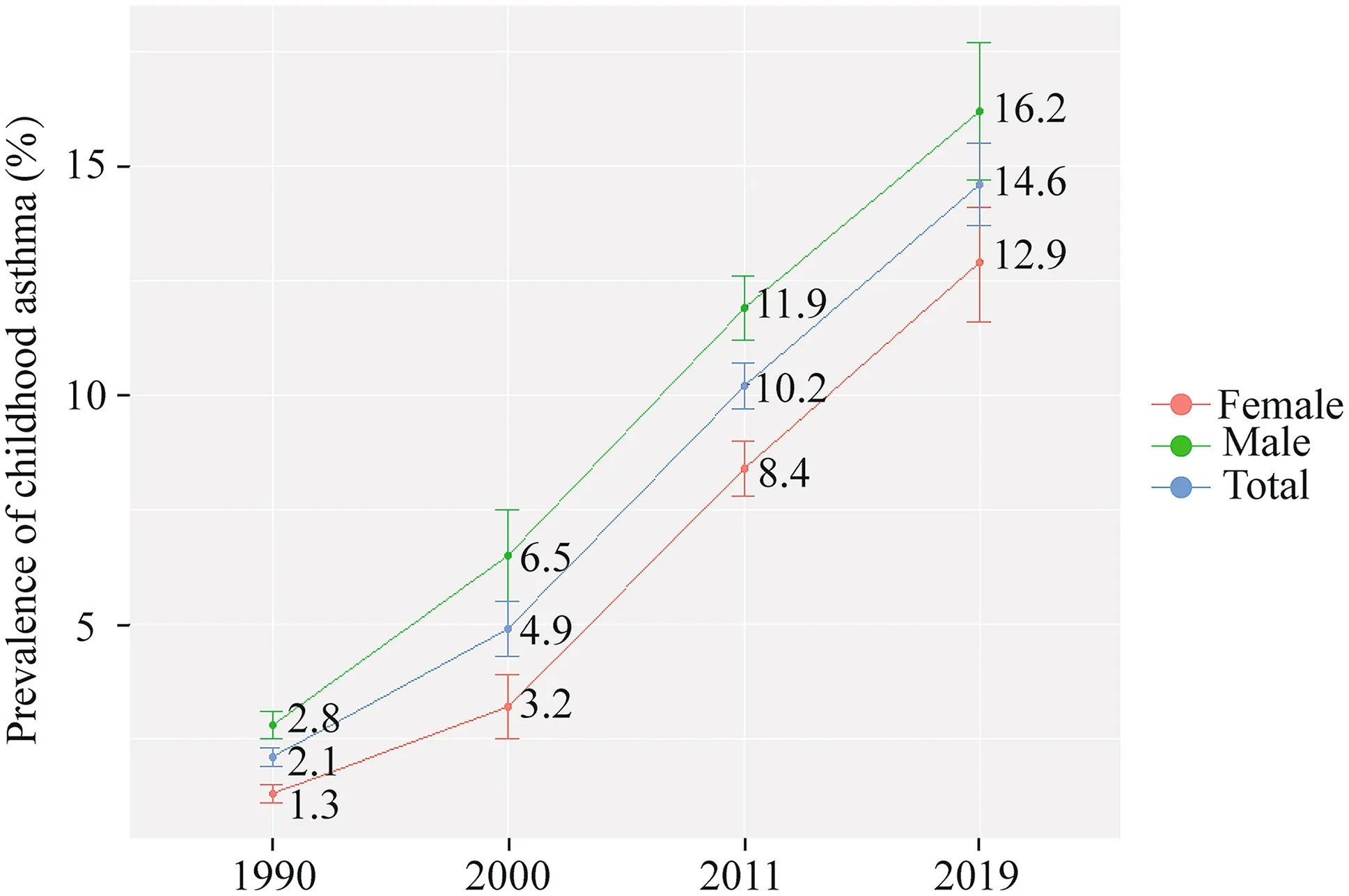
Fig. 2 Prevalence of childhood asthma aged 3-7 years in Shanghai during 1990-2019. Previous data were collected from two nationwide cross-sectional surveys (1990 and 2000) [ 5] and one big multicenter survey (2011) [ 8]
Discussion
In this city-wide cross-sectional study, we found that the overall prevalence of asthma among children aged 3-7 years was 14.6% in Shanghai, China, which has increased more than six folds since 1990. Being male,younger age, preterm delivery, being born in spring or autumn, being delivered by elective cesarean section without indication, having miscarriage history, high SES,allergy history of child or family, and exposure to passive smoking, latex paint, and dust were potential risk factors for childhood asthma, while spending more time outdoors,having indoor plants, and cleaning rooms more frequently were potential protective factors for childhood asthma.
The prevalence of childhood asthma in Shanghai has been rising dramatically over recent decades [ 8]. Three national surveys conducted in 1990, 2000 and 2010 found that children aged 0-14 years in Shanghai had the highest prevalence of asthma across the country with 2.0%, 4.5%and 7.6%, respectively [ 5, 7]. In particular, the prevalence of asthma among the children aged 3-7 years has increased remarkably from 1990 to 2019 (Fig. 2), suggesting possible impacts of rapid socioeconomic development and urbanization on asthma, even though increased awareness and improved diagnosis of childhood asthma may also account for some of these increments [ 5, 7, 8]. The ISAAC study found that the global prevalence of asthma among children of 6-7 years was 14.1% in 2002 [ 22],which is similar to the prevalence observed in this study(14.0%). Recently, the Global Asthma Network Phase I study in Mexico reported that the prevalence of wheezing ever or current wheeze was 26.2% (95% CI 25.8%,26.7%) and 10.2% (95% CI 9.9%, 10.5%) in school children (6-7 years), respectively [ 23].
We found that being male, younger age, higher SES, having allergy history and exposure to passive smoking were key risk factors of childhood asthma, which were consistent with many previous studies [ 6, 12- 16]. Genotype is thought to account for 40-60% of variance in the allergen-specific IgE level, which is an important risk factor for childhood asthma [ 24]. The hygiene hypothesis might partly explain the association between higher SES and higher prevalence of childhood asthma [ 25, 26]. For example, a western lifestyle was associated with a lack of infections, where microbes inducing Treg cells, Th1 cells and allergen cross-reactive antibody responses. Environmental and microbial stimuli during early life are sensed and integrated by barrier tissues of the lung, the skin and the gut, resulting in promoting either inflammatory or tolerogenic immunity [ 26]. A recent review reported that exposure to passive smoking increases the risk of childhood asthma and involves multiple mechanisms including impaired fetal lung development, endocrine disorders, abnormal immune responses, and epigenetic modifications [ 27].
Previous studies reported that household mold/dampness and renovation were associated with an increased risk for childhood asthma [ 9, 10]. Zhang et al. confirmed that using cellulose based materials in home decoration and renovation, and adding new furniture during early childhood and pregnancy could increase the risk of childhood asthma[ 11]. Similarly, we found that the prevalence of childhood asthma was higher in the group exposed to mold/dampness or latex paint compared to those without these exposures.The rapidly increasing prevalence of childhood asthma is likely to be related to changes of lifestyle and environmental exposures during pregnancy or in early life. Accumulating evidence suggests that early life exposure not only has shortterm effects on fetal growth but also long-term impacts on an individual’s health and disease susceptibility in later life[ 27, 28].
In addition, we found preterm births, being born in spring or autumn and delivered by elective cesarean section without indications, miscarriage and exposure to dust were associated with the increased risk of childhood asthma. Preterm children, perhaps accompanied by incomplete development of the respiratory and immune systems,are more likely to develop asthma in their lifetime [ 29].Children born in spring or autumn might be exposed to pollen more frequently compared to those born in winter[ 30]. Pollen exposure may weaken innate defense against respiratory viruses and may trigger childhood asthma [ 31].Children born by elective cesarean section without indication, not exposed to stress and flora during childbirth, and often accompanied with antibiotic use, may be more likely to develop childhood asthma [ 32- 35]. Children exposed to dust frequently, which may contain dust mites and respiratory viruses, may be at higher risk of suffering respiratory diseases or symptoms. Meng and Rosenwasser found that 30-60% of variance in the allergen-specific IgE level in the positive skin test could be attributed to dust mite etiology[ 24].
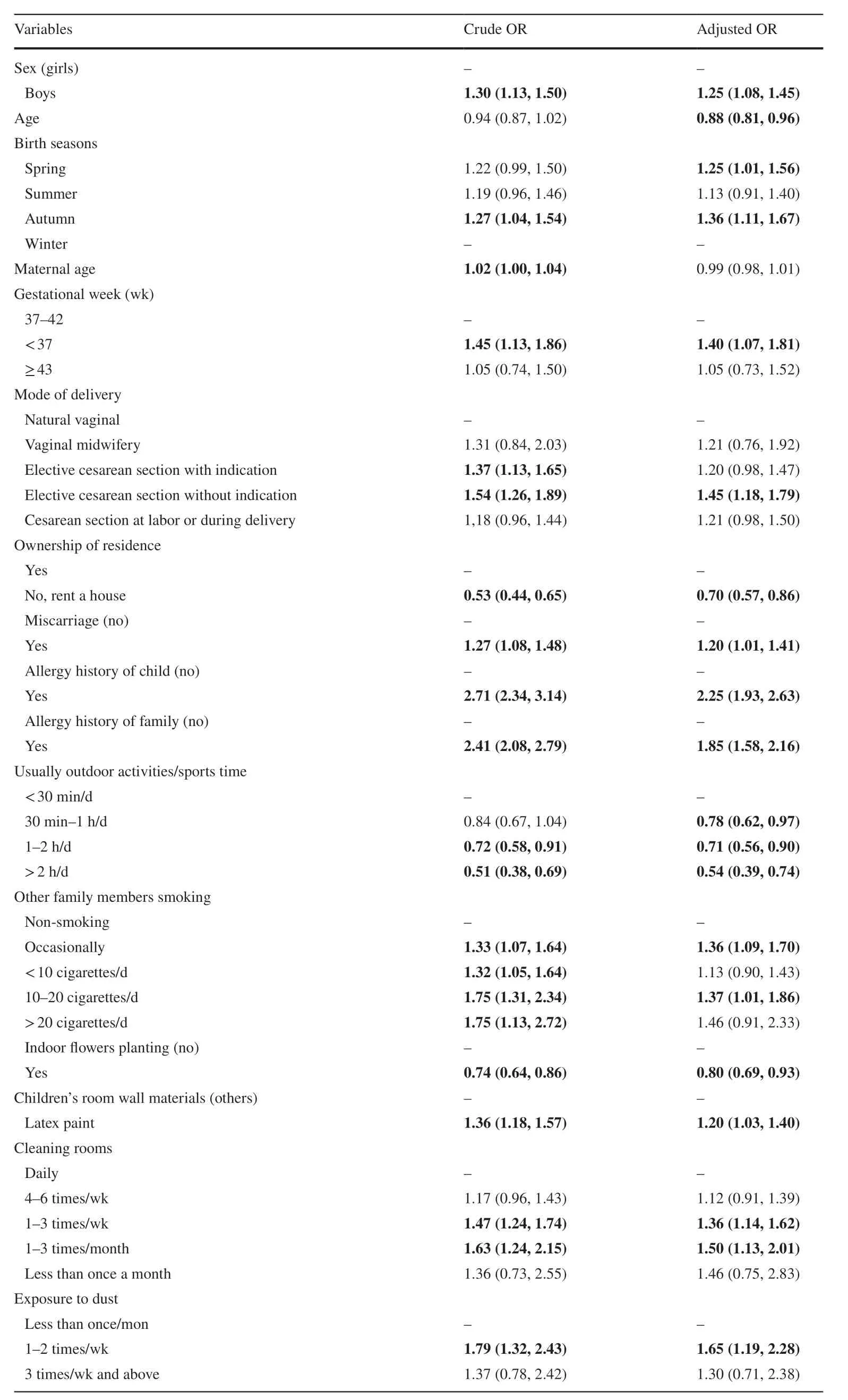
Table 2 Odds ratios(OR) from univariate and multivariate logistic regression of influencing factors for childhood asthma
We also found that spending more time outdoors(> 30 minutes/day), having indoor plants and cleaning rooms more frequently (daily) were potential protective factors for childhood asthma. Spending more time on outdoor activities/sports can strengthen the immunity, to protect children from viral/bacterial infection [ 36]. Having indoor plants and cleaning rooms more frequently could reduce exposure to fine particles and dust, which is associated with improved airway and reduced respiratory inflammation in children [ 24, 37].
There are three major strengths in this study. First, a representative sample was likely obtained through a stringent sampling approach with a multi-stage and multi-strata random sample size. Second, this population-based cross-sectional study achieved a high response rate (96.5%), so selection bias was probably minimal. Third, a wide range ofindependent variables including both risk and protective factors for childhood asthma were considered in this study to explore how they influenced the prevalence of asthma.
This study also has several limitations. First, the crosssectional study design cannot establish a causal relationship. However, the independent variables included in the multivariable model (e.g., exposure during pregnancy, SES and allergy history) were significantly earlier than the diagnosis of childhood asthma, thus suggesting a certain temporal relationship. Second, recall bias was inevitable to some extent as all the information was obtained from questionnaires. As pregnancy was a special event and the China’s one-child policy in the past decades, the potential for recall bias might not be extensive. In addition, we conducted two questionnaire surveys (2 weeks apart) with 290 preschool children in the same kindergarten, and found that the validity and reliability of the questionnaire were quite high.Finally, the nature and severity of childhood asthma was not measured in this study, and therefore, the determinants of mild and severe asthma cases cannot be distinguished.
Despite these limitations, the results from this study have demonstrated the contemporary prevalence of childhood asthma and its influencing factors in Shanghai. Until further research is undertaken, these findings may provide evidence that can be used to develop appropriate strategies to prevent and control childhood asthma in metropolitan cities like Shanghai. For example, children should spend more time on outdoor sports and should keep home clean while trying to avoid exposure to passive smoking.
In conclusion, the prevalence of childhood asthma in Shanghai has increased rapidly during the past decades. The findings about risk and protective factors of childhood asthma may be useful for developing and implementing targeted and appropriate interventions to prevent and control childhood asthma in Shanghai and in other similar metropolitan cities.
Supplementary InformationThe online version contains supplementary material available at https:// doi. org/ 10. 1007/ s12519- 021- 00436-x.
AcknowledgementsThe authors thank the kindergarten teachers and parents who participated in this study.
Author contributionsYH and YC contributed equally to the paper. YH performed the statistical analysis and drafted the manuscript. YC was involved with study design and participants recruitment. ST and SL conceived the study, developed its design, supervised the field work and revised the manuscript. All authors have read and approved the final manuscript.
FundingThe study was funded by special grant for Preschool Children’s Health Management from Shanghai Municipal Education Commission, grants from National Natural Science Foundation of China(81874266, 81673183), and key project from Shanghai Municipal Science and Technology Commission (18411951600).
Data availabilityData are only available on an approved request to the corresponding author.
Compliance with ethical standards
Ethical approvalThe ethical application and the consent procedure of this study were approved by the Ethics Committees of Shanghai Jiao Tong University School of Medicine and Shanghai Children’s Medical Center.
Conflict of interestNo financial or nonfinancial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
 World Journal of Pediatrics2021年4期
World Journal of Pediatrics2021年4期
- World Journal of Pediatrics的其它文章
- Increased asprosin is associated with non-alcoholic fatty liver disease in children with obesity
- Mosaic trisomy 12 diagnosed in a female patient: clinical features,genetic analysis, and review of the literature
- Responsible genes in children with primary vesicoureteral reflux:findings from the Chinese Children Genetic Kidney Disease Database
- Pediatric interfacility transport effects on mortality and length of stay
- Impact of probiotics supplement on the gut microbiota in neonates with antibiotic exposure:an open-label single-center randomized parallel controlled study
- Characteristics of immune and inflammatory responses among different age groups of pediatric patients with COVID-19 in China
