Increased asprosin is associated with non-alcoholic fatty liver disease in children with obesity
Lu-Jie Liu · Yu-Rong Kang · Yan-Feng Xiao
Abstract
Keywords Adipokine · Asprosin · Non-alcoholic fatty liver disease · Obesity
Introduction
The dramatically increasing prevalence of obesity in childhood and adolescents represents a major problem worldwide[ 1]. Being obese is commonly associated with metabolic diseases, such as type 2 diabetes mellitus, cardiovascular disease, non-alcohol fatty liver disease (NAFLD) and metabolic syndrome. NAFLD is the most frequent liver disease in children and adolescents. The global incidence of pediatric NAFLD from the general population is 7.6% and is 34.2%in obese children [ 2]. NAFLD encompasses a wide spectrum of abnormalities including simple hepatic steatosis to non-alcoholic steatohepatitis (NASH), cirrhosis, end-stage of liver disease and hepatocellular carcinoma, which has become an increasing public health issue also in pediatrics[ 3, 4]. However, the etiology and pathogenesis of obesity and NAFLD are not fully understood.
Adipose tissue is an active endocrine organ secreting a lot of pro-inflammatory and anti-inflammatory adipokines,which play a pivotal role in the pathogenesis of metabolic disease. Asprosin is a novel adipokine discovered by Romere et al. in a group of patients with an extremely lean condition called neonatal progeroid syndrome [ 5]. As a fastinginduced glucogenic and orexigenic hormone, asprosin is secreted from white adipose tissue and targets the liver and the brain. Asprosin stimulates hepatic glucose release and raises insulin levels and sensitivity to insulin by activating G-protein-cyclic-AMP dependent protein kinase A (cAMP)- protein kinase A (PKA) pathway in the liver. In contrast,asprosin activates agouti-related protein (AgRP) neurons to increase food consumption in the brain [ 5, 6]. Although asprosin has been positively correlated with obesity and with alterations in glucose metabolism, both in mouse models and in humans, the pathophysiology related to the action of this adipokine is not entirely clear, especially in pediatric ages [ 5- 12]. There are conflicting results regarding the correlation between plasma asprosin levels and obesity in pediatric cohorts [ 7, 11- 13]. Ke et al. discovered that serum asprosin was significantly higher in adult NAFLD patients,but no report has identified the relationship of circulating levels of asprosin with NAFLD in children [ 14].
Consequently, we conducted this cross-sectional study to evaluate serum levels of asprosin both in lean controls and obese children, as well as to investigate the potential association of asprosin with NAFLD in obese children.
Methods
Subjects
A total of 110 children (aged 6-18 years), including 35 children with normal weight and 75 obese children were enrolled at the Second Affiliated Hospital of Xi’an Jiaotong University. Individuals with a history of alcohol consumption,endocrine disorders, genetic obesity syndromes, fever or acute infection, viral hepatitis, autoimmune hepatitis or liver disease for causes other than simple obesity were excluded from this study. All the subjects or their legal guardians provided written informed consent. Our study was approved by the Ethics Committee of Xi’an Jiaotong University.
Anthropometric collections
Standing height, body weight and waist circumference (WC)were measured while the children wore only underclothes without shoes. Body mass index (BMI) and waist to height ratio (WHR) were calculated as body weight (kg)/the square of height (m 2 ) and waist circumference (cm)/height (cm),respectively. According to the BMI reference for Chinese children and adolescents, children with a BMI > 95% for their age and sex were diagnosed as having obesity. The BMI data were standardized to age- and sex-specific centiles and were converted into a BMI standard deviation score (BMISDS) [ 15]. Systolic and diastolic blood pressures (SBP and DBP, respectively) were measured after a 10-min rest.
Biochemical measurements
Blood samples were obtained at 8:00 am following 12 hours of overnight fasting. Triglyceride (TG), total cholesterol (TC),high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), fasting blood glucose (FBG),alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were tested by the Hitachi 747 autoanalyzer.Serum asprosin, adiponectin and tumor necrosis factor-α(TNF-α) levels were determined by enzyme-linked immunosorbent assay kits (USCN Life Science Inc. Wuhan, China).
Liver ultrasonography
The upper abdominal ultrasonographic examinations were performed for all patients using an ultrasound (GE Medical Systems, Ultrasound and Primary Care Diagnostics LLC,Wauwatosa, WI, USA) by one of the three trained sonographers. NAFLD was defined according to Vajro et al. as follows: marked liver-kidney echo discrepancy, attenuated echo penetration and visible diaphragm, and vague hepatic vessel structures using the right kidney as the reference organ [ 16].
Statistical analysis
Statistical analysis was performed using SPSS 22.0 software and Graphpad Prism 8.0. A Kolmogorov-Smirnov test was used to test for normality of data. Given that TG levels did not follow a normal distribution, the data were ln-transformed prior to statistical analysis. Continuous variables were expressed as mean ± standard deviation (SD),whereas categorical variables were shown as count (percent). Independent-Samplettest or chi-square test was used for comparisons between subgroups. Correlations between asprosin and other variables were assessed by Pearson’s correlations or Spearman correlations. The risk factors of NAFLD were determined by binary logistic regression analysis. Independent variables were included in the regression model if a significant contribution to the model.Pvalues(two tailed) < 0.05 were regarded as statistically significant.
Results
The clinical and laboratory data of all participants are shown in Table 1. There were no significant differences in age and gender among three groups. The obesity groups had significantly higher BMI-SDS, WHR, SBP, DBP, TG, FBG and TNF-αthan those of in the lean control group, whereas HDL-C and LDL-C were similar among the three groups.In addition, there were significant differences between the NAFLD and non-NAFLD groups in terms of ALT and AST, which strongly suggested that liver injury in obesity with NAFLD was more severe than in obesity without NAFLD.
Compared with the lean group, serum asprosin concentrations were significantly higher in the obesity group(Fig. 1). There was no significant difference in asprosin levels between controls and obese children without NAFLD(1.12 ± 0.43 vs. 1.20 ± 0.35,P> 0.05). However, serum asprosin levels were significantly elevated in the obese children with NAFLD when compared with the obese children without NAFLD (1.50 ± 0.43 vs. 1.20 ± 0.35,P< 0.05).In addition, when using 40 U/L as the ALT cut-off level,asprosin levels were higher in the group with ALT > 40 U/L(1.43 ± 0.45 vs. 1.22 ± 0.42,P< 0.05) among all subjects.
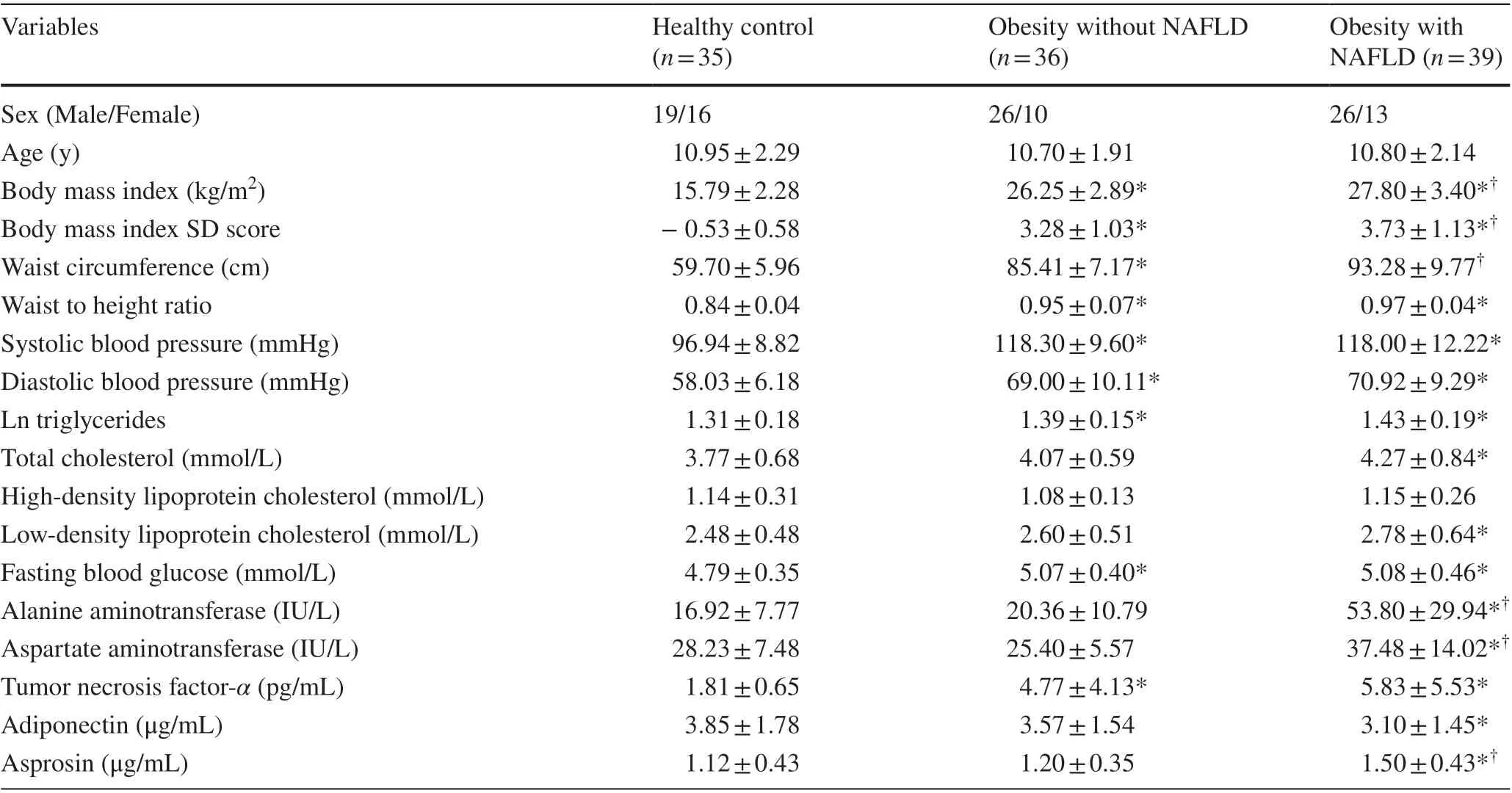
Table 1 Clinical and laboratory parameters of study population
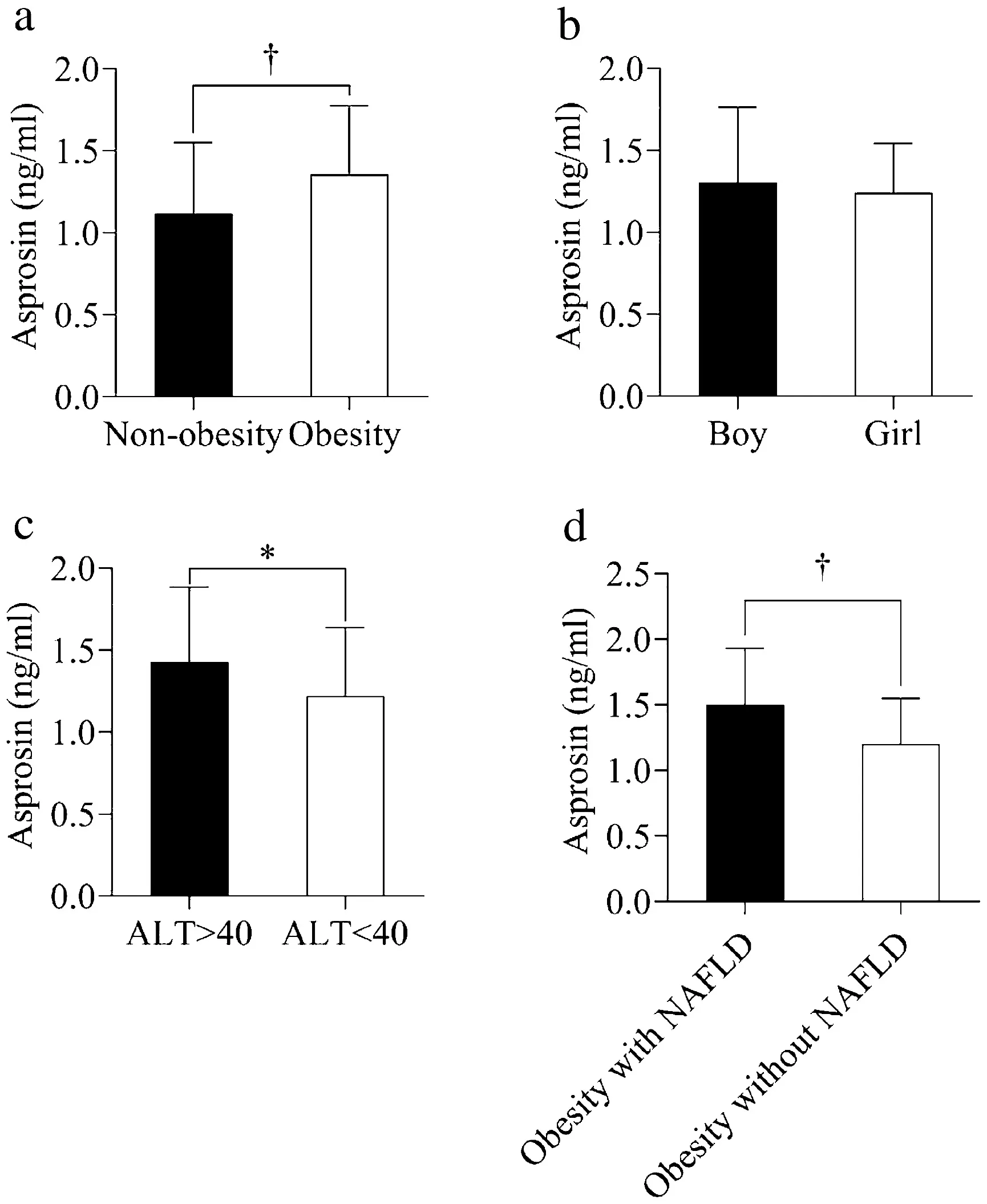
Fig. 1 The serum asprosin levels in different groups. Data are shown as mean ± standard deviation (SD). * P < 0.05. † P < 0.01. ALT alanine aminotransferase
Correlation analyses were assessed between serum asprosin levels and clinical and biochemical variables of all studied subjects using Pearson’s correlation or Spearman’s correlation (Table 2). Serum asprosin concentrations were positively correlated with BMI, WHR, FBG,ALT and TNF-α(Fig. 2). Furthermore, a negative correlation was observed between adiponectin and asprosin. No significant correlations were found between asprosin levels and TG, TC, HDL-C, LDL-C and AST levels.
A stepwise forward model in binary logistic regression analysis was used to examine the predictors of NAFLD in children with obesity. NAFLD was used as the dependent variable and BMI-SDS, WC, WHR, SBP, TG, ALT, FBG,asprosin, adiponectin and TNF-αwere independent variables. We found that ALT (OR = 1.184,P< 0.001), WC(OR = 1.133,P= 0.019), and asprosin (OR = 7.348,P=0.033) were predictors of NAFLD.
Discussion
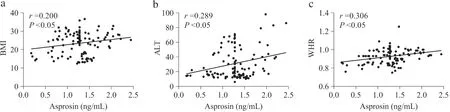
Fig. 2 Correlation analysis between asprosin and clinical and biochemical parameters in children with obesity and without obesity. WHR waist to height ratio, BMI-SDS body mass index SD score, ALT alanine aminotransferase
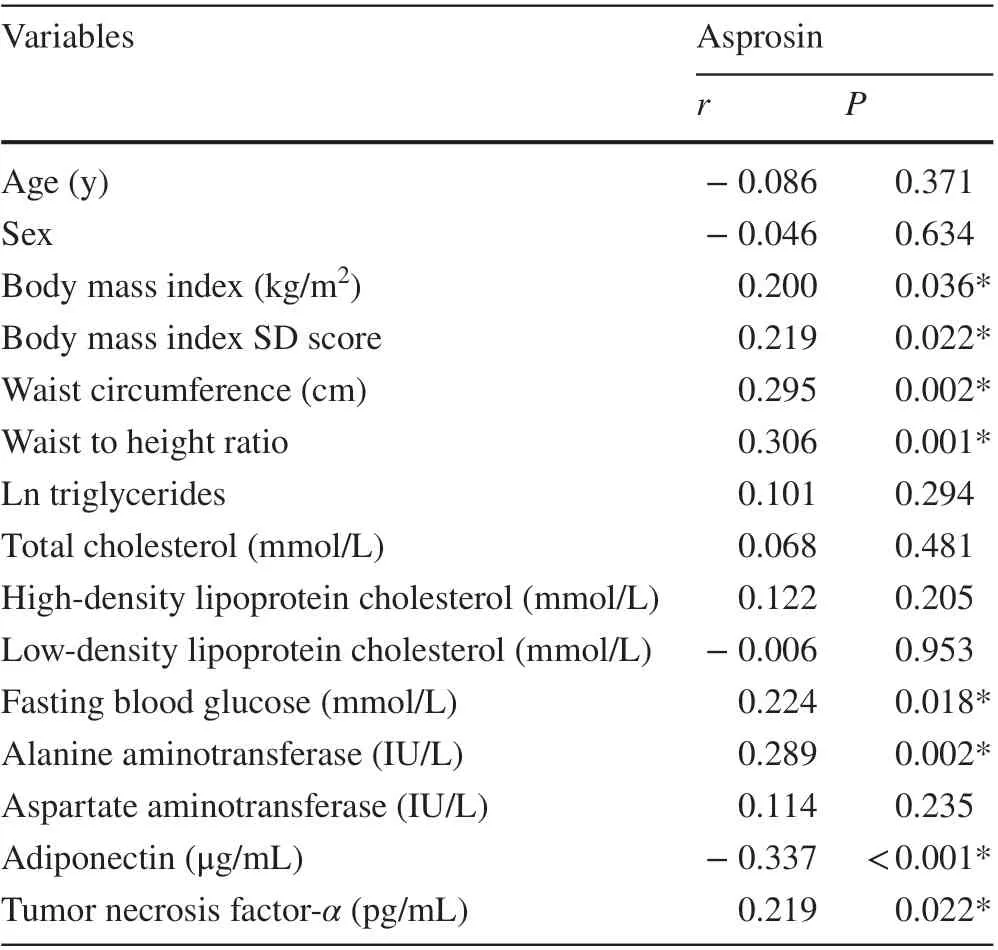
Table 2 Correlations of asprosin with clinical variables
Evidence of serum asprosin levels is scarce and inconclusive in children and adolescents. Our data revealed that circulating asprosin levels were significantly higher in children with obesity compared with those in children with normal weight. In addition, a positive correlation was found between asprosin and obesity-related characteristics, such as BMI, WC and WHR, which confirmed that asprosin plays a crucial role in obesity. More importantly,our study firstly identified that the serum asprosin levels were elevated in obese children with NAFLD. TNF-αis an elevated pro-inflammatory factor in chronic inflammation of NAFLD, and the destruction of liver cells further leads to an increase in ALT [ 3]. We confirmed that serum asprosin concentrations were positively correlated with TNF-αand ALT, which suggested that asprosin was closely related to the pathological mechanism of NAFLD in children and adolescents.
Asprosin is mainly secreted from the white adipose tissue and is significantly increased in case of starvation.OLFR734 (a G-protein-coupled receptor) has been identified as the receptor for asprosin in liver [ 17]. The elevation of asprosin exerts a glycogenic effect in liver through G-protein-cAMP-PKA pathway and indirectly affects insulin levels or sensitivity to insulin. Asprosin also promotes appetite via activating AgRP and POMC neurons,although the receptor in the brain is not clear. In our study,obese children had significantly higher circulating asprosin levels than lean controls. This is consistent with prior results in children, adults and animal models [ 6, 7, 10,
11, 14]. Moreover, concentrations of serum asprosin are pathologically elevated in obese mice, and neutralization of asprosin could reduce body weight using a monoclonal antibody [ 6]. Increasing concentrations of asprosin are found in obesity-associated disorders, such as polycystic ovary syndrome, type 2 diabetes mellitus, insulin resistance (IR), and acute coronary stroke in adults [ 10, 19- 23].Both bariatric surgery and exercise can lead to a reduction in asprosin levels among obese people [ 8, 24]. A prospective study conducted among Turkish children shows that obese or overweight children have higher serum asprosin than healthy controls, which is consistent with our results[ 11]. In contrast, serum asprosin levels in obese children and adolescents are decreased in another reference [ 13].Specifically, the mean BMI-SDS in the obese group studied by Long et al. is about two, whereas our obese children have BMI-SDS greater than three on average, suggesting that our children might be in a severe obese state [ 13].In other studies, asprosin levels in overweight show no significant difference between overweight patients and controls [ 25]. These data suggest that asprosin may have a dynamic process in the pathophysiology of obesity in children. However, few studies have conducted a systematic analysis of the variety of asprosin under different nutrition states. Therefore, further studies with larger samples are needed for clarification.
Our study also found that serum asprosin levels were significantly higher in children with NAFLD than in those without NAFLD. When divided into three groups, the obese children with NAFLD showed highest levels among all children. The accumulation of fat in liver promotes the development of NAFLD. There are different types of obesity including whole body obesity and visceral obesity. The latter is more closely associated with NAFLD [ 3]. In our study,obese children had a wider waist circumference, suggesting that our children were predominantly abdominal obesity.ALT is considered as a great indicator of NAFLD and is strongly associated with damage severity to hepatic cells[ 3]. We observed that ALT levels in children with NAFLD were significantly higher than those without NAFLD. Consistent with our results, a study detects increasing serum asprosin levels in NAFLD adult patients and demonstrates that asprosin could be used as a non-invasive indication to predict the occurrence of NAFLD [ 14]. Thus, it is reasonable to assume that there is a potential association between asprosin and NAFLD in obese children.
In addition, serum asprosin levels were positively correlated with BMI, WHR, FBG, ALT and TNF-α. The mechanism of asprosin’s contribution to NAFLD is not clear. There are three possible hypothetical mechanisms.First, excessive asprosin in obese patient increases release of hepatic glucose and leads to partial or systemic IR [ 5]. It has been reported that hyperinsulinemia and hyperglycemia induced by IR created lipid input-to-output imbalance and promoted hepatic steatosis [ 26]. Second, de novo lipogenesis (DNL) is one of the important parts of fat accumulation in the liver [ 26]. Elevated glucose can be produced and stored in triglycerides through glycolysis. Considering that asprosin functions by raising sugar and increasing appetite,we hypothesize that asprosin is involved in DNL formation. Third, a recent study reports that recombinant asprosin promotes metabolic disorders through TLR4/JNK mediated inflammation [ 27]. Toll-like-receptors (TLR) signaling is activated in the liver while its downstream molecules are increased in NAFLD [ 28]. Asprosin might mediate the pathological process of NAFLD caused by initial reasons through TLR4. It is obvious that asprosin is closely related to NAFLD, but further researches need to explore their relationship and mechanisms.
Finally, we used binary logistic regression analysis to explore the potential predictors of NAFLD. Besides the widely recognized ALT and WC, it was surprising to find that the serum asprosin was also a good predictor of NAFLD in obese children. The gold-standard indicator of NAFLD relies on biopsy, an invasive examination that can cause irreversible damage. Thus, an urgent need for non-traumatic diagnostic indicators is required. Adipokines, such as resistin, visfatin and omentin, can be used to predict inflammation in NAFLD patients [ 3]. However, the accuracy of the predictions still needs to be improved, and it might even require the joint prediction of multiple adipokines. Here, our data provided evidence that asprosin could be used as one of the predictors of NAFLD in children.
Our study has some limitations. As a case-control study, this research could not reflect the causal relationship between asprosin, obesity and NAFLD. Second, we choose liver ultrasonography to diagnose NAFLD, which has little lower diagnostic value than biopsy but is non-invasive.In conclusion, our results suggested that serum asprosin levels were elevated in obese children, especially in those with NAFLD, and may be involved in the pathogenesis of NAFLD in children with obesity. Further studies are needed to explore the molecular mechanism of asprosin in children’s obesity and NAFLD.
AcknowledgementsThe authors thank all the staff at the Department of Pediatrics in The Second Affiliated Hospital of Xi’an Jiaotong University for their logistic support, and the cooperation and participation of all children, and their parents.
Author contributionsXYF conceived the study; designed and supervised experiments; performed the acquisition, analysis, and interpretation of data; and drafted and revised the article. LLJ and KYR performed the recruitment of blood and analyzed the data. LLJ analyzed the data and contributed to discussion. All authors reviewed and approved the manuscript.
FundingThis study was supported by funding from the Chinese National Natural Science Foundation (81673187).
Compliance with ethical standards
Ethical approvalThis was a spontaneous study conducted in conformity with World Medical Association Declaration of Helsinki for medical research involving human subjects. The study protocol is currently being evaluated by the Ethical Committee of the Second Affiliated Hospital of Xi’an Jiaotong University.
Conflict of interestThe authors have no conflicts of interest to declare.
Data availabilityThe datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
 World Journal of Pediatrics2021年4期
World Journal of Pediatrics2021年4期
- World Journal of Pediatrics的其它文章
- Application of Kaiser Sepsis Calculator in culture-positive infants with early onset sepsis
- Similarities and differences between multiple inflammatory syndrome in children associated with COVID-19 and Kawasaki disease: clinical presentations, diagnosis, and treatment
- Extent, reasons and consequences of off-labeled and unlicensed drug prescription in hospitalized children: a narrative review
- Clinical characteristics of COVID-19 in family clusters: a systematic review
- Cerebral palsy in children born after assisted reproductive technology:a meta-analysis
- Characteristics of immune and inflammatory responses among different age groups of pediatric patients with COVID-19 in China
