A comparative study of methods for remediation of diesel-contaminated soil*
Fan-xu MENG, Yan SONG, Li-juan MAO, Wen-jun ZHOU, Dao-hui LIN†‡,3
1Department of Environmental Science, Zhejiang University, Hangzhou 310058, China
2Analysis Center of Agrobiology and Environmental Sciences, Zhejiang University, Hangzhou 310058, China
3The Institute of Zhejiang Ecological Civilization, Huzhou 313300, China
Abstract: Soil pollution by diesel fuels is a worldwide environmental problem, but little research has been carried out into on-site techniques for remediation of soil polluted by waste solvents. This study compared chemical oxidation and soil washing methods for their efficiency and environmental and economic impacts. Soil was spiked with 0# diesel to simulate an actual pollution level of about 1260 mg/kg total petroleum hydrocarbon (TPH). Fenton-like oxidation eliminated 90.4% of the TPH with a Fe2+׃H2O2 ratio of 1׃10 in 5 d compared with 25.8% removal by the activated persulfate method under the same conditions. In washing tests,sodium dodecylbenzenesulfonate and Tween 80 were both unsuitable for TPH washing, while ultrapure water removed 36.1% of TPH in 75 min. Only the Fenton-like oxidation technique met remediation goals based on the screening values of the Guideline for Risk Assessment of Contaminated Sites. The environmental impact and economic assessment of techniques demonstrated the superiority of water washing for dealing with low-degree TPH contamination.
Key words: Total petroleum hydrocarbon (TPH); Chemical oxidation; Soil washing; Environmental risk; Soil remediation https://doi.org/10.1631/jzus.A2100087 CLC number: X53
1 Introduction
Diesel is a petroleum by-product used worldwide. Its production, smelting, transportation, and incomplete combustion produce sites polluted with total petroleum hydrocarbon (TPH). Diesel is more toxic to plants than crude oil because of its higher proportion of light hydrocarbons (Kirk et al., 2005).The extensive occurrence of soil pollution caused by TPH and its relevant derivatives has attracted attempts to seek and establish efficient, economical,and green remediation strategies to restore polluted sites.
Confronted by several different types of TPHpolluted soils, various technologies including chemical oxidation (Mirzaee et al., 2017; Picard and Chaouki, 2017; Lominchar et al., 2018), chemical washing (Li et al., 2016; Patowary et al., 2018),thermal desorption (Samaksaman et al., 2016), bioventing (Österreicher-Cunha et al., 2004), biopiling(Li et al., 2019), and other combined approaches (Guo SH et al., 2014; Dos Santos et al., 2017) have been put into practice. Bioremediation (Gojgic-Cvijovic et al.,2012; Bahadure et al., 2013) is generally considered an environmentally friendly technology thanks to its effectiveness in preventing future pollution events,but its relatively long remediation time (a dozen weeks to several months) restricts its application.Taking the real-world application of different techniques into account, conventional remediation methods have frequently been based on successful precedents. Considering each technology’s maturity and convenience, chemical oxidation and soil washing are optimum options due to the strong interactions between oxidants and TPH and their time-saving characteristics. However, comparative studies of the multifarious methods for remediation of TPHpolluted soils are rare.
Among multiple advanced oxidation processes(AOPs), Fenton/Fenton-like techniques and the increasingly prevalent activated persulfate oxidation technique have shown a benign remediation potential to eliminate diesel. Representative studies on removing TPH from soil are shown in Table 1. The Fenton-like technique can achieve a 92% TPH removal efficiency rate in one week, compared with 98% in eight weeks by activated persulphate oxidation (Usman et al., 2012a; Lominchar et al., 2018).The different removal rates of these oxidizing agents are attributed mainly to the different free radicals generated in the reactions. In Fenton/Fenton-like oxidation, the reaction process is intense because it is caused by the second most reactive chemical species(·OH) (Yap et al., 2011). In activated persulfate oxidation, the sulphate radical (SO4−, oxidationreduction potential (ORP)=2.6 V) is gentler than H2O2(ORP=2.8 V), but the reagents may penetrate deeper into the source zone (Osgerby, 2006). In addition, sulphate radicals (SO4−) are more stable than·OH because of their low affinity with soil organic matter (Usman et al., 2012a). Therefore, both methods may achieve similar remediation efficiency rates over a relatively long span. Soil washing is gaining attention because of its relative rapidity (Khan et al.,2004; Österreicher-Cunha et al., 2004). Its remediation efficiency is directly related to its washing agents.Surfactants are an effective washing agent for organic-polluted soil (Bruell et al., 1997). Anionic and non-ionic surfactants show a favourable removal rate of petroleum hydrocarbons due to solubilizationabove the critical micelle concentration (CMC) (Befkadu and Chen, 2018). Faced with non-negligible adsorption in soil (Kang and Jeong, 2015; Zhang et al.,2018), research on mixed surfactants is of great interest due to their synergistic effects in proper ratios.The adsorption of non-ionic surfactants to soil decreases in the presence of anionic surfactants (Yang et al., 2006). In contrast, non-ionic surfactants may reduce the precipitation of anionic surfactants with cations simultaneously (Huang et al., 2014). Besides surfactants, water washing also allows removal of TPH in accidentally polluted zones (Hernández-Espriú et al., 2013). Different classes of surfactants used for TPH washing are summarized in Table 2.Even though many studies have concentrated on TPH-polluted soil remediation via the two methods evaluated here, a comprehensively comparative study is lacking.
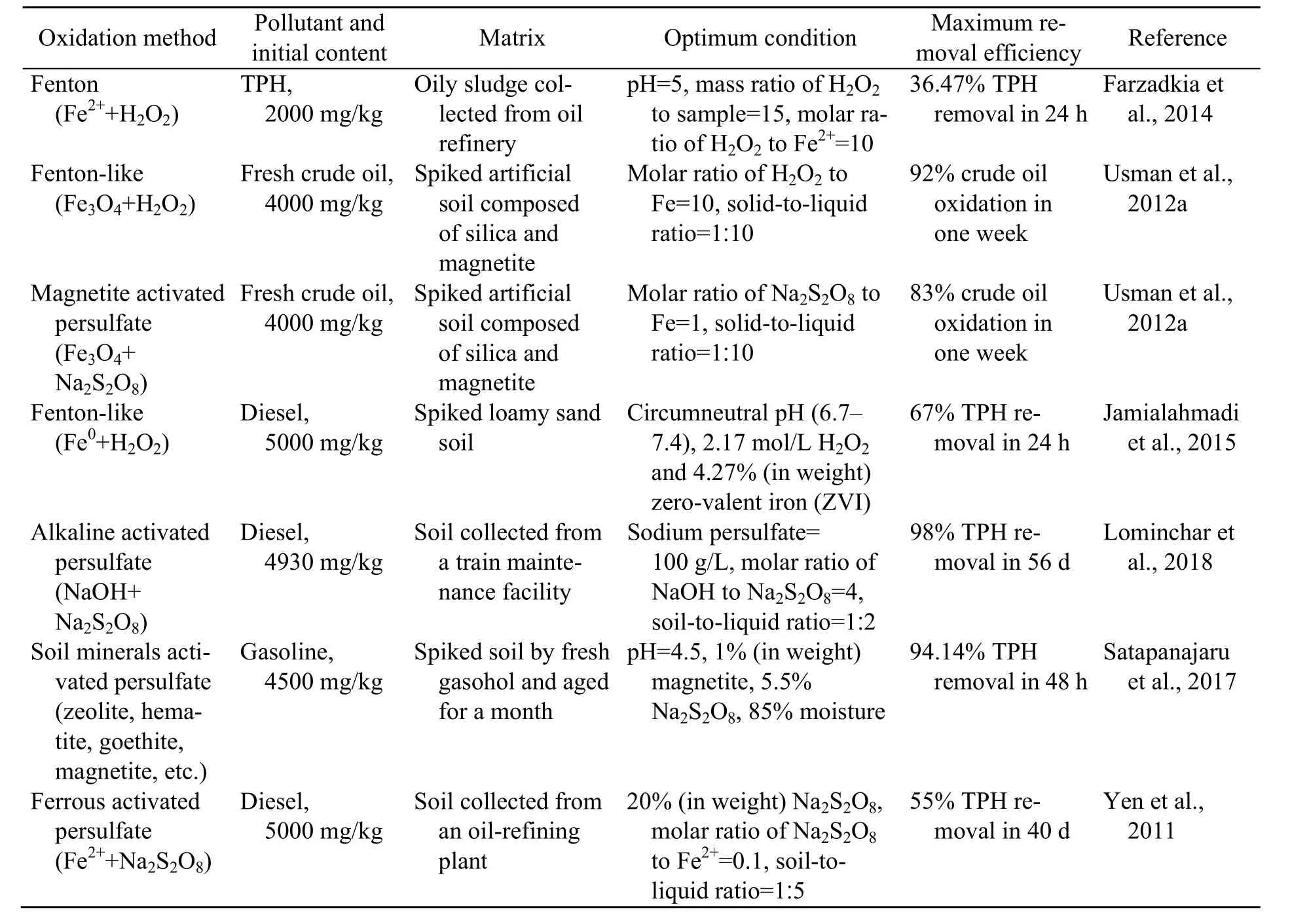
Table 1 Summary of studies using Fenton, Fenton-like, and activated persulfate oxidations to remove TPH from soil
Previous comparative studies focused mainly on optimizing experimental condition parameters, as several mathematical experimental design methods,including response surface methodology, artificial neural networks, and particle swarm optimization,had been applied (Moreira et al., 2007). However, few studies have compared the efficiency, environmental impacts, and economic requirements of conventional methods, especially for chemical-contaminated sites.In this study, classical chemical oxidation and soil washing technologies were applied to remediate spiked soil collected from waste solvent-polluted sites.0#diesel was spiked to simulate actual contamination conditions based on similar components (C10-C36).Sodium dodecyl benzene sulfonate (SDBS) and Tween 80 were chosen as representative anionic and nonionic surfactants, respectively, in consideration of their wide applications in soil remediation. The aims of this study were (1) to compare the treatment effects of two classical soil remediation methods to seek optimum conditions for waste solvent-polluted sites;and (2) to assess their environmental friendliness and economy to provide a reference remediation method for such sites. This study aimed to provide a decision blueprint for application to similarly polluted sites.
2 Experimental design
2.1 Chemicals
0#diesel was purchased from a local petrol station. Its density was 0.833 g/cm3and kinematic viscosity 3.049 mm2/s at 20 °C. Other chemicals were purchased and used without further purification, including hydrogen peroxide (H2O2, 30%; Sinopharm Chemical Reagent Co., China), ferrous sulphate heptahydrate (FeSO4·7H2O, analytical reagent (AR);Shanghai Lingfeng Chemical Reagent Co., China),citric acid (C6H8O7, ≥99.5%; Macklin, Shanghai,China), sodium persulfate (Na2S2O8, 99%; Macklin,Shanghai, China), SDBS (C18H29NaO3S, 90.0%;Macklin, Shanghai, China), Tween 80 (C12H10CINO3,viscous liquid; Aladdin, Shanghai, China), methanol(CH3OH, high performance liquid chromatography(HPLC) grade, ≥99.9%; Sigma-Aldrich, USA), carbon tetrachloride (CCl4, AR, 99.5%; Aladdin,Shanghai, China), and a TPH mixture (standard solution, 30.5 μg/mL in carbon tetrachloride; Aladdin,Shanghai, China). Deionized (DI) water (18.2 MΩ·cm,Milli-Q) was used in the experiments.
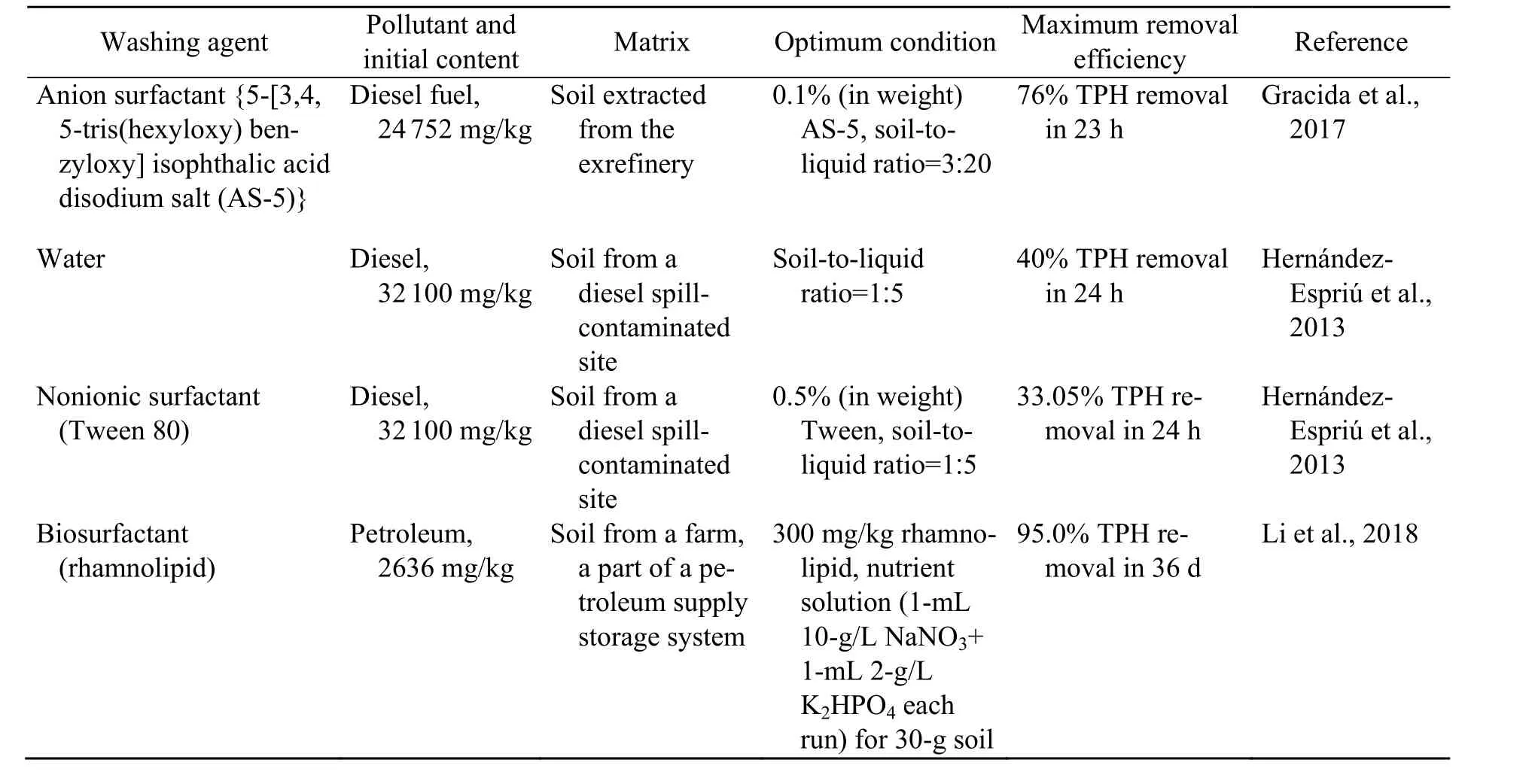
Table 2 Summary of studies using soil washing methods to remove TPH from soil
2.2 Soil pre-treatment and characterization
Contaminated soil was collected from a local waste solvent-polluted site in Zhejiang Province,China. The main pollutant in the soil had been determined to be TPH at a concentration of 1260 mg/kg.This TPH level exceeded the screening value of TPH(C<16 (carbon atom amount less than 16 in alkane of TPH), 620 mg/kg) for commercial and industrial sites according to DB 33/T 892-2013 (Quality and Technology Supervision Bureau of Zhejiang Province,2013). The soil was air dried for one week and then homogenized using a 60-mesh sieve (0.25 mm). The pre-treated soil was kept at ambient temperature before use. After the pre-treatment, the soil TPH content was below the limit of detection of the analytic method (Section 2.5). To simulate the original pollution situation, corresponding amounts of 0#diesel dissolved in carbon tetrachloride were added to the sieved soil. The spiked soil was thoroughly stirred with a glass rod and aged for 2 d. The TPH content was determined before use.
Basic soil properties were measured. The soil humidity was calculated as the ratio of weight loss at 105 °C for 12 h. The soil pH and redox potential(ORP) were measured with DI water (soil׃water=1׃2.5) using a METTLER TOLEDO™ Seven Compact pH meter S220. Porosity was assessed by the ring knife testing method (Meng et al., 2016). Total organic carbon (TOC) was analysed via a TOC-V CPH SSM5000A (SHIMADZU, Japan). The mechanical composition of the soil was measured using a laser particle size analyser (Microtrac S3500, USA).
2.3 Chemical oxidation experiments
For Fenton-like oxidation, experiments were conducted in pots for 5 d. Each pot contained 200 g of the spiked soil. The amounts of citric acid and oxidant(H2O2) were fixed at 0.24 mmol/g and 6 mmol/g,respectively. By changing the additional amount of FeSO4·7H2O, the Fe2+׃oxidant molar ratio was set to 1׃10, 1 ׃50, 1 ׃100, and 1 ׃500 to evaluate its effect on oxidation activity. All reagents were added in solution form, and the soil׃solution weight ratio was controlled at 1׃1. Blank experiments were performed without the addition of any reagents.
For activated persulfate oxidation, Fe2+from FeSO4·7H2O was used as the activator. The Fe2+׃oxidant (Na2S2O8) molar ratios were set the same as for the Fenton-like oxidation, and the Na2S2O8amount was fixed at 2 mmol/g. The other experimental conditions were the same as for the Fentonlike oxidation.
2.4 Chemical washing experiments
DI water, SDBS, and Tween 80 were used as washing agents. In DI water washing batch experiments, the effects of the soil-to-liquid ratio and the washing time were examined simultaneously. Specifically, spiked soil samples (10 g, 5 g, and 2.5 g)were added to 50-mL centrifuge tubes. Then, 50 mL of DI water was poured into the centrifuge tubes(soil-to-liquid ratios: 1 ׃5, 1׃10, and 1׃20) and shaken in a horizontal shaking incubator (180 r/min, 25 °C)for 75 min. To examine the influence of the washing time, soil was collected at 15, 45, and 75 min by centrifugation (4000g, 20 min (gis the gravitational acceleration)) and freeze dried for 24 h. Then, the residual TPH in the soil was measured.
For SDBS and Tween 80 washing batch experiments, the impacts of the washing agent concentration, washing time, and soil-to-liquid ratio were examined. Specific experimental conditions and relevant parameters are shown in Table 3. The best washing time (TB) was determined in the washing agent concentration and washing time test carried out first and used in the washing agent concentration and soil-to-liquid ratio test. The residual TPH in the treated soil was measured.
Following confirmation of the optimum conditions for single surfactant washing, further mixed washing agent batch experiments were carried to obtain more comprehensive data regarding the synergistic effects of anionic and non-ionic surfactants(Zhou and Zhu, 2007; Shi et al., 2015). Corresponding experimental conditions are listed in Table 3.
2.5 Analytical methods
TPH in soil was extracted by vibration and sonication. In detail, 0.5 g of soil was weighed in a 50-mL centrifuge tube, and 50 mL of carbon tetrachloride were added to the tube. Then, the tube was shaken and sonicated for 1 h. Afterwards, the mixture was centrifuged at 4000gfor 20 min and 2 mL of supernatant was placed in a 2-mL screw top vial with a cap. The TPH content was measured using a gas chromatograph (Agilent 7890B) equipped with an automatic liquid sampler (7693A), flame ionization detector(FID), and HP-5 capillary column (30 m×0.25 mm×0.25 μm) (Agilent Technologies, Palo Alto, CA,USA). The ramping temperature procedure and other settings were the same as those used by Lai et al.(2009). The limit of quantification (LOQ) was 0.7 mg/L (70 mg/kg in soil).
2.6 Statistical analysis
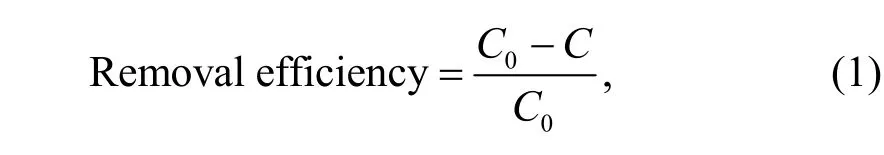
The treatment efficiency rates of the different methods are expressed by the removal efficiency,calculated by whereC0indicates the initial TPH content (chemical washing experiments) or the TPH content in blank experiments (chemical oxidation experiments), andCindicates the residual TPH content under different treatment conditions.
All experiments were carried out twice, and the data in figures are presented as the means±standard errors. Analysis of variance (ANOVA) with different chemical oxidation treatments for TPH was performed using the SPSS 20.0 software package for Windows. Statistical significance was determined byp<0.05.
3 Results and discussion
3.1 Soil characterization
The basic soil properties are shown in Table 4.The background concentration of TPH in the aged blank soil was below the limit of quantification(70 mg/kg). Blank spiked experiments demonstrated that the recovery rate of TPH was 80.7%–99.0% after 2 d of ageing.
3.2 Chemical oxidation of contaminated soil
Fenton-like oxidation results (Fig. 1a) showed aslight decrease in TPH after 1 d of treatment. After another 2 d, a significant difference (p<0.05) was generated among different Fe2+׃H2O2ratios. After 5 d of treatment, the differences between different treatments and blank groups became even more obvious.When the molar ratio increased, the TPH removal efficiency increased from 29.4% to 78.2% in 3-d and from 66.5% to 90.4% in 5-d treatments. The maximum removal of TPH was achieved with an Fe2+׃H2O2ratio of 1׃10, which was similar to that of previous studies (Goi and Trapido, 2004; Yap et al.,2011; Farzadkia et al., 2014). The lowered removal efficiency at lower Fe2+׃H2O2ratios was caused mainly by the reaction between excess H2O2and hydroxyl radicals (Lin and Gurol, 1998). For the TPH removal efficiency and screening value of TPH, the optimal Fe2+׃H2O2molar ratio was 1׃10.
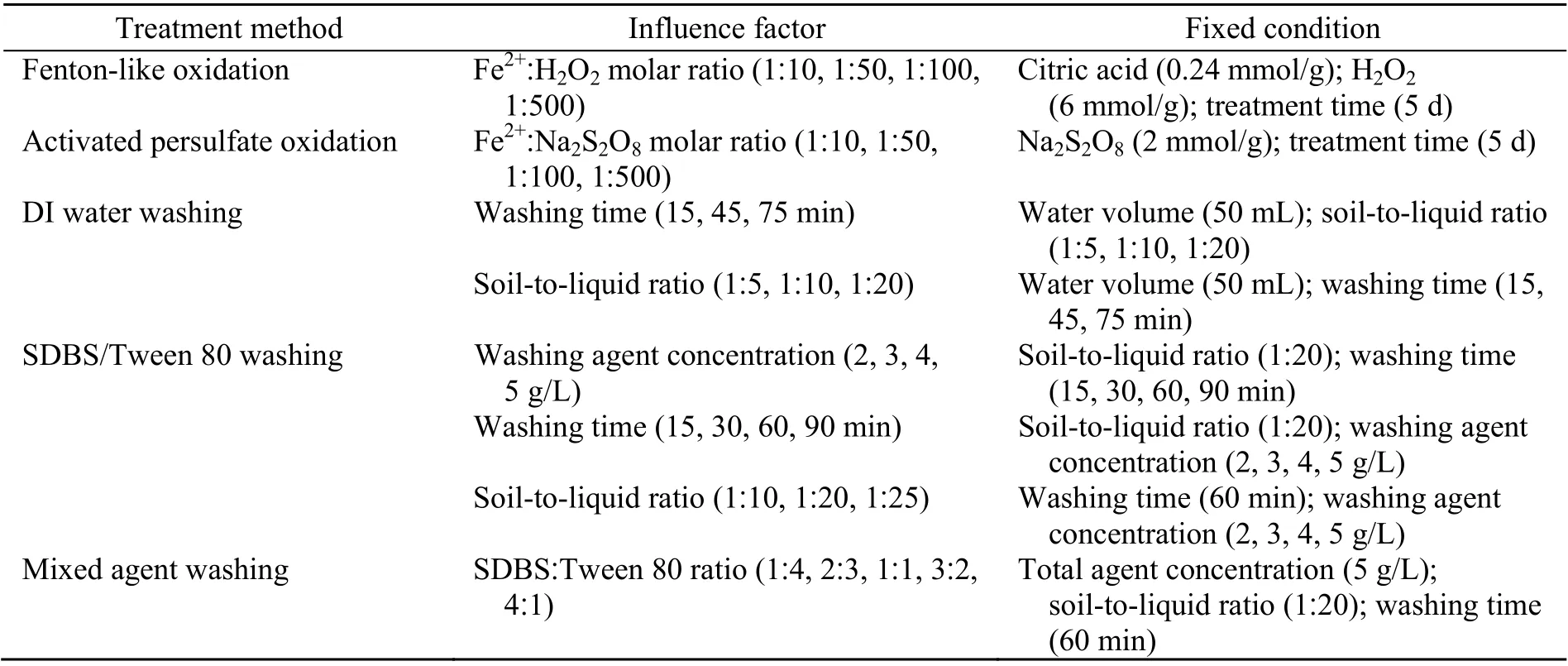
Table 3 Summary of soil remediation experimental conditions and relevant parameters
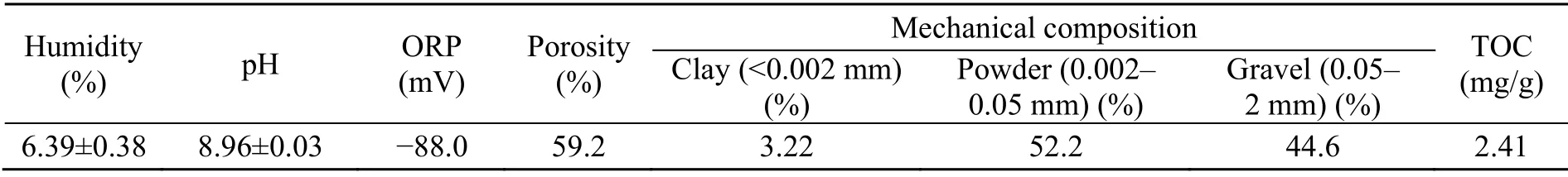
Table 4 Basic physicochemical properties of original soil
With the Fe2+-activated persulfate method, TPH levels decreased much more slowly (Fig. 1b). However, as with the Fenton-like method, the removal efficiency was enhanced with an increase in the molar ratio. The maximum removal efficiency of TPH was only 39.5% with an Fe2+׃S2O82−ratio of 1׃10 and 5 d of ageing, which was less than half that of the Fentonlike disposal. This is because of the differences in the selective behaviour of the two oxidation processes. A series of studies (Usman et al., 2012a, 2012b, 2012c)demonstrated that activated persulfate oxidation is a selective oxidation process aimed mainly at lowmolecular-weight n-alkanes, while in Fenton-like treatments, the oxidation process is unselective and relatively thorough. Another key factor is the longevity of free radicals in experiments. For Fenton-like reactions, the main free radical (·OH) lasts only 10−9–10−6s in the liquid phase (de Laat and Gallard, 1999; Du et al., 2007; Wu et al., 2010). For activated persulfate,sulphate radicals (SO4−·) have a longer lifetime(3×10−5–4×10−5s) (Zhou et al., 2015). Correspondingly, further scavenging tests showed a faster reaction rate for hydroxyl radicals compared with sulphate radicals (9.7×108L/(mol·s) vs. 3.2×106L/(mol·s),second-order rate constants) when methanol was the quenching agent (Liang and Su, 2009). This dynamic also coordinated with the exothermic degree when all reagents were added to the spiked soil. Based on these results, activated persulfate oxidation is a relatively weak oxidation method of TPH degradation and cannot meet the screening value. For elimination impact, Fenton-like oxidation performed better than activated persulfate oxidation in this short-term trial.
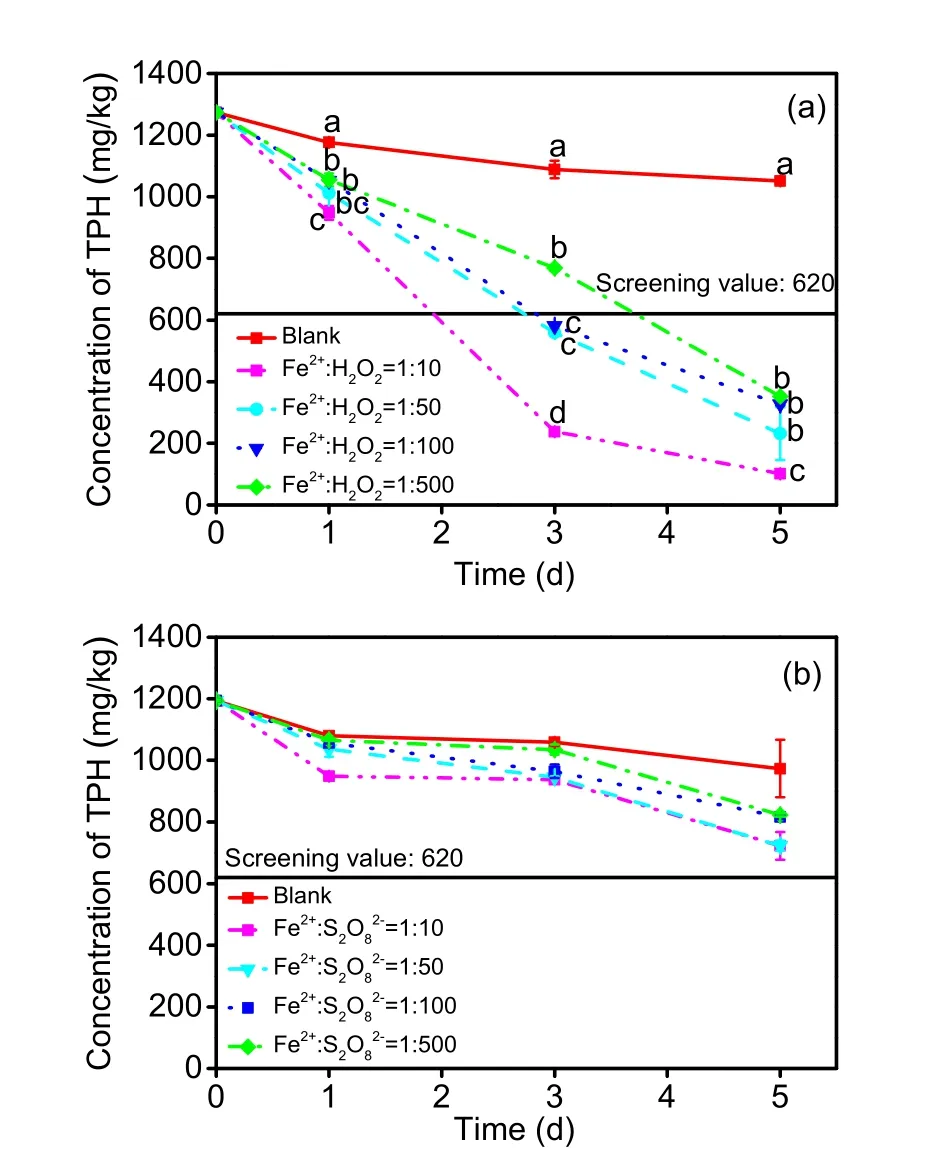
Fig. 1 Residual concentrations of TPH in spiked soil with chemical oxidation: (a) effects of the Fe2+ to H2O2 molar ratio in Fenton-like oxidation; (b) effects of the Fe2+ to persulfate molar ratio in activated persulfate oxidation.Different letters noted for the same time point in Fig. 1a represent a significant difference
3.3 Chemical washing of contaminated soil
The DI water washing experiment results are presented in Fig. 2. With a decrease in the soil-toliquid ratio, the removal efficiency rose first and then decreased. The best result was obtained with a soil-toliquid ratio of 1׃10. An unexpected finding was that water washing was so efficient for TPH disposal that the ultimate result was close to that of 5 d of activated persulfate treatment (726 mg/kg vs. 723 mg/kg). The maximum removal efficiency reached 36.1% with a soil-to-liquid ratio of 1 ׃10 and 75-min washing, which was slightly less than that obtained with highconcentration diesel-contaminated soil (40%; initial TPH content: 32 100 mg/kg) (Hernández-Espriú et al.,2013) and approximated the result (32.9%) of column experiments (Khalladi et al., 2009). Although the result did not achieve the remediation target (screening value) within the experimental time, water flushing appears to be suitable for remediation of a low level TPH-polluted soil (TPH<916 mg/kg), according to its removal efficiency.
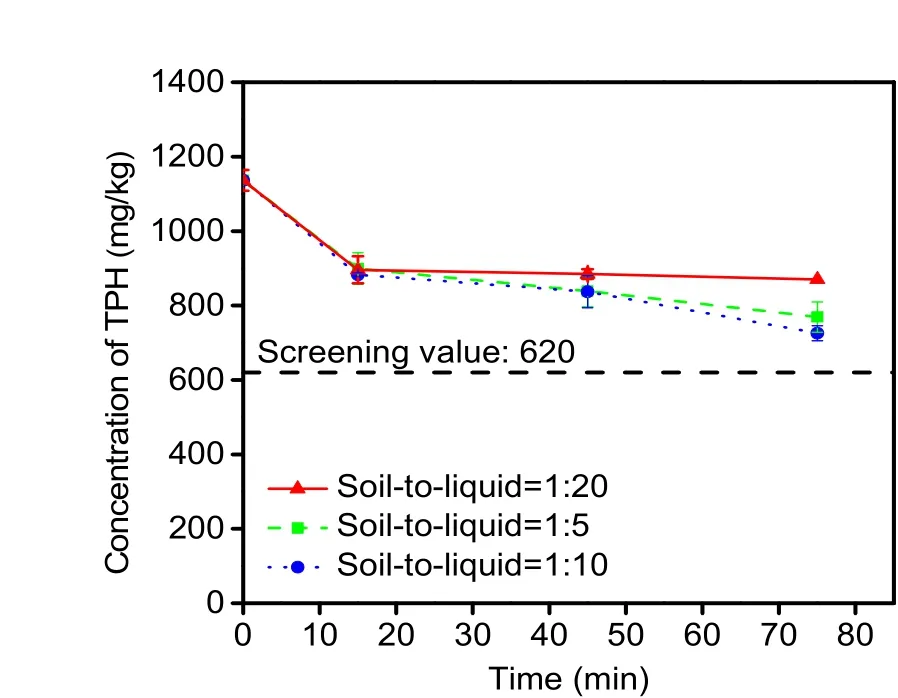
Fig. 2 Effects of the soil-to-liquid ratio and washing time on the removal of TPH from spiked soil by DI water washing
The SDBS-washing spiked soil results are presented in Fig. 3. From Fig. 3a, 60 min (TB) was the balance moment in most cases, when the TPH removal efficiency was between 5.52% and 13.2%.SDBS showed a prior solubilization effect on TPH with the increase in SDBS concentration, except for the 4-g/L SDBS treatment. SDBS washing produced a maximum removal efficiency of 13.5% under 5-g/L SDBS washing within 90 min. Furthermore, the elevated solution concentration was not helpful in clearing TPH in spiked soil (Fig. 3b), which might be due to more adsorption sites forming on the surface of soil derived from increased micelles/admicelles (Guo P et al., 2014).
The detergency of Tween 80 on TPH was low(Fig. 4), with a maximum reduction of only 12.1% in 1 h (TB). With increasing Tween 80 concentrations,the TPH removal efficiency decreased first and then increased (Fig. 4a). The removal efficiency increased continuously with increasing soil-to-liquid ratio, except for 5-g/L Tween 80 (Fig. 4b). In the final washing result, the maximum removal efficiency achieved was only 11.3%. This phenomenon reflects a high affinity between non-ionic surfactants and organically polluted soil (Guo et al., 2009; Chen and Sun, 2016;Cheng et al., 2018). Although adsorption occurs for anionic surfactants on soil as well, the amount is slightly lower than that of non-ionic surfactants (Yu et al., 2007; Chen and Sun, 2016), so the detergency of SDBS is slightly higher than that of Tween 80.
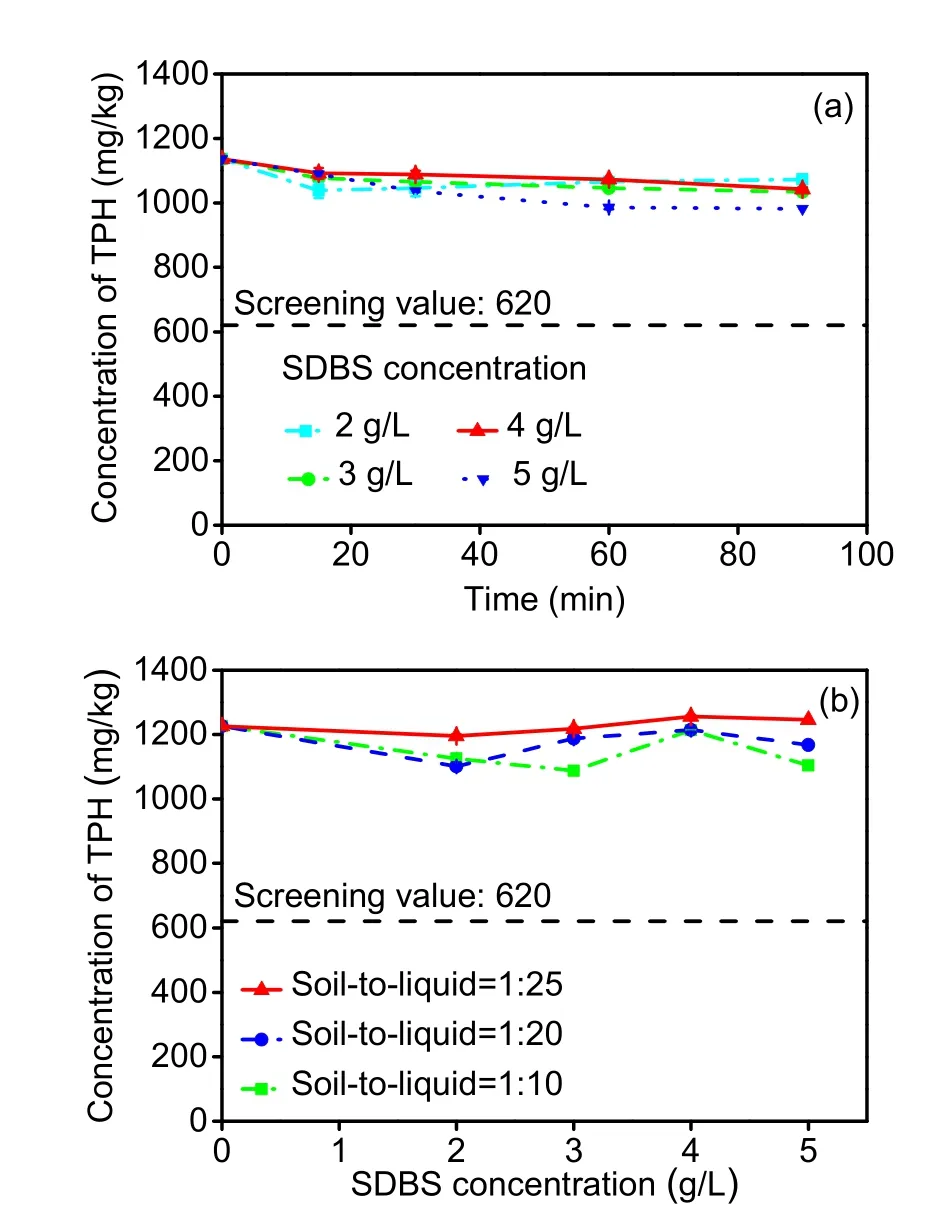
Fig. 3 Effects of the washing agent concentration and washing time on the removal of TPH (soil-to-liquid ratio=1:20) (a) and effects of the washing agent concentration and soil-to-liquid ratio on the removal of TPH (washing time=60 min) (b) in spiked soil by SDBS washing
Based on the single washing agent experiment, a further mixed surfactant washing experiment was conducted based on the optimal conditions (Fig. 5).With an increase in the ratio of Tween 80, the washing efficacy tended to be elevated. However, a co-solubilization effect did not appear for decontaminating TPH in spiked soil. The highest removal efficiency was only 6.44%, which was lower than the removal efficiency rates of SBDS and Tween 80(8.23%) used separately under the same conditions.One possible explanation is that the extremely strong micelle generation caused by the high surfactant concentration (nearly 10 and 143 times higher than those of SDBS and Tween CMC, respectively) and their gathering behavior was responsible for the sorption and solubilization of the high carbon content chains (C21-C40) (Huguenot et al., 2015). For 0#diesel, the major component is n-alkanes (C13-C20).
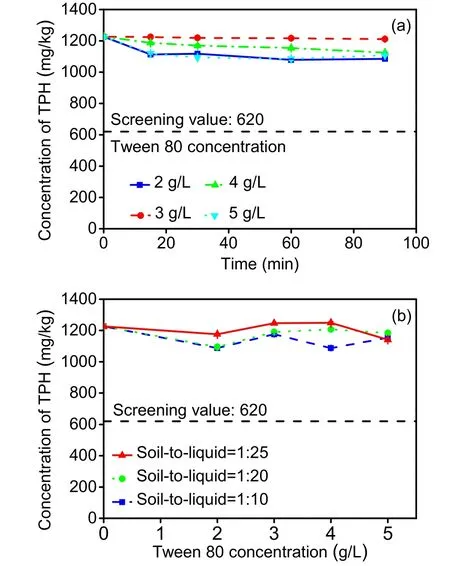
Fig. 4 Effects of the washing agent concentration and washing time on the removal of TPH (soil-to-liquid ratio=1:20) (a) and effects of the washing agent concentration and soil-to-liquid ratio on the removal of TPH (washing time=60 min) (b) in spiked soil by Tween 80 washing
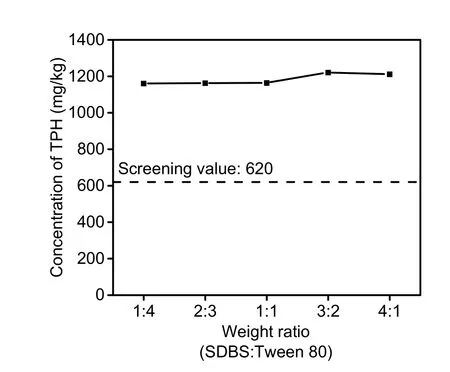
Fig. 5 Composite agent leaching results with different proportions under optimized washing conditions (total concentration=5 g/L, washing time=60 min, and soil-toliquid ratio=1׃20)
According to the efficiencies of the different washing agents, a single SDBS or Tween 80 dosage could not adequately remediate diesel-polluted soil in this site. Also, the mixed surfactants did not show a synergistic effect on TPH removal. However, DI water showed a strong detergency effect on the TPH.In an earlier study, Hernández-Espriú et al. (2013)used several different kinds of surfactants to deal with diesel-contaminated soil (32 100 mg/kg) and found that the washing efficiency of DI water surpassed that of a high concentration of Tween 80 (10 g/L) by about 10%. The phenomenon illustrates the validity and convenience of water washing.
3.4 Comparative assessment of remediation efficiency and feasibility
When specific soil remediation technology is implemented, the possible environmental risks and ecological toxicity should be taken into account to make a wise decision. For the Fenton-like process,although the iron used in the process is highly abundant with low toxicity and hydrogen peroxide is an environmentally friendly oxidant, the ‧OH formed is a biocide (Büyüksönmez et al., 1998). With its exothermic process, the Fenton reaction will increase the soil temperature during treatment (Palmroth et al.,2006), as observed in the experiment. Also, a drastic oxidation process will cause phosphorus deficiency(Alef, 1991), an increase in the toxicity of leachates,and the death of soil microorganisms (Vibrio fischeri)(Palmroth et al., 2006). Compared with the Fentonlike process, activated persulfate oxidation is mild.Persulfate can stay active in soils for weeks to months,is not obviously influenced by pH, and can oxidize a variety of contaminants (Stroo and Ward, 2011).Nonetheless, Fenton-like oxidation is still a better alternative than activated persulfate for a waste solvent-polluted site in terms of performance.
Environmental impacts, ecological toxicity, and biodegradation should be considered when choosing washing agents to apply to a target contaminated soil.Adsorption behaviour is common in the soil washing process and becomes the main factor in influencing the transport and distribution of surfactants at the subsurface (Kang and Jeong, 2015; Chen et al., 2016).The loss portion cannot lower the interfacial tension,and so does not favour mobilizing oil from soil(Gogoi, 2011). A recent study (Zhang et al., 2018)demonstrated the irreversible sorption of SDBS on two kinds of sediments (retained maximum: 865 and 9674 μg/g), which was related to the entrapment of the surfactants during the conformational changes of the organic matter. Considering the recalcitrant molecular structure and long retention time (García et al.,2009), their ecological toxicity should not be neglected. Similar to oxidizing agents, ecological toxicology research on surfactants is carried out with microorganisms. Toxicity research onFolsomia fimetariarevealed that the survival of juveniles(700 mg/kg exposure) was only 20% compared with 90% in the control, and reproductive output decreased by about 65% with a 1000-mg/kg linear alkylbenzenesulfonate (LAS) treatment (Holmstrup and Krogh, 1996). The study illustrated the importance of a rational dosage of LAS. SDBS has low biodegradability (García et al., 2009), but some studies(Haggensen et al., 2002; González et al., 2007;Mungray and Kumar, 2008) showed that the aerobic condition favoured LAS degradation, with a removal efficiency of over 90% through the classical membrane bioreactor technique. Thus, the degradability of SDBS need not be a point of focus.
For non-ionic surfactants, the sorption of Tween 80 on soil is spontaneous (Gibbs free energy change ΔG<0) and exothermic, so clay-rich soils (>20%) are not suited to the Tween 80 washing disposal (Yang et al., 2010). In addition, Tween 80 adsorption may decrease the permeability of soils (montmorillonite content: 1.0%–1.4%) by about 5% (Gardner and Arias,2000). Changes in soil properties are not beneficial for surfactant-enhanced remediation and make the remediation course costly and ineffective. A further concern is the eco-toxicological effects that may result from residuals. The potential toxicological impacts of Tween 80 were examined for 18 different fungal strains (Garon et al., 2002), and no growth inhibition arose. Furthermore, Tween 80 showed a growthpromoting effect on plants in some circumstances(Gao et al., 2007; Ni et al., 2014), presumably because it contains bioavailable carbon. In general, the ecological toxicity of Tween 80 is negligible in the soil matrix. For biodegradability, both aerobic and anaerobic environments support the biodegradation of Tween 80 (Lee et al., 2004; Franzetti et al., 2006), so the use of Tween 80 in soil washing is safe overall.Nevertheless, the bad detergency performance on TPH limits the use of Tween 80 in real-world polluted soil.
To maintain a dynamic balance between environmental friendliness, technical efficiency, and costbenefit analysis during soil remediation, the economic cost is another concern for ex-site soil remediation. A simplified cost estimation was necessary for this study. The costs of two of the most efficient remediation soil technologies were estimated per weight (ton, t). As shown in Tables 5 and 6, the cost of water washing (24.30 $/t) is less than one third of that of Fenton-like oxidation (79.10 $/t), so waterflushing is an optimum choice for prompt TPH reduction from mildly diesel-contaminated soil. Nevertheless, labour costs comprise 79.7% of the total costs in the water flushing method. The percentage of labour costs will continue to increase in future soil ex site remediation projects because overall labour costs in China are continuously increasing (Chen et al.,2013). Hence, future research should be concentrated on enhancing the level of mechanization to save engineering costs.
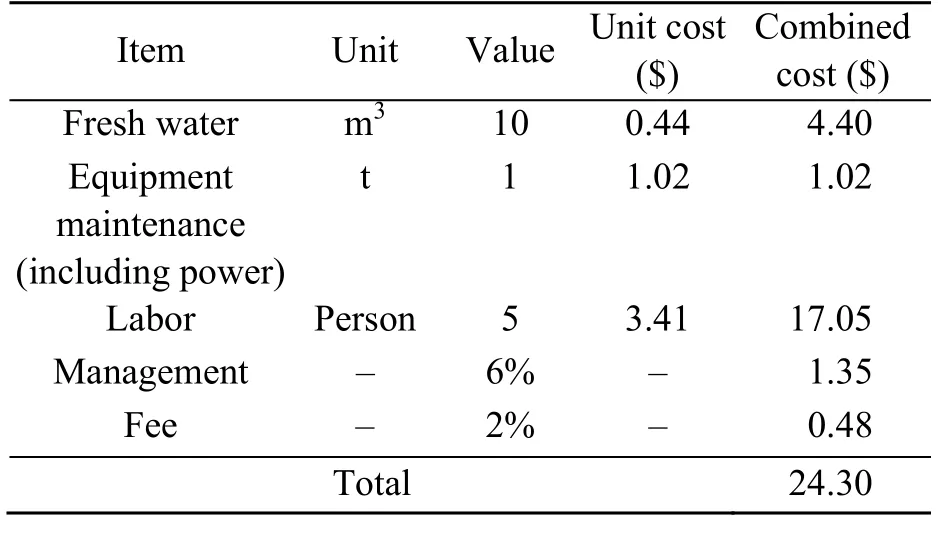
Table 5 Cost calculation for water washing remediation per ton of diesel-contaminated soil under optimum conditions
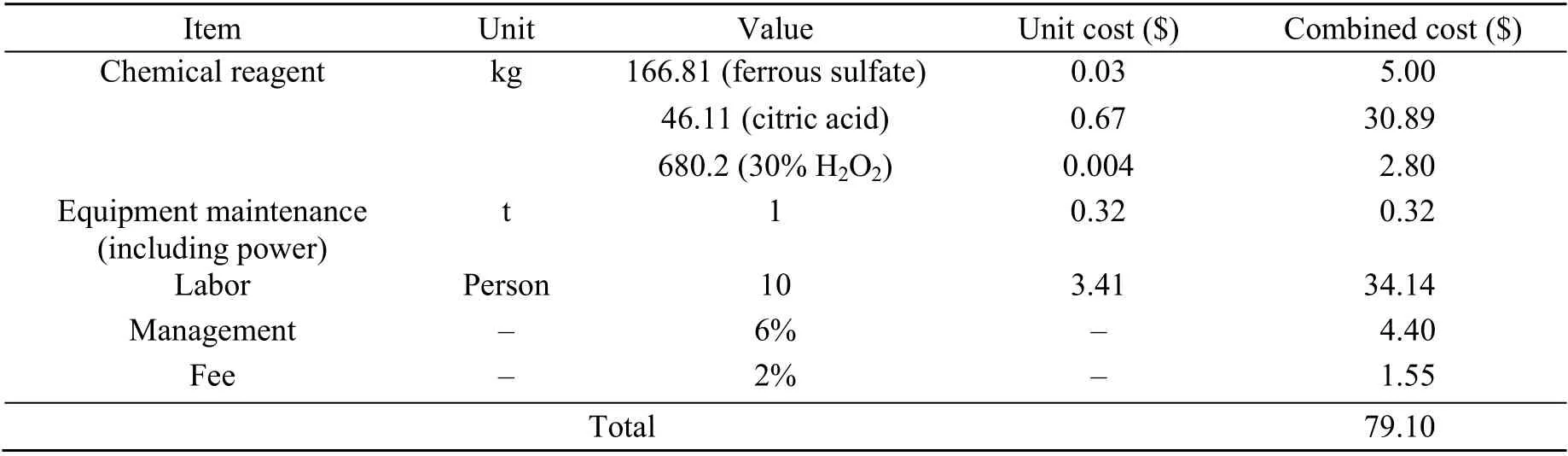
Table 6 Cost calculation for Fenton-like oxidation remediation per ton of diesel-contaminated soil under optimized conditions
4 Conclusions
Two conventional soil remediation technologies were implemented in diesel-contaminated soil and thoroughly investigated for technique efficiency,environmental impacts, and economic viability.Chemical oxidation tests showed a drastic reaction generated by the Fenton-like process and a relatively mild and slow process generated by activated persulfate oxidation. The optimal oxidation treatment condition for both techniques was a Fe2+׃oxidation reagent molar ratio of 1׃10. For washing experiments,the maximum TPH removal was achieved with DI water washing. Overall, only the Fenton-like process could achieve the remediation goal based on the screening value. However, for low-level TPH pollution (<916 mg/kg), DI water washing would be a better alternative. The environmental friendliness assessment showed that activated persulfate oxidation and pure water washing were the greenest methods when considering their environmental impacts, ecological toxicity, and biodegradation simultaneously.In terms of estimated economic cost, water washing(24.30 $/t) was considered the best choice for polluted site remediation on account of its high efficiency and environmental and economic superiority. This study provided a basic blueprint for diesel-contaminated soil remediation, and further studies should concentrate on seeking a balance between engineering, nature, and society.
Contributors
Fan-xu MENG and Dao-hui LIN designed the research.Fan-xu MENG and Yan SONG conducted the experiments.Li-juan MAO provided the instrumental analysis method.Wen-jun ZHOU helped to organize the manuscript. Dao-hui LIN revised and edited the final version.
Conflict of interest
Fan-xu MENG, Yan SONG, Li-juan MAO, Wen-jun ZHOU, and Dao-hui LIN declare that they have no conflict of interest.
 Journal of Zhejiang University-Science A(Applied Physics & Engineering)2021年10期
Journal of Zhejiang University-Science A(Applied Physics & Engineering)2021年10期
- Journal of Zhejiang University-Science A(Applied Physics & Engineering)的其它文章
- Wheeled jumping robot by power modulation using twisted string lever mechanism∗
- A graphics processing unit-based robust numerical model for solute transport driven by torrential flow condition*
- A novel time-span input neural network for accurate municipal solid waste incineration boiler steam temperature prediction*
- Adsorption of tetrodotoxin by flexible shape-memory polymers synthesized from silica-stabilized Pickering high internal phase emulsion*#
