A new automatic cell smear and laser release system for near-infrared light responsive release of nucleated red blood cells
GUO Zhong-yang,YOU Qian-nan,GE Ming-feng,WANG Guo-wei,MEI Qian ,DONG Wen-fei
(1. School of Biomedical Engineering (Suzhou), Division of Life Sciences and Medicine,
University of Science and Technology of China, Hefei 230026, China;2. Suzhou Institute of Biomedical Engineering and Technology,Chinese Academy of Science, Suzhou 215163, China)
* Corresponding author,E-mail: qmei@sibet.ac.cn
Abstract: In order to realize the separation and release of nucleated red blood cells from peripheral blood and develop a safe and effective non-invasive technique to separate nucleated red blood cells for prenatal diagnosis of fetal diseases, an automatic cell smear preparation system based on hydrogel material was established, and a laser focusing and microscopic imaging system for recognizing and releasing nucleated red blood cells was constructed. Firstly, the mechanical structure of cell smear preparation machine was designed, the upper computer control software was designed based on single chip microcomputer, and a hydrogel membrane substrate smear was prepared by optimizing the slide-pushing angle and speed. MXene, a twodimensional material, was introduced into temperature-sensitive hydrogel gelatin, and the near-infrared light response was realized on the surface of hydrogel membrane by using the near-infrared photothermal conversion characteristics of MXene. Then, the whole cell smear experiment was carried out on the surface of the hydrogel substrate membrane. A monolayer cell smear was prepared by optimizing the parameters of blood slide. Finally, the optical path of laser focusing and microscopic imaging was established. After the nucleated red blood cells were recognized and located, the light from an 808 nm laser source passed through a collimator lens and a convergent lens and was focused on the surface of the cell smear, which released cells under photothermal effect. A monolayer cell smear was processed and prepared, and then a photothermal effect was produced under the near-infrared light of 808 nm. After the control of the laser focusing system, a fixed cell-releasing area with a spot diameter of 300 μm was finally obtained. In this paper, the automatic slidepushing technology was applied to the preparation of a monolayer cell smear based on hydrogel membrane,
Key words: cell smear; hydrogel; prenatal diagnostics; near-infrared light response; cell release
1 Introduction
Prenatal diagnosis is essential for the early diagnosis and screening of birth defects such as Down syndrome, neural tube defects and single-gene diseases[1]. The existing prenatal diagnoses can be divided into invasive diagnoses and non-invasive diagnoses. Invasive diagnoses include amniocentesis,umbilical cord blood sampling, and chorionic puncture, etc.[2]. They are performed by invasive methods, and are often accompanied by miscarriage, amniotic fluid overflow, infection and other risks[3].Therefore, establishing a non-invasive prenatal diagnosis method is the focus and mainstream trend of the current prenatal diagnosis technology development[4]. Fetal Nucleated Red Blood Cells (NRBCs)contain the entire fetal genome and the early gestational expression (expressed in peripheral blood at the 6th week and maximized between 12thand 14thweek). They are highly distinguishable in maternal blood cell population and are hard to be confused with maternal cells. With obvious cellular morphological characteristics, they are easy to identify and are the preferred fetal cells for prenatal diagnosis[5].However, fetal NRBCs are very few in maternal peripheral blood. How to identify and separate NRBCs from peripheral blood is a major challenge in prenatal diagnosis[6].
The existing methods for separation and enrichment of peripheral blood cells include density gradient centrifugation, magnetic activated cell sorting, fluorescence activated cell sorting, microfluidic chip technology, etc[7-9]. Among these methods, the separation and detection are mainly realized by the specific binding of antibody and cells. The procedures have a complex operation process, long detection cycle and high cost. Simplifying the process of cell separation and enrichment is an urgent problem to be solved for non-invasive prenatal diagnosis.
Using the slide-pushing technique to make a good cell smear for blood cell morphology examination is critical in clinical application[10]. The traditional man-made cell smear cannot be standardized due to the uncontrollability of manual operation,which leads to cell overlap and rupture. The automatic cell smear preparation machine can standardize the preparation of monolayer cell smears and identify the NRBCs by optical imaging based on their special morphology. In order to achieve the collection of NRBCs for downstream application,the thermal response release system reported so far can release the captured cells at physiological temperature, and help the released cells maintain enough activity. This method can release cells in a large area, however, it is hard to release cells in a fixed area. Correspondingly, light-stimulated response system is considered as an ideal controllable release system due to its operability[11]. Compared with ultraviolet light, near-infrared light produces less damage to cells and has stronger penetration, so it is more suitable for the construction of light-responsive release system. By introducing a light response system into the thermal response release system, more efficient fixed-point release can be achieved. This paper proposes the use of the filmforming and photothermal properties of hydrogel[12],in which MXene two-dimensional material is introduced into thermo-sensitive hydrogel gelatin, the near-infrared photothermal effect of MXene can be combined with the thermal response of hydrogel substrate. Thus an automatic monolayer cell smear preparation machine is prepared, and a laser convergence and microscopic imaging system are established to solve the traditional problem of cell capture, separation and release depending on the specific binding of antigen and antibody. By optimizing the structure of each module and material properties,the recognition and fixed-point release of NRBCs provide a new approach for noninvasive prenatal testing.
2 Design of cell smear preparation machine system and recognition &release system
2.1 Overall design of control system
The scheme of automatic pushing and laser convergence system used to recognize and release the NRBCs is shown in Figure 1. The automatic slide pushing system consists of a mechanical motion subsystem, an adaptive smear system, a microstructural slide-loading platform and an electronic control subsystem. The movement of the XY stage is controlled by the mechanical motion subsystem,which controls the pushing speed and angle. The adaptive smear structure has two DOFs, which can adaptively fine-tune the condition of the smear and the surface in contact with it. By combining the two-dimensional motion of the motion subsystem, the three-dimensional motion of the smear and slide can be controlled. The microstructural slide-loading platform can carry the standard microscope slides(25 mm × 75 mm) for the preparation of hydrogelbased smears and cell smears. The electronic control subsystem is used to realize the communication and interactive operation among the modules of the whole smear system.
Firstly, a hydrogel membrane is prepared on a slide by using the automatic slide-pushing system.The membrane is taken as substrate, and then covered with a monolayer blood cell by spreading the blood sample. In this way, a cell smear is obtained. Secondly, the blood cell membrane is stained by Wright-Giemsa solution to examine cell morphology. As a clinical hematological detection method,this method has the advantages of good staining effect, obvious nucleo-cytoplasm contrast, easy operation and rapid staining[13]. After the staining of the cell smear, the NRBCs can be identified and located under optical microscope according to unique cell morphology. Then the cell smear is placed in a laser convergence and microscopic imaging system.According to the near-infrared response properties of hydrogel substrate, an 808 nm laser is selected for fixed-point irradiation to realize the photothermal conversion and release NRBC in the spot area.
2.2 Design of an automatic smear system
The automatic smear system includes four parts: a mechanical motion subsystem, an adaptive smear system, a microstructural slide-loading platform and an electronic control subsystem. The design principle of mechanical motion subsystem is shown in Figure 2. The system is divided into two modules: speed regulation and angle adjustment.The speed regulation module, which consists of an X stage, a carrier base and a closed-loop stepper motor, can adjust the speed within the range of 0−150 mm/s. The angle adjustment module is composed of a Y stage, a smear structure base, a closedloop stepper motor and an R-axis decelerating stepper motor. It can tune the angle within the range of 20°−50° when spreading the blood sample.
The adaptive smear system consists of smear clips and two sets of hinges that are perpendicular to each other in the pushing direction. With two DOFs,the system can adaptively fine-tune the smear while keeping it fit to the contact surface. The microstructural slide carrier is designed with a slide-loading groove and a waste liquid collection groove.
A 200 μm height difference between the slideloading groove and the slide surface is designed to ensure a hydrogel substrate membrane with uniform thickness during pushing. The waste liquid collection groove is used to collect excess hydrogel when pushing the slide to the end, so as to avoid the backflow-caused damage and contamination. The electronic control subsystem consists of an upper computer control system, a motion control card, a motor driver, a photoelectric limit switch and a power supply. The upper computer control software is programmed based on a single chip microcomputer. The serial port is connected with the motion control card to realize the interactive operation and control of pushing speed module and angle module.
2.3 Design of cell recognition and release system
According to the properties of hydrogel substrate membrane and the design principle of cell smear, the cell recognition and release system shown in Figure 3 has been designed in order to achieve the recognition and fixed-point release of NRBCs. It is composed of a laser-focusing subsystem, a bright field illumination subsystem and a microscopic imaging subsystem. According to the near-infrared response characteristics of hydrogel substrate membrane, an 808 nm laser source is combined with a collimating lens and a convergent lens(C1) into a laser convergence subsystem to irradiate a fixed area of the cell smear, which, in turn, produces the photothermal effect and releases the cells.In order to observe the cell release, a bright field illumination subsystem and a microscopic imaging subsystem are constructed on the basis of the laser convergence subsystem. The bright field illumination subsystem is composed of the bright field source and Kohler lens group. Through the application of dichromatic mirror, the bright field source and the 808 nm laser source can share an optical path. Subsequently, the microscopic imaging subsystem module composed of a microscopic objective, a reflecting mirror, a convergent lens (C2) and an imaging detector is used to observe the cell-releasing effect.
The laser convergence system is built to produce the near-infrared response, which enables the cell release at a fixed point. The laser light is focused to form a tiny light spot. A smaller light spot means a smaller spot area projected on the cell smear and higher efficiency and precision of cell release. To control the size of the light spot, the spot diameter should be reduced as much as possible so as to narrow the range of cell release at a fixed point. According to Gauss formula[14]:

whereω1is the spot diameter after focusing;Fis the focal length of the convergent lensC1,F=F1+F2,whereF1is the distance from the convergent lens to the dichromatic mirror andF2is the reflection distance from the dichromatic mirror to the cell smear;andω0is the waist radius of the laser Gaussian beam. The spot size and light intensity distribution on the equiphase planes on both sides of the convergent lensC1 are the same, soω0is the spot radius after collimation. By substituting the laser wavelengthλ(808 nm) and the collimated spot radiusω0(850 μm) into Equation (1), the spot diameter after laser convergence can be obtained as follows:

The bright field light is radiated to the cell smear surface through the Kohler lens group, and then is reflected to the detector by the imaging system composed of a microscope objective, a mirror and a convergent lens. By moving the stage, the whole cell smear can be imaged and the NRBCs can be identified and located. The laser light irradiates the identified target area through the collimating lens and convergent lens, and triggers the near-infrared response and photothermal conversion followed by fixed-point cell release. Finally, the cellreleasing performance is characterized by a microscopic imaging system.
3 Experiment and results
3.1 Preparation and optimization of hydrogel pusher
The experimental hydrogel was two-dimensional MXene composite gelatin (C102H151O39N31,gelatin) prepared by our group. The gelatin and MXene were stirred and mixed evenly at a mass ratio of 200:1 in a water bath at 38 ℃ to obtain the hydrogel. The prepared hydrogel was kept at 38 ℃to ensure the melting state through magnetic stirring, pushed and spread on the slide surface by a home-made automatic pushing machine, and then naturally cooled to form a layer of hydrogel membrane with smooth surface and uniform thickness.The prototype machine designed and fabricated based on a single-chip microcomputer is shown in Figure 4. The slide-carrier is located in a horizontal position. The pusher-loading platform has an angle to the slide carrier, but with a base parallel to the slide carrier. The preparation of hydrogel membrane was optimized by adjusting the pusher’s speed and angle. At first, a certain amount of hydrogel solution was draw with a pipette, and dropped evenly on the front end of the slide. Then the pusher was started and descended until its bottom touched the slide horizontally. The pusher was pushed forward with an angle to the slide so that a hydrogel membrane was formed on the surface of the slide.
In the experimental process, orthogonal experiments were carried out at different pushing speeds(20 mm/s, 30 mm/s and 40 mm/s), pushing angles(25°, 30° and 35°) and hydrogel amounts (200 μL,250 μL and 300 μL) to optimize the preparation parameters of hydrogel membrane. The results showed that a faster pushing speed and a larger pushing angle could cause the damage to the hydrogel membrane more easily. The hydrogel amount is mainly determined whether the hydrogel membrane could completely cover the slide. After repeated experiments, the preparation parameters of hydrogel membrane were optimized at a pushing speed of 30 mm/s, a pushing angle of 30° and a hydrogel amount of 250 μL.
The prepared hydrogel membrane was observed and characterized by Scanning Electron Microscope (SEM) and Atomic Force Microscope(AFM). As shown in Figure 5 (a, b), the hydrogel membrane had a uniform thickness of about 200 μm and an average surface roughness ofRa= 1.31 nm,providing a good surface smoothness for subsequent whole blood experiment. An ultraviolet absorption test was carried out on the slide spread with a composite gelatin. As can be seen from the test results in Figure 5(c), the prepared composite gelatin has an obvious absorption peak at about 800 nm, which indicates an achievable near-infrared response and photothermal conversion. Based on the above optimized conditions, a hydrogel film was prepared.Its photothermal properties were tested by 808 nm laser irradiation, and the characterization results are shown in Fig. 5(d), including the photothermal response curves of gelatin, composite gelatin and stained composite gelatin under the irradiation of 808 nm laser (laser power: 150 mW/mm2). Under the laser irradiation, the temperature of gelatin was almost unchanged, and no photothermal effect was produced. However, within the irradiated area of composite gelatin, the temperature increased and quickly rose to 37 ℃ within 120 s and even up to 47 ℃ within 360 s (this temperature could be applied to the subsequent cell release). To verify whether the Wright-Giemsa staining method would affect the photothermal properties of stained composite gelatin, a laser irradiation experiment was performed under the same conditions. The stained composite gelatin exposed to 150 mW/mm2laser irradiation could be heated to 37 ℃ within 20 s and up to 62.9 ℃ within 320 s. When the laser power was reduced to 100 mW/mm2, the photothermal conversion effect on the stained composite gelatin was similar as that on the unstained composite gelatin.

Fig. 1 Schematic diagram of cell smear preparation and laser response system图 1 细胞涂片制备及激光响应系统示意图

Fig. 2 Schematic diagram of mechanical system of cell smear图 2 细胞涂片机械系统原理示意图

Fig. 3 Schematic diagram of laser focusing and microscopic imaging system图 3 激光会聚与显微成像系统示意图

Fig. 4 Prototype of preparation machine of automatic cell smear图 4 自动细胞涂片制备机样机
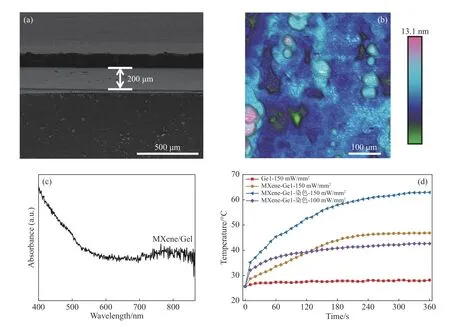
Fig. 5 (a) Characterization by SEM; (b) surface roughness characterization by AFM; (c) ultraviolet absorption spectrum;(d) photo-thermal curves图 5 (a)SEM表征;(b)AFM表面粗糙度表征;(c)紫外吸收图谱;(d)光热曲线
3.2 Preparation and optimization of a cell smear on hydrogel membrane
An automatic cell smear preparation machine was designed to carry out blood slide-pushing experiment on the surface of hydrogel membrane. The blood samples were from the peripheral blood with a hematocrit (HCT) ranging from 0.39 to 0.45 examined in a clinic. The reference value of HCT for normal females is 0.35−0.45. However, during pregnancy, the HCT value will vary within 0.33−0.46 as the mother undergoes a series of physiological changes with the growth and development of her fetus[15].
4 groups of peripheral blood samples with the HCT values of 0.39−0.45 were pushed on the surface of hydrogel substrate. According to the parameters mentioned above, the pushing speed, the pushing angle and the whole-blood volume were investigated and optimized in the process of cell smear preparation. After optimization, the pushing effect was shown in Figure 6 (Color online) (smear speed: 100 mm/s, pushing angle: 40°, blood volume:5 μL). The Fig. 6 compares the effects of a stained cell smear on slide substrate, a cell smear on hydrogel membrane substrate and a standard cell smear prepared by a fully automatic blood analyzer Mindray SC-120 which is currently used in clinical tests. The front, middle and tail ends of the three cell smears were analyzed by microscopic imaging system, repectively. Five sampling points were randomly selected from each part within the 0.3 mm2FOV (field of view) of the imaging system to obtain the cell distribution images of the smears. The cells were then counted using ImageJ software. The cell distributions on the smears with 4 groups of blood samples are shown in Fig. 7. As can be seen from the figure, the cells aggregate and overlap on the smear with slide substrate, while the cells are in a single layer format adhere to hydrogel membrane substrate and are distributed uniformly. By comparing the front and tail parts of the cell smears, it can be seen that the cell smear with hydrogel membrane substrate shares the same monolayer cell distribution with the standard smear. In addition, the average of cell density is 19.3% higher than that of the standard smear, thus improving the efficiency of the NRBC observation and detection based on cell smear.

Fig. 6 Comparison of self-made cell smear and Mindray SC-120 standard cell smear: (a-c) slide; (d-f) hydrogel membrane; (g-i) Mindray SC-120图 6 自制细胞涂片与迈瑞SC-120标准细胞涂片效果比对分析:(a-c)玻片;(d-f)水凝胶膜;(g-i)迈瑞SC-120

Fig. 7 Cell distribution statistics图 7 细胞分布统计
The performance parameters of the prototype machine proposed in this paper were compared with those of the commercial Mindray SC-120 automatic pushing machine. The comparison results are summarized in Table 1. Compared with Mindray SC-120, the proposed prototype has the following advantages: (1) The pusher-loading platform is modularized so that the various substrates (glass/hydrogel/releasable) can be made by changing the platform; (2) the scraping operation in traditional scraping-pushing process is not necessary, so the blood consumption is reduced down to only 4 μL; (3) the pusher can be a standard medical type, rather than a customized product, so the maintenance cost is reduced.
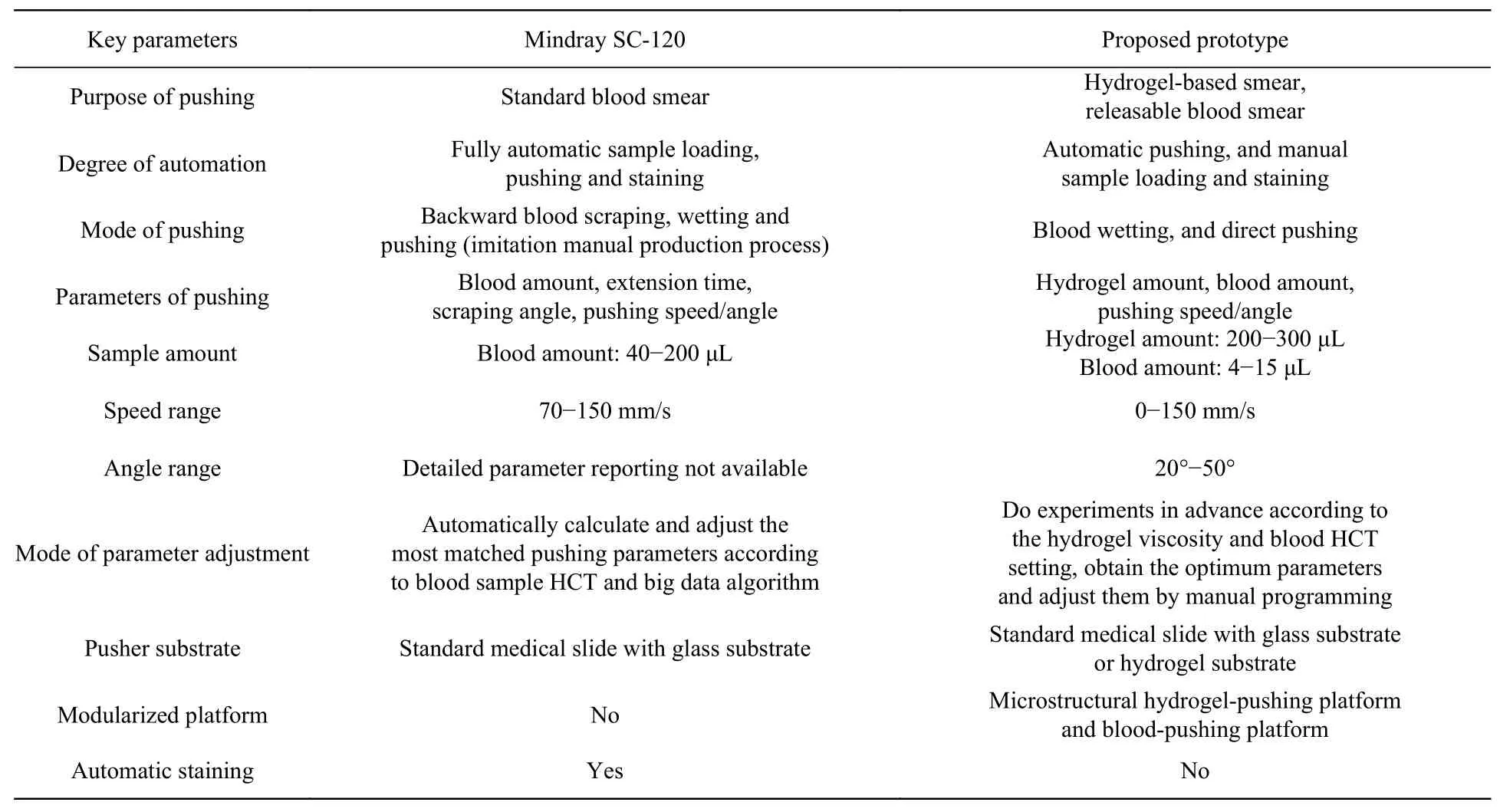
Tab. 1 Performance comparisons between the proposed prototype and Mindray SC-120 automatic pushing-staining machine表 1 本样机与迈瑞SC-120全自动推片染色机的性能参数对比
3.3 NRBC release under near-infrared response
3.3.1 Recognition and release of NRBCs
The NRBC recognition and release based on cell smear preparation technique were achieved by using the photothermal response properties of hydrogel. The prepared cell smear was placed in the microscopic imaging system. Then the NRBC was identified and determined according to its morphological features, such as round nucleus, a nucleuscytoplasm ratio of less than 1/2, no cytoplasmic granules, and nucleus amesiality[16]. As shown in Fig. 8(a), the NRBCs in the cell layer on the smear were identified and located, and then irradiated for 90 s by an 808 nm laser source (laser power:100 mW/mm2) fixed at a distance where a 1.7 mm spot was formed. Finally, they were rinsed with deionized water and dried, and observed under a microscope.
As can be seen from the characterization results (Fig. 8(b)), almost all the cells fall off with the photothermal melting of the hydrogel membrane within the fixed area of hydrogel membrane irradiated by laser. Therefore, the fixed-point release of cells can be achieved by introducing photothermally responsive hydrogel.

Fig. 8 (a) Recognition and localization of NRBC; (b-d)comparison of cell release areas before and after laser convergence with different spot diameters.(b) D = 1 700 μm; (c) D = 600 μm; (d) D = 300 μm图 8 (a)NRBC识别定位结果;(b-d)激光会聚前后细胞释放区域比较:(b)光斑直径D = 1 700 μm;(c)光斑直径D = 600 μm;(d)光斑直径D = 300 μm
3.3.2 Optimization of laser convergence system
The fixed-point release of NRBCs was achieved through direct irradiation of 808-nm laser.However, the spot diameter directly projected onto the cell smear surface was 1.7 mm and required further adjustment relative to the cell size. Therefore, a laser convergence system was built by connecting the laser with a collimating lens and a convergent lens to reduce the spot diameter on the smear surface. This system could not only ensure the function of cell release, but also improve the accuracy of NRBC release. By substitutingω0= 300 μm andω0= 150 μm into Equation (2) respectively, the focal lengthsF= 100 cm andF=50 cm can be obtained. SinceF2is a fixed distance (F2= 20 cm), the focus diameter can be adjusted by adjustingF1=80 cm andF1= 30 cm, whereF1is the distance between the convergent lensC1 and the dichromatic mirror. The photothermal releases generated by the converged spots of about 600 μm and 300 μm are shown in Figure 8(c) and Figure 8(d) respectively. The micro-characterization results show that the proposed laser convergence system can scale down the cell-releasing area to about 271.2 μm (in diameter), while achieving the same photothermal conversion effect.
4 Conclusion
In this paper, a monolayer cell smear based on hydrogel membrane was prepared by automatic pushing technique. The prepared hydrogel membrane had the property of near-infrared light response and showed photothermal conversion under 808 nm laser irradiation. The processing parameters of hydrogel membrane substrate and cell smear were optimized. The whole blood cells were spread into a single layer on the surface of the hydrogel membrane substrate with a uniform thickness of 200 μm. A laser convergence system and a microscopic imaging system were constructed using an 808 nm laser source and applied to cell smears. By designing and constructing the light path for laser convergence and microscopic imaging, the light source was focused on a fixed area of the cell smear to realize the cell recognition and release. The results showed that when the pushing speed was 100 mm/s and the pushing angle was 40°, the prepared cell smear had uniform cell distribution and an average cell density 19.3% higher than a standard smear. While keeping laser power at the cell-releasing level, this technique can reduce the spot diameter to about 300 μm, so as to realize the cell release and enrichment. This paper provides a new technical approach that can be combined with automatic microscanning imaging and microneedle extraction in subsequent studies to efficiently and accurately extract the NRBCs for noninvasive prenatal diagnoses.
——中文对照版——
1 引 言
产前诊断对于唐氏综合征、神经管缺陷、单基因疾病等出生缺陷的早期诊断和筛查至关重要[1],目前产前诊断主要分为有创性诊断和无创性诊断。有创性诊断包含羊膜枪穿刺、脐血取样以及绒毛膜穿刺等[2],是通过侵入式方法进行的,在诊断过程中往往伴随着流产或羊水溢出、感染等风险[3]。因此,开发以非侵入式进行无创性产前诊断是当前产前诊断技术发展的研究重点和主流趋势[4]。胎儿有核红细胞(NRBCs)包含了胎儿全部基因组,具有孕早期表达(6周在外周血中表达,12−14周表达量达到最高),在母血细胞群中可鉴別性高,不会出现与母体细胞混淆的情况,具备明显的细胞形态学特征,易识别,是应用于产前诊断的首选胎儿细胞[5]。然而,其在母体外周血中数量极其稀少,如何从外周血中对有核红细胞进行识别分离是产前诊断中的一大挑战[6]。
目前已有的对外周血细胞进行分离富集的方法主要有密度梯度离心富集、磁激活细胞分选法、荧光激活细胞分选法以及微流控芯片等[7-9],这些方法多以抗体与细胞特异性结合为核心进行分离检测,操作流程复杂、检测周期较长且成本较高。在已有的研究基础上简化细胞分离和富集过程是当前提高产前诊断亟待解决的问题。
采用推片技术制作良好的细胞涂片用于血液细胞形态学检查是临床应用中一种重要的细胞检测技术[10]。传统的人工制作细胞涂片受限于人工操作的不可控性,常导致细胞重叠、破裂等而无法标准化。通过自动化细胞涂片制备机可以实现单层细胞涂片的标准化制备,结合光学成像对有核红细胞进行识别。为了实现有核红细胞后续的分离以进行进一步测定应用,目前如热响应释放系统,可以在生理温度下实现捕获细胞的释放,并使释放细胞保存足够的活性,这种方法虽然能够实现大面积的细胞释放,但是无法对固定区域进行定点释放。相对应的,光刺激响应系统由于其可操作性目前已被作为一种理想的可操控释放体系[11],其中,近红外光相较于紫外光对细胞的伤害较小,穿透力更强,更适用于构建光响应释放体系,通过在热响应释放系统中引入光响应体系,达到实现更高效的定点释放目的。本文提出利用水凝胶材料的成膜以及光热特性[12],在温敏水凝胶明胶中引入MXene二维材料,将MXene的近红外光热效应和热响应凝胶基质相结合,在此基础上制备自动化单层细胞涂片制备机,建立激光会聚和显微成像系统,解决了传统依赖于抗原抗体特异性结合的捕获与分离释放的问题,通过对各模块的结构和材料的性能优化,实现了有核红细胞的识别与定点释放,为无创产前检测提供了一种新的途径。
2 细胞涂片制备机系统设计和识别释放系统设计
2.1 控制系统总体设计
图1 所示为应用于识别释放有核红细胞的自动推片与激光会聚系统结构示意图。其中,自动推片系统由机械运动子系统、自适应涂片系统、微结构玻片载台以及电控子系统组成。XY轴滑台的二维运动由机械运动子系统控制,进行推片速度和角度的调控。自适应涂片结构具备双自由度,可实现涂片与其接触表面之间的自适应状态微调,结合运动子系统的二维运动,可达到调控涂片、载玻片三维运动的目的。微结构玻片载台可承载(25 mm × 75 mm)标准显微镜载玻片,用于水凝胶基底涂片以及细胞涂片的制备。电控子系统用于整体涂片系统各模块之间的通信与交互操作的控制。
通过自动推片系统在载玻片制备水凝胶膜,以此为基底膜,在其表面进行推片获得具备单层血细胞膜的细胞涂片。其次,选用Wright-Giemsa染色法对血细胞膜进行细胞形态学染色,该方法作为临床血液学检测方法具备染色效果清晰、核质对比明显、操作简便以及染色快速等优点[13]。细胞涂片经过染色可依据细胞形态于光学显微镜下进行NRBC的识别与定位。识别定位后的细胞涂片置于激光会聚与显微成像系统下,根据水凝胶基底的近红外响应性质,选取808 nm激光进行定点照射,实现定点区域的光热转换释放有核红细胞。
2.2 自动推片系统设计
自动推片系统包括机械运动子系统、自适应涂片系统、微结构玻片载台以及电控子系统4部分。其中,机械运动子系统设计原理如图2所示,该系统分为速度调节和角度调节两大模块。速度调节模块包括X轴滑台、载体底座和闭环步进电机,可实现0~150 mm/s范围内的速度调控;角度调节模块由Y轴滑台、涂片结构底座、闭环步进电机以及R轴减速步进电机组成,推片时可实现20°~50°内的角度调节。
自适应涂片系统具备双自由度,包含涂片夹、推进方向上相互垂直的两组铰链,其可自适应微调涂片,与接触底面保持贴合的状态。微结构玻片载台整体设计包含玻片承载槽和废液收集槽,其中,玻片承载槽表面与玻片表面经由设计加工呈现200 μm的高度差,用于推片制备厚度均匀的水凝胶基底膜;废液收集槽用于推片到底部时收集多余的水凝胶样品,避免回流破坏水凝胶膜、造成污染。电控子系统包含上位机控制系统、运动控制卡、电机驱动器、光电限位开关以及供电电源。通过单片机制作上位机控制软件,将串口与运动控制卡连接,实现推片速度与角度两大模块的交互操作调控。
2.3 细胞识别释放系统设计
根据水凝胶基底膜的性质与细胞涂片的设计原理,为了实现有核红细胞的识别与定点释放,设计如图3所示的细胞识别释放系统,分为激光会聚子系统、明场照明子系统和显微成像子系统3部分。根据水凝胶基底膜的近红外响应特性,以808 nm激光光源为主体,结合准直镜和会聚镜(C1)组成激光会聚子系统,用于定点照射激发细胞涂片产生光热效应,从而释放细胞。为了观察细胞释放情况,在激光会聚子系统的基础上构建明场照明子系统和显微成像子系统。选用明场光源和科勒镜组搭建明场照明子系统,通过二向色镜达到明场光源和808 nm激光光源公路共用的目的。随后,通过由显微物镜、反射镜、会聚镜(C2)以及成像探测器构建的显微成像子系统模块进行后续细胞释放效果的观察。
构建激光会聚系统实现近红外响应定点释放细胞,激光被聚焦后形成一个微小光斑,光斑越小,投射到细胞涂片表面的面积就越小,释放细胞的效率和精度也越高。为了调控照射光斑大小,尽可能缩小光斑直径,从而缩小定点释放的细胞范围,根据高斯公式[14]:
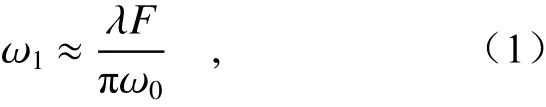
其中,ω1为聚焦后的光斑直径,F为会聚镜C1的焦距,F=F1+F2,其中F1为会聚镜到二向色镜的距离,F2为二向色镜到细胞涂片的反射距离,ω0为激光高斯光束的束腰半径,会聚镜C1两侧等相位面上的光斑大小和光强分布相同,因此ω0又为准直后的光斑半径。将激光波长λ(808 nm)、准直后的光斑半径ω0(850 μm)作为常数代入式(1)可得激光会聚后的光斑直径为:

基于自动推片机制备的单层细胞涂片,明场光源经由科勒镜组照射到细胞涂片表面,再结合显微物镜、反射镜和会聚镜的成像系统被探测器获取。通过位移平台的移动,实现细胞涂片的全载片成像,从而进行有核红细胞的识别与定位。激光光源经由准直镜和会聚镜准直会聚照射在已识别定位的目标区域,产生近红外响应光热转换,从而实现细胞定点释放。最后由显微成像系统成像表征细胞释放性能。
3 实验与结果
3.1 水凝胶推片的制备与优化
实验用水凝胶为课题组前期制备的二维材料MXene复 合 明 胶(C102H151O39N31, gelatin)材料,将明胶与MXene以200:1的质量比在恒温38 ℃的水浴条件下搅拌混合均匀,制备得到水凝胶。制备好的水凝胶经磁力搅拌后保持在38 ℃的恒温溶融状态,通过搭建的自动推片机推涂在玻片表面,自然冷却形成一层表面平整且厚度均匀的水凝胶薄膜。图4为基于单片机控制软件设计制造的样机结构,玻片载台位于水平位置,推片载台与玻片载台形成夹角,其底部与玻片载台平行,通过调控推片的速度和角度对水凝胶膜的制备进行优化。首先手动用移液器吸取一定量的凝胶溶液,均匀滴在玻片前端,启动推片,推片向下,底部水平接触到玻片,推片与玻片形成一定的角度,向前推动从而在玻片表面制备水凝胶薄膜。
实验过程中,选取不同的推片速度(20 mm/s、30 mm/s和40 mm/s),推片角度(25°、30°和35°)以及凝胶用量(200 μL、250 μL和300 μL)进行正交实验,优化水凝胶膜制备参数。实验结果表明涂片速度越快、涂片角度越大则越易导致凝胶膜破损;凝胶用量则主要影响凝胶膜是否能完整覆盖玻片。经重复实验后,优化水凝胶推片参数如下:涂片速度为30 mm/s,涂片角度为30°以及凝胶用量为250 μL。采用扫描电子显微镜(SEM)和原子力显微镜(AFM)观察表征制备好的水凝胶推片,如图5(a),5(b)所示,所制备的水凝胶膜厚度大约在200 μm左右,且厚度均匀,其表面平均粗糙度Ra= 1.31 nm,为后续全血展开提供了较好的表面平整光滑性。对复合明胶推片进行紫外吸收测试,结果如图5(c)所示。从图5(c)的测试结果可以看到,所制备的复合明胶在~800 nm处具有明显的吸收峰,可以实现近红外响应产生光热转化。基于上述优化条件制作了水凝胶推片,采用808 nm激光照射对其进行光热性质测试,表征结果如图5(d)所示。图中分别为明胶、复合明胶以及复合明胶染色后在808 nm激光照射下的光热响应曲线(激光功率为150 mW/mm2),可见:明胶在激光照射下温度基本不变,无光热效应;复合明胶照射区域的温度随着激光照射时间变长而逐渐升高,在120 s内可快速升温到37 ℃,360 s内最高温度可达47 ℃,可应用于后续的细胞释放。同时为验证Wright-Giemsa染色法是否会对材料的光热特性造成影响,在相同实验条件下对染色后的复合明胶进行了激光照射实验。可见,在150 mW/mm2功率下,染色后的复合明胶在20 s内可升温至37 ℃,320 s内最高温度可达到62.9 ℃;降低激光功率到100 mW/mm2,此时光热转换效应与未染色的复合明胶基本一致。
3.2 基于水凝胶膜的细胞涂片制备与优化
采用自行设计的自动细胞涂片制备机在水凝胶膜表面进行血液推片实验,血液样本来自于医院临床检验的红细胞比容(HCT)范围在0.39~0.45的外周血样。正常女性的HCT参考值为0.35~0.45,而妊娠期间随着胎儿的生长发育,母体会发生一系列的生理变化,HCT指标会随妊娠期进展的不同在0.33~0.46范围内变化[15]。
采用4组HCT值均在0.39~0.45内的外周血血样,在水凝胶基底表面进行推片,根据上述制作优化水凝胶推片的方法,对细胞涂片制备过程中的涂片速度、角度以及全血样本用量进行考察优化,优化后(涂片速度为100 mm/s,涂片角度为40°,血液用量为5 μL)推片效果如图6(彩图见期刊电子版)所示。图6分别为玻片基底、水凝胶膜基底染色后 与目前在临床检验中所使用的全自动血液分析仪迈瑞SC-120制备的玻璃基底标准细胞涂片的效果对比。通过显微成像系统分别对这3种细胞涂片的前端、终端、尾端进行取样分析,在该成像系统拍摄视野为0.3 mm2内分别在各部分随机选取5处作为取样点,得到细胞涂片的细胞分布图像,随后利用ImageJ软件进行细胞计数。图7为4组血液样本的细胞涂片细胞分布情况,从图中可以看出,以玻片为基底的细胞涂片出现了细胞聚集重叠现象,而基于水凝胶膜基底的细胞涂片呈现细胞单层粘附且分布均匀紧密的形态。对比细胞涂片的前端和尾端部分可以看出,以水凝胶膜为基底所制作的细胞涂片与标准涂片具备相同的单层细胞分布形态,且平均细胞密度比标准涂片增加了19.3%,提高了以细胞涂片为基础观察检测有核红细胞的应用效率。
本文搭建的样机与目前迈瑞SC-120全自动推片机的性能参数对比结果如表1所示。本文所搭建的样机相较于迈瑞SC-120有以下几方面优点:(1)推片载台模块化,可通过更换不同载台制作玻璃/凝胶/可释放等多类涂片;(2)推片模式无需先刮后推,降低用血量,最少仅需4 μL血液;(3)推片可使用标准医用推片,无需使用配套定制推片,降低维护成本。
3.3 近红外响应释放有核红细胞
3.3.1 有核红细胞的识别与释放
利用水凝胶的光热响应特性,实现以细胞涂片制备技术为基础的有核红细胞识别与释放。将所制作的细胞涂片置于显微成像系统下,根据有核红细胞的核型圆润,核质比小于1/2,胞质无颗粒且细胞核偏向一侧的形态学特征[16]进行识别并确定有核红细胞区域。如图8(a)所示,对细胞涂片细胞层中的NRBC进行了识别定位,然后使用808 nm激光光源进行照射,将激光器固定在光斑直径为1.7 mm大小的距离,激光功率为100 mW/mm2,照射时间为90 s,最后用去离子水冲洗并干燥,在显微镜下进行观察。
从表征结果(图8(b))可以看出,激光照射的定点区域随着水凝胶膜的光热熔化,表面细胞也随之脱落,基本无残留细胞。因此,通过在细胞涂片制作中引入光热响应水凝胶可以实现细胞的定点释放。
3.3.2 激光会聚系统的优化
采用808 nm激光直接照射达到了有核红细胞定点区域释放的效果,然而,直射到细胞涂片表面的光斑直径为1.7 mm,相较于细胞尺寸还需要进一步调整。通过准直镜和会聚镜连接激光器共同搭建激光会聚体系,缩小激光照射到细胞涂片表面的光斑直径,在保证细胞释放功能的同时,提升对有核红细胞释放的准确度。ω0分别取300 μm和150 μm,计算得到F分别为100 cm和50 cm,其中F2= 20 cm为固定距离,通过调节使F1分别为80 cm和30 cm,即会聚镜C1到二向色镜的距离,实现会聚直径的调节。图8(c)、8(d)分别是经过会聚后直径约为600 μm以及300 μm的光斑产生的光热释放结果,显微表征结果表明所搭建的激光会聚系统可将细胞释放区域缩小到直径为271.2 μm左右,且达到了同等的光热转换效应。
4 结 论
本文采用自动推片技术制备以水凝胶膜为基底的单层细胞涂片,所制备的水凝胶膜具备近红外光响应特性,在808 nm激光照射下将产生光热转换。优化水凝胶膜基底以及细胞涂片的加工设计参数,在200 μm厚度均一的水凝胶膜基底表面将全血细胞进行了单层展开。基于808 nm激光响应光源构建了应用于细胞涂片的激光会聚系统和显微成像系统,通过设计和制作激光会聚和显微成像光路,将光源聚焦到细胞涂片定点区域,以实现细胞的识别与释放。结果表明,当推片速度为100 mm/s、角度为40°时制备出细胞分布均匀且平均细胞密度较标准片提升19.3%的细胞涂片。在保证激光功率达到可释放细胞效果的同时,将光斑直径缩小到~300 μm,从而实现细胞的释放富集。本文为后续研究中结合自动显微扫描成像技术以及显微微针提取系统,高效、精准提取有核红细胞,并应用于无创产前诊断提供了一种新的技术途径。
- 中国光学的其它文章
- Surface plasmon resonance characteristics of a graphene nano-disk based on three-dimensional boundary element method
- Design of optical wedge demodulation system for fiber Fabry-Perot sensor
- Photon-assisted Fano resonance tunneling periodic double-well potential characteristics
- Formation mechanism of the continuous spectral profile of lightning plasma
- 中国遥感卫星辐射校正场敦煌戈壁场区光环境变化研究
- 油气井下光纤光栅温度压力传感器

