Role of glycolysis in retinal vascular endothelium, glia,pigment epithelium, and photoreceptor cells and as therapeutic targets for related retinal diseases
Ting-Ting Yang, Hui Li, Li-Jie Dong
1Editorial Department of Chinese Journal of Ocular Fundus Diseases, West China Hospital of Sichuan University, Chengdu 610041, Sichuan Province, China
2Tianjin Key Laboratory of Retinal Functions and Diseases,Tianjin Branch of National Clinical Research Center for Ocular Diseases, Eye Institute and School of Optometry, Tianjin Medical University Eye Hospital, Tianjin 300384, China
Abstract
● KEYWORDS: glycolysis; microglia; retinal pigment epithelium; photoreceptor cells
INTRODUCTION
Glucose is an important energy source in cells, and its homeostasis is maintained by glycolysis, tricarboxylic acid cycle, and oxidative phosphorylation coordination[1].The oxidative phosphorylation of glucose is a thorough and highly productive metabolic process, whereas glycolysis is the opposite, but it produces large amounts of adenosine triphosphate (ATP) in a short period. Aerobic glycolysis is the process by which cells choose to undergo glycolysis even in an aerobic environment[2]. This phenomenon was first observed in tumor cells, and studies have linked cell proliferation in tumor tissue to aerobic glycolysis[3]. And the phenomenon of aerobic glycolysis is also found in retinal endothelium[4]. As Wanetet al[5]says, the pattern of cell metabolism is related to its fate[5]. Aerobic glycolysis is closely related to cell division,and the rate of glycolysis growth is closely related to the rate of retinal growth in chicken embryos[6]. Among the retinal cells, only vascular endothelial cells rely on aerobic glycolysis to provide ATP. Due to the different tissue environment, other cells cannot carry out the process of aerobic glycolysis, but the effect of glycolysis on these cells is still very important and has been proved to be an effective target for the treatment of many retinal diseases. Our latest research shows that pyrimidine bundle-binding protein-associated splicing factors(PSF) compounds control angiogenesis by influencing cell metabolism and vascular endothelial growth factor (VEGF)expression[7]. At the same time, we also found that PSF could inhibit angiogenesis by recombining mitochondrial bioenergy and glycolysis to reduce the effect of reactive oxygen species[8].Based on these understandings, this review focuses on the metabolic patterns of glucose in various types of retinal cells,focusing on the important role of glycolysis in various types of cells.
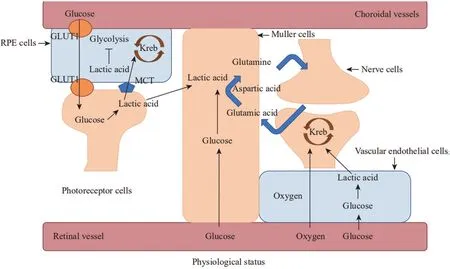
Figure 1 The metabolic pattern of retinal cells The outer segment of photoreceptor cells, the Müller cells and the endothelium cells all produce adenosine triphosphate through glycolysis. The retinal pigment epithelium cells and nerve cells of the outer segment of photoreceptor cells produce adenosine triphosphate through oxidative phosphorylation lactate. In addition, Müller cells penetrate the entire retina, it takes the glutamate between nerve cells, converts it into L-glutamine, and then transports it to the presynaptic cells. GLUT1: Glucose transporter 1; MCT:Monocarboxylic acid transporter; Kreb: Tricarboxylic acid cycle; RPE: Retinal pigment epithelium.
METABOLIC PATTERN OF VARIOUS RETINAL CELLS
The capillary endothelium area of the retina is undoubtedly the most oxygen-rich, but it has been found that the energy expenditure of endothelial cells is satisfied by glycolysis alone[2]. On the one hand, endothelial cells normally proliferate very little and require only a small amount of energy from glycolysis[9]; on the other hand, the absence of aerobic oxidation of glucose also contributes to the availability of more oxygen to deeper tissue cells and reduces the effects of oxidative stress on endothelial cells themselves[10-11].Retinal pigment epithelium cells (RPE), optic nerve cells,and Müller cells are metabolically integrated and depend on each other[12-13]. The outer segments of photoreceptor cells rely mainly on glycolysis for energy metabolism[14-15], and the lactic acid produced is transferred to RPE by monocarboxylic acid transporter 1 (MCT1), which is oxidized and metabolized to provide energy for RPE[16], some of the lactic acids were transferred to the Müller cells[1]. Far from the blood vessels, the optic nerve cells produce energy mainly through lactic acid oxidation produced by glycolysis in the Müller cells[17-19]. Lactic acid, which enters RPE cells, is transferred to mitochondria to provide energy for oxidative phosphorylation,and it inhibits glycolysis in RPE cells[20]. The RPE is close to the choroidal vessels, and the glucose in the blood passes through the glucose transporter 1 (GLUT1) from the blood through the RPE cells to the photoreceptor cells. Unlike the RPE, the Müller cells obtain energy mainly by the glycolytic process[21](Figure 1).
可恰恰相反,李老先生在美国生活得有滋有味,不仅身体硬朗,还活出了一片新天地。原来,他自己有一套在美国生活的独特方式。
In 2001, the National Institutes of Health’s Institute of Neurological Diseases and Stroke introduced the concept of a neurovascular unit, emphasizing the relationship between brain nerve cells and the cerebrovascular system (https://www.ninds.nih.gov/About-ninds/Strategic-Plans-Evaluations/Strategic-Plans/Stroke-Progress-Review-Group). In the retina, endothelium, pericytes, retinal nerve cells, and neuroglia (mainly Müller cells) make up the neurovascular units. Neurodegeneration and microvascular damage are interdependent[22], highlighting the role that certain cells play in the whole. Retinal endothelium needs only a small amount of energy from glycolysis to meet its needs[2]. The rest of the glucose in the retinal vessels is transported to Müller cells,where it is converted into glycogen and stored in Müller cells, in addition to consuming the glucose itself to supply its ATP[21]. Lactic acid is transferred from endothelial cells and Müller cells to optic nerve cells, where it supplies ATP to the nerve cellsviathe tricarboxylic acid cycle[23]. Glycolysis plays an important role in neurovascular units. Glutamate is an excitatory neurotransmitter in the retina. If glutamate accumulates between synapses, it will cause excitotoxicity.The Müller cells convert glutamate into non-toxic L-glutamine through glycolysis, plays an important role in maintaining the stability of the neuroendocrine environment[24]. The induction of aerobic glycolysis and the stabilization of endothelial cells can significantly decrease the activation of hypoxia-inducible factor-1α (HIF-1α) and thus stabilize the neurovascular units[25].
ROLE OF GLYCOLYSIS IN RETINAL VASCULAR E N D O T H E L I U M A N D A S A TA R G E T F O R TREATMENT OF NEOVASCULARIZATION
文中针对低功耗可穿戴设备[21],提出了一种低复杂度、高效率的血压测量算法。此方法通过滑动均匀滤波、周期分割、基线校准、归一化等处理,识别出特征点并计算出特征值,进而回归分析建立SBP、DBP各自与特征值的关系表达式,实现无创连续的血压测量。本文的血压测量算法与实际的电子血压计具有较好的一致性,SBP与DBP一致性占比均达到95%以上,且SBP与DBP的精确度分别为0.45±6.57 mmHg和 0.09±4.75 mmHg,满足血压标准差不大于8 mmHg的要求。
In the physiological state, the ATP required for the survival of RPE cells is oxidized by lactic acid produced by photoreceptor cells in RPE mitochondria. In the pathological state, RPE cells increase the expression level of glycolysis to avoid oxidative damage, however, a large number of glycolysis products have toxic effects on photoreceptor cells, which can stabilize the function of RPE mitochondria and provide a feasible target for retinal degeneration and other diseases.
ROLE OF GLYCOLYSIS IN MÜLLER CELLS AND FOR NEUROPROTECTION AND MAINTENANCE OF THE BLOOD-RETINAL BARRIER
Retinal nerve cells are oxygen-intensive cells, and glucose in retinal blood vessels enters retinal nerve cells through GLUT1 of RPE cells[58]. Under physiological conditions, unused glucose entering the RPE is transferred to photoreceptor cells along a concentration gradient[13,20]. Photoreceptor cells produce energy by aerobic glycolysis, and lactic acid, the product of glycolysis, is transferred to RPE cells through MCT1. Lactic acid inhibits the glycolysis in RPE cells and is oxidized to provide energy for RPE cells[1,59]. Therefore,mitochondrial oxidative phosphorylation is very important for RPE in the physiological state. The researchers observed the process by which electron transport chain III inhibitors block oxidative phosphorylation, and found mitochondrial edema and damage to RPE cells[60]. Mitochondria are the oxidative phosphorylation of cells, and they are involved in the glucose tricarboxylic acid cycle, lipid oxidation, and lactic acid oxidation, so mitochondrial function is critical for the survival of RPE cells. miRNA-451a has been found to have protective effects on mitochondria of RPE cells and may provide a therapeutic target for pathological changes of DR RPE cells[61].On the other hand, metabolic disorders also play an important role in retinal degenerative diseases, retinal degeneration is delayed by adenosine monophosphate which can stabilize energy metabolism in RPE cells by increasing the copy number of mitochondrial DNA and ATP level in RPE cells[62].
The glycolytic gene expression of RPE cells was upregulated according to RNA analysis in retinal degenerative diseases[63]. To resist oxidative damage, RPE cells promote aerobic glycolysis to produce less reactive oxygen species[64].With the increase of aerobic glycolysis metabolism, a large amount of lactic acid is transported to photoreceptor cells[1].The endogenous coding factor H protein helps to maintain the transcriptional and metabolic homeostasis of RPE cells, to protect RPE cells from oxidative stress, or by up-regulating glycolysis when RPE cells were exposed to mild hydrogen peroxide, the level of glycolysis was decreased by knockout of complement protein-encoding factor H protein, the researchers suggest that endogenous coding factor H may protect RPE cells from oxidative stress by up-regulating glycolysis expression[65]. Retinal metabolism and remodeling are early features of age-related macular degeneration. NAD+induces filamentous phagocytosis to restore homeostasis in RPE cells, mitotic phagocytosis is important for promoting glycolysis, which is necessary for metabolic differentiation[66].However, lactic acid, a product of glycolysis, is cytotoxic.Excessive lactic acid is transported to RPE cells by MCT, and immature MCT binds to the basic protein encoded by Bsg to mature and be transported to RPE cell membrane, RPE cells,and cones/rods in Bsg deficient mice[67].
“我们这次展出的PulseTM流体处理系统主要应用领域在汽车后市场,为汽车维修保养及工业生产提供完美解决方案。”固瑞克公司润滑设备&流体输送设备亚太区销售市场总监RichardFoulis先生表示,“我们的产品系统帮助工厂实现透明化生产,无论是在操作者方面还是管理人员,都能让他们更清晰地了解到工厂的流体设备处于何种状态,工作是否正常稳定,包括存量的流体是否报警、是否处于低液位状态等。”
Pathologic neovascularization is an important cause of vision loss in many fundus diseases, such as proliferative diabetic retinopathy, age-related macular degeneration, and neonatal retinopathy[26]. Pathological neovascularization has many defects, such as vascular insufficiency and leakage, which can lead to complications such as retinal hemorrhage, exudation,and retinal detachment. Proliferation and germination of endothelial cells are one of the important steps to induce pathological neovascularization[27-28]. It is found that glycolysis plays an important role in the pseudopodia of neovascular tip cells. Because of the small size of the pseudopodia,which cannot bear the volume of mitochondria, glycolysis is the only way to obtain ATP, the differential expression of glycolysis between the tip cells and their adjacent cells induced the tip effect in favor of neovascularization[29]. In budding angiogenesis, the tip of the endothelial cell and the stalk cell remodel their cell-to-cell connections to allow the budding organism to migrate and extend and maintain the integrity of the blood vessels, deletion of the glycolytic key enzyme pyruvate kinase M2 reduced the amount of ATP required for the internalization/transport of VE-cadherin at the endothelial cell-cell junction[30]. Both hyperglycemia and hypoxia aggravate glycolysis, VEGF has long been recognized as an important factor in promoting neovascularization, and plays an important role in diabetic retinopathy (DR), especially in the pathogenesis of proliferative DR[31]. VEGF was found to double the amount of glycolysis induced by endothelium[32-33].In addition to the above-mentioned advantages of glycolysis,which is the major source of energy metabolism for endothelium, glycolysis also provides the necessary conditions for the rapid generation of new blood vessels[29].
2012—2015年,四省区建设高效节水灌溉工程面积3 800万亩 (黑龙江1 500万亩,吉林900万亩,辽宁600万亩,内蒙古800万亩),其中喷灌面积1 650万亩,微灌面积1 890万亩,管道输水灌溉工程面积260万亩。在项目区中,地表水灌区面积211万亩,占5.6%;地下水灌区面积3 589万亩,占94.5%。大田粮食作物面积3 722万亩,占98%;大田经济作物面积78万亩,占2%。
The Müller cells are the neuroglia cells in the retina, which play an important role in supporting the optic nerve cells as well as maintaining the blood-retinal barrier. Aerobic glycolysis has a protective effect on Müller cells, and the aerobic glycolysis of Müller cells provides targets for visual protection and maintenance of the blood-retinal barrier.
ROLE OF GLYCOLYSIS IN RPE CELLS AND AS A TARGET FOR TREATMENT OF RETINAL DEGENERATION
Müller cells are the neuroglia cells in the human retina, which run through the entire retina and play an important role in supporting nerve cells[47]. Müller cells not only maintain the stability of intercellular glutamate but also decompose stored glycogen into retinal nerve cells to provide nutrients and produce antioxidants and neurotrophin[48-49]. The lactic acid produced by the photoreceptor cells mentioned above is transferred to the Müller cells, and the number of literature also considers that the Müller cells metabolize energy through glycolysis, and lactic acid can be used as an energy source[21].Aspartic acid-glutamic acid carriers transfer glycolytic products into mitochondria and completely oxidize glucose, but these carriers are highly expressed in retinal neuron carriers and less or absent in Müller cells, lactic acid produced by glycolysis in Müller cells was transferred to optic nerve cells and completely oxidized[50]. ATP produced by glycolysis plays an important role in maintaining glutamate uptake by Müller cells,glutamate is an important excitatory transmitter between the retina, and glutamate homeostasis will produce excitotoxicity to the optic nerve cells[51]. However, the mitochondria of Müller cells also play an important role in metabolism[52]. Oxygen-induced Müller cells reduced glycolysis into mitochondria, and increased L-glutamine consumption resulted in increased ammonia release and retinal tissue damage[53].
Recently study found that the changes in energy metabolism in macroglial cells, such as Müller cells, may have an important effect on the support function of the DR mice model, causing retinal leakage and loss of visual potential in optic nerve cells[54]. Selective excision of Müller cells revealed the disappearance of proteins involved in glycolysis and the sirolimus target protein (mTOR) pathway in the extracellular segments of photoreceptor cells, the ablation of Müller cells was also accompanied by destruction of blood-retinal barrier and neovascularization[55-56]. Glucose transporters transfer glucose from one side of the cell membrane to the other,and glucose transporters undoubtedly play a primary role in intracellular glucose metabolism. GLUT1 is also an important downstream target for controlling the energy metabolism pathway. Inhibition of glucose uptake by GLUT1 has been found to slow the development of retinal neovascularization[44].Retinal binding protein, a retinal transporter secreted by photoreceptor cells, plays an important role in the protection of DR. By binding to GLUT1, resulting in decreased glucose uptake, to prevent inflammatory factors from damaging the retinal endothelium and Müller cells[57]. Müller cells protect endothelial cells from HIF-1α damage by inhibiting glycolysis in the mTOR signaling pathway and stabilizing glycolysis in the endothelium in hypoxic retinal nerve endothelial cells[25].
1)改善办学条件工程计划得到全面贯彻落实。国务院明确提出,这一工作由国家统一部署、省级政府统筹安排、县级政府具体实施。要强化省级统筹,省级政府要从实际出发,统筹使用、合理分配中央和省级财政资金,科学配置教育资源,细化目标任务,明确完成时限,层层抓好落实,有力有序推进。做好改善基本办学条件建设需求与相关资金的统筹和对接,防止资金、项目安排重复交叉或支持缺位。除教育专项以外,在中央一般性转移支付中,拿出一定比例用来改善薄弱学校基本办学条件。鼓励社会力量参与和支持贫困地区义务教育发展,形成政府、学校、社会共同推进的良好格局。
Energy metabolism of endothelial cells is an important regulator of angiogenesis[34]. Hypoxia-inducible factors play an important role in retinal pathology and are implicated in both endothelium and neuronal dysfunction[35-36], glycolysis is a downstream effect of hypoxia-inducible factors[37].VEGF induces the proliferation and migration of endothelial cells[38], which more than doubles the amount of glycolysis endothelium[32-33]. The increased glycolysis has a significant effect on VEGF expression in DR patients[39]. At the same time, the researchers suggest that glycolysis disorder could be an early predictor of DR vascular disease[39]. Not only in mature endothelial cells, but some studies have also found that knock-out of peroxisome proliferator-activated receptor(PPAR) α, the expression of threonine kinase (Akt) and its downstream signal [nuclear respiratory factor 1 (NRF), NRF2,SIRT1, and GLUT1] decreased, which caused endothelial progenitor cell injury[40]. The absence of endothelial progenitor cells is thought to be associated with neovascularization[41].Wnt signaling plays an important role in both physiological and pathological neovascularization, and it was found that Wnt signaling affects endothelial progenitor cells by up-regulating the function of mitochondria and reducing glycolysis[42].Many key enzymes in glycolysis are hypoxia downstream factors. In oxygen-induced retinopathy, adenosine A2A receptors increase glycolysis levels in the retina by acting on HIF-1α, thus driving neovascularization[43]. Extracellular regulated protein kinase (ERK)/Akt/HIF-1α adenosine A2A receptor signaling pathway plays an important role in ischemic diseases[43]. High expression of adenosine A2A receptor was found in vitreous neovascularization. Glucose is transported to the cellviaglucose transporter 1, an important speed-limiting substance in glycolysis. It was found that the interference of uncoupling protein with glucose transporter 1 resulted in impaired glucose uptake and delayed the development of physiological retinal blood vessels in oxygen-induced and room-cultured neonatal mice[44]. Some of the substances that inhibit glycolysis in endothelial cells, 3-(3-pyridinyl)-1-(4-pyridinyl)-2-protein-1-one (3PO) not only inhibits the activity of phosphofructokinase-2/fructose-2,6-bisphosphonate 3, an important rate-limiting enzyme activator for glycolysis but also acts on the Notch-VEGF signaling pathway and inhibits neovascularization[45]. Endothelium relies heavily on glycolysis to produce ATP, and many studies have pointed to glycolysis as a therapeutic target for neovascularization, but the role of mitochondrial oxidative phosphorylation remains significant.Oxidative respiratory chain complex III knock-out studies have shown that endothelial cells breathe less, with decreased endothelial cell proliferation and retinal neovascularization[46].As noted above, in the physiological state of the retina,endothelial cell primarily provides energy to itself through aerobic glycolysis because of its low ATP requirements;in the pathological state, primarily neovascularization,aerobic glycolysis provides rapid and abundant ATP support for angiogenesis and inhibits glycolysis in endothelial cells or provides an important target for the treatment of neovascularization.
ROLE OF GLYCOLYSIS IN PHOTORECEPTOR CELLS FOR DELAYING VISION LOSS
Photoreceptor cells are energy-consuming cells, they are the main sites of light transmission in the retina. Although photoreceptor cells contain a large number of oxidative phosphorylation enzymes and mitochondria, they consume most of their glucose in glycolysis first[15]. In photoreceptor cells, much of the glucose consumed is metabolized not by oxidative phosphorylation but by aerobic glycolysis[14].When oxygen is reduced, it can be observed that glycolysis significantly increases Na+transport in compensatory dark current, but when oxygen is completely deprived, the disappearance of light-induced nerve impulses in retinal nerve cells other than photoreceptors can be observed[23]. Blocking aerobic glycolysis for further compensation in a mouse model of glaucoma revealed that the axonal mitochondria of the optic nerve could not maintain the function of the cells because of its efficient ATP production, although it could make up for the high energy demands of some cells[68]. It has also been reported that aerobic glycolysis in cone and rod cells is not a necessary metabolic option for cell survival, but the function of rod cells is affected[69]. A study of type 2 diabetes has shown a significant association between metabolic disorders associated with increased lactic acid and decreased vision[70]. Age-related degeneration of the optic nerve was accelerated by glycolysis deficiency and endoplasmic reticulum unfolded protein effect in photoreceptor cells, bipolar cells and retinal ganglion cell cells in a mouse model[71]. The results showed that the increase of glycolysis, photoreceptor cell death, and Wnt signal activation during DR proliferation promote the survival of retinal cells[72].
Aerobic glycolysis was found in photoreceptor cells to have the same protective effect as endothelial cells against oxidative stress, Wnt/β-catenin signaling pathway protects photoreceptor cells by stimulating P13K/Akt signaling pathway to activate glycolytic key the enzyme of HIF-1α[73].The lack of hexokinase, the first rate-limiting enzyme in glycolysis, is significantly associated with neurodevelopmental disorder and visual impairment[74]. Hexokinase-mediated aerobic glycolysis is essential to maintain the function of photoreceptor cells. Long-term inhibition of aerobic glycolysis leads to photoreceptor cell degeneration[15]. Pyruvate kinase is one of the key enzymes in glycolysis, and the pyruvate kinase M2 subtype regulates visual function by regulating phosphodiesterase 6β, in mice with the pyruvate kinase M2 subtype knocked out, the decrease in both pyruvate kinase M2 and phosphodiesterase 6β was accompanied by a decrease in rod cell function, whereas in mice with pyruvate kinase M2 subtype enhanced, the reduced/oxidized redox ratio reduced[75]. Also, the absence of pyruvate kinase M2 leads to a reduced glycolysis rate, and the segments of cone cells lacking pyruvate kinase M2 are significantly shorter than those of normal cone cells[76]. Mitochondrial pyruvate carriers link glycolysis and mitochondrial metabolism, and the absence of mitochondrial pyruvate carriers in the retina leads to the loss of function of cone and rod cells in the retina, which leads to the loss of vision[77]. The binding of glucose with GLUT1 induces the increase of glucose in the cone cells. The cone active factor is a protective factor secreted by the cone cells and has a protective effect on them, cone-active factors act by binding to base proteins, promote the survival of cone cells by increasing aerobic glycolysis, and are also found to supplement glucose in rod cells[78-80].
The integrity of retinal nerve cells is related to vision.Photoreceptor cells produce ATP mainly through aerobic glycolysis. The decrease of aerobic glycolysis leads to the decline of photoreceptor cell function and impaired vision,therefore, aerobic glycolysis of stable photoreceptor cells provides a reliable target for delaying vision loss in disease states.
ACKNOWLEDGEMENTS
Conflicts of Interest: Yang TT, None; Li H, None; Dong LJ,None.
 International Journal of Ophthalmology2021年9期
International Journal of Ophthalmology2021年9期
- International Journal of Ophthalmology的其它文章
- Five-year results of refractive outcomes and visionrelated quality of life after SMlLE for the correction of high myopia
- One-step viscoelastic agent technique for lCL V4c implantation for myopia
- miRNA-26b suppresses the TGF-β2-induced progression of HLE-B3 cells via the PI3K/Akt pathway
- Pediatric ocular trauma with pars plana vitrectomy in Southwest of China: clinical characteristics and outcomes
- Socio-economic disparity in visual impairment from cataract
- Disseminated hydatid disease in the orbit and central nervous system
