Characteristics of MSWI fly ash with acid leaching treatment
CAO Yi-nan,LUO Jin-jing,SUN Shi-qiang
(College of the Environment &Ecology, Xiamen University, Xiamen 361105, China)
Abstract:The chemical and mineralogical characteristics of fly ash from a municipal solid waste incineration (MSWI) in China and the influence of processing parameters on heavy metals removal during leaching were investigated in this work.The fly ash particles had complex surface structure with limited specific surface area.The alkali chloride and metal salts in MSWI fly ash showed evidently impact on leaching efficiency.Metal leachability was related to their properties and speciation in fly ash.Water-soluble salts such as KCl,NaCl and CaCl2 in fly ash were easily washed out.In this study,removal efficiency by water washing was achieved to 93.1% for Cl,41.4% for Na,48.5% for K and 24.8% for Ca,respectively.Mineralogical analysis also revealed change of fly ash mineral phases and specification distribution after water washing.Under liquid to solid ratio of 40∶1 L/kg and treatment time of 120 min,the leaching process achieved high dropping yields of toxicity characteristic leaching procedure (TCLP) concentrations for Cu,Zn Cd and Pb (80%-100%),moderate dropping yields for As (30%-80%) and relatively low dropping yields of Ni (< 30%).In addition,heavy metals such as Pb and Zn in fly ash with twice water washing treatment at a low liquid-solid ratio could reach lower TCLP concentrations.The result indicated that the combination process of twice water washing and one acid washing could significantly reduce the environmental risk of MSWI fly ash.
Key words:MSWI fly ash;characteristic;heavy metals removal;water-flushing pretreatment;chemical leaching
The large amount of municipal solid waste(MSW) generating is considered as a global environmental issue.For those countries with high population density and limited landfilling space,the disposal of MSW leads to serious environment problems[1].Incineration process is an effective MSW disposal method and is widely employed in the world.During the incineration,MSW is burned with the emission of NOx,SOx,HCl,CO,CO2,H2O,etc.[2].It can decrease 90% volume and 70% mass of original MSW[3].
Complex constitutes of MSW determine its complex components of municipal solid waste incineration (MSWI) fly ash.Heavy metals in MSW accumulate in the combustion residues after burning process because of efficient mass and volume dropping.Some kinds of heavy metals such as Pb,As,Cd and Cr in fly ash are considered as the priority substance due to their persistence,bioaccumulation and toxicity.High temperature environment during incineration could transform organic compounds into inorganic compounds.Therefore,the source and formation of heavy metals in fly ash are complicated and varied.The existing forms of heavy metals in fly ash are affected by flue gas temperature,components,air mass and so on[4].In general,fly ash particles with small size have higher environmental risk due to the fact that they have larger specific surface area to accumulate toxic substance compared to those with large size[5].In this way,the heavy metals accumulated on the surface of fly ash could be easily leached out and drain into nature environment by raining,which resulted in groundwater or soil contamination.MSWI fly ash has been classified as hazardous substance because of its high environmental risk.
Generally,current fly ash treatment methods can be categorized into three groups: solidification/stabilization,heavy metal separation and recycling[6-8].In recent years,a number of researches focus on solidification/ stabilization,such as sintering and vitrification.Bie et al[9]found cement solidification could bind heavy metals in the hydration structure and the leaching concentrations of heavy metals in fly ashcement compound could reach a relatively low level under alkalescent condition.Czop et al[10]found immobilization of dangerous compounds through the C-S-H phase of concrete significantly reduced the migration of dangerous substance into environment and minimized its toxicity.Zhang et al[11]adopted sulfur oxidizing bacteria to recover sulfur in MSWI fly ash and toxicity characteristic leaching procedure (TCLP)concentrations of heavy metals decreased.Usually,the fly ash is reused as building materials after treatment.However,the chloride and heavy metals are still in fly ash and become barriers in further application of recycled materials.Before reusage of fly ash,there must be pre-treatment to lower its environmental risk and fulfill the industrial requirement[12].Therefore,the elimination of some harmful components such as heavy metal and chlorine compounds is a crucial step of recycling disposal.It has been found that most of the environmental constraints regarding MSWI fly ash applications are related to the leaching behavior of the final products.Compared to storage underground,the leaching of heavy metals by chemical solution is a more economical and land resource saving method.Shi et al[13]adopted various testing methods such as XRD and SEM to analyze the effects on leaching efficiency of fly ash attribution.Saqib et al[14]found water and acid leaching could remove most types of heavy metal in fly ash.Funari et al[15]used acid leaching to remove heavy metal and obtained high leaching efficiency for a number of elements in fly ash.
This study focused on investigating the chemical and mineralogical characteristics of fly ash including the structure of fly ash surface,components,chemical functional groups,soluble alkali materials in fly ash and the components variation during water-extraction process.TCLP was adopted to assess samples environmental risk.By evaluating environmental risk of fly ash after water-extraction and acid washing process,we can have a better understanding about how treatment methods affect heavy metals in fly ash and reduce its environmental risk.The mechanism of heavy metal removal was analyzed according to the detailed characteristic research.
1 Materials and methods
1.1 Materials
The raw fly ash in this study was collected from a MSWI plant in Fujian province,China.The solid waste that was burned contained kitchen garbage,wood and plastic solid waste which were primarily categorized.The treatment procedure of MSWI system was semidry process which achieved flue gas deacidification.The flue gas flowed through spray tower which led the temperature drop to 50-60 ℃ and the acid stripping tower where lime was sprayed into and reacted with the acid components.After that,flue gas went through carbon injecting system.Finally,the fly ash was captured by bag filters.The sample was taken from the fly ash silo.The origin sample noted as RA was grey with visual observation and the particle size varied.In order to remove water in fly ash,the original sample was dried in oven at a suitable temperature (105 ℃) for 24 h before being tested in following examination.
1.2 Analytical apparatus and methods
1.2.1 Characterization
Scanning electron microscopy with energydispersive spectrometry (SEM-EDS,JSM-6390LV)was used to identify phases of compounds on the surface and element constitutes.The sample with KBr mixed in a tablet was detected by Fourier transform infrared spectroscopy (FT-IR,NEXUS 470) in the 4000-400 cm-1range.The particle size was determined by Laser Particle Size Analyzer (MASTERSIZER 2000)and the pore size distribution was measured at 77 K by nitrogen adsorption analyzer (ASAP2020).The fly ash sample was examined by X-ray powder diffraction(XRD,D8 DISCOVER,CuKα radiation,30 kV,15 mA)with scans conducted from 10° to 70° at a rate of 10(°)/min.The 10∶1 liquid-solid ratio (L/S) and 10 min washing time were adopted to examine the soluble alkaline material in RA.The solid residue and the liquid were separated by air pressure filtration and 0.45 μm glass-fiber filters.After separation,the liquid sample was tested by pH value meter (MP512-01) and the dried fly ash sample was used for following treatment.
1.2.2 Environmental risk assessment
According to TCLP from HJ/T300-2007,the matching extraction agent was chosen to operate leaching experiment.A 20∶1 liquid-solid ratio (L/S)and 18 h toxicity characteristic leaching time were used.The rotation speed was 110 r/min at 23 ℃.The fly ash and the extraction agent were separated by air pressure filtration and 0.45 μm glass-fiber filters.After separation,the liquid was collected and preserved at 4 ℃.20 mL extraction liquid sample and 5 mL concentrated nitric acid were mixed in test tubes to digest liquid sample.Then the extraction agents were cooled down to room temperature.Finally,several elements were examined in the leachate (Cr,Ni,Zn,Cu,As,Cd,Pb)by inductively coupled plasma (ICP-MS,Agilent 7900).
1.2.3 Pre-treatment methods
In order to remove water-soluble salts,the raw fly ash was treated with following two steps and termed as WA.The pre-treatment methods include.
Step 1:the fly ash sample was mixed with deionized water at room temperature with the 2∶1 liquid-solid (L/kg) ratio and rotation speed of 200 r/min for 1 h.Then the solid phase and the water phase were separated by a vacuum pump filter.After separation,the sample was dried at 105 ℃ for more than 24 h.
Step 2:the pretreated fly ash sample from step 1 was mixed with deionized water at room temperature with the 10∶1 liquid-solid (L/kg) ratio for 10 min.The mixture was stirred and mixed fully.After washing process,the mixture is separated and dried with the same condition of step 1.
1.2.4 Chemical leaching
The mixed extractant of 0.2 mol/L HCl and 0.06 mol/L H2SO4simulating solvents of incineration flue gas dissolved in water were chosen to treat fly ash and the treatment time and solid-liquid ratio were set as variation.In this way,the cost could be reduced to some extent after industrial application.Rotation speed of 150 r/min and room temperature were set,since temperature has little influence on leaching efficiency in acid leaching of MSWI fly ash.The solid residue and acid solvent were separated by air pressure filtration.After separation,the treated samples were dried at 105 ℃ for more than 24 h and used for TCLP to assess its environmental risk.
The RA sample was put into deionized water at room temperature with the 10∶1 liquid-solid (L/kg)ratio for 10 min.Then the mixture was separated and dried.The leaching time was set as 15,30,60,120 and 240 min with the 10∶1 liquid (hydrochloric and sulfuric acid) -solid (L/kg) ratio.In other cases,the leaching ratio was set as 10∶1,20∶1,30∶1,40∶1,50∶1,60∶1(L/kg) and 120 min treatment time was chosen to evaluate their influences on TCLP concentration of fly ash.
The sample treated by water washing with 10∶1 liquid-solid (L/kg) ratio for 10 min and the leaching process was noted as LA.The WA treated by leaching process was noted as WLA.A 40∶1 liquid-solid (L/kg)ratio was adopted with 120 min treatment time during leaching process.The sample preparation procedure is shown in Figure 1.

Figure 1 Flow chart of samples preparation
The samples were tested by TCLP to evaluate heavy metal removal efficiency and environmental risk.Higher heavy metal leaching value indicates lower environmental safety.
1.2.5 Loss on ignition
In order to examine the mass of organic matter in raw fly ash sample,loss on ignition was measured in triplicate by furnace set at (950 ± 25) ℃ for 15-20 min.Before weighing,the sample was dried and cooled down to room temperature.The sample was burnt repeatedly until the sample’s weight was constant.
2 Results and discussion
2.1 Characterization of the RA and WA
2.1.1 Soluble alkali and particle size of ash
Liquid was separated from the water extraction with 10∶1 liquid-solid (L/kg) ratio of RA fly ash and was measured directly.This liquid-solid (L/kg) ratio was enough to dissolve most water-soluble salts include soluble alkali in fly ash[15].The pH value of separated liquid was 11.29 which demonstrated that soluble alkali in fly ash could create alkaline circumstance in solution.It was caused by the injection of lime in the MSWI flue gas treatment system during the incineration process for removing the acidic gas in it[16].In addition,the process of solid waste incineration was a violent oxidation process which generated different metal oxides by chemical reactions.The flue gas contained water gas because of spray tower.Some water in flue gas reacted with metal oxides to generate alkali such as Ca(OH)2and NaOH[17].Therefore,fly ash contained different kinds of soluble alkaline compounds.The acid neutralization ability of fly ash resulted from these materials and some metal oxidations[18].The particle size distribution of RA sample is presented in Figure 2.It indicated that more than 70% of fly ash particles from RA had small size under 200 μm and the average particle size was 138 μm.In general,there were few particles with diameter higher than 200 μm.
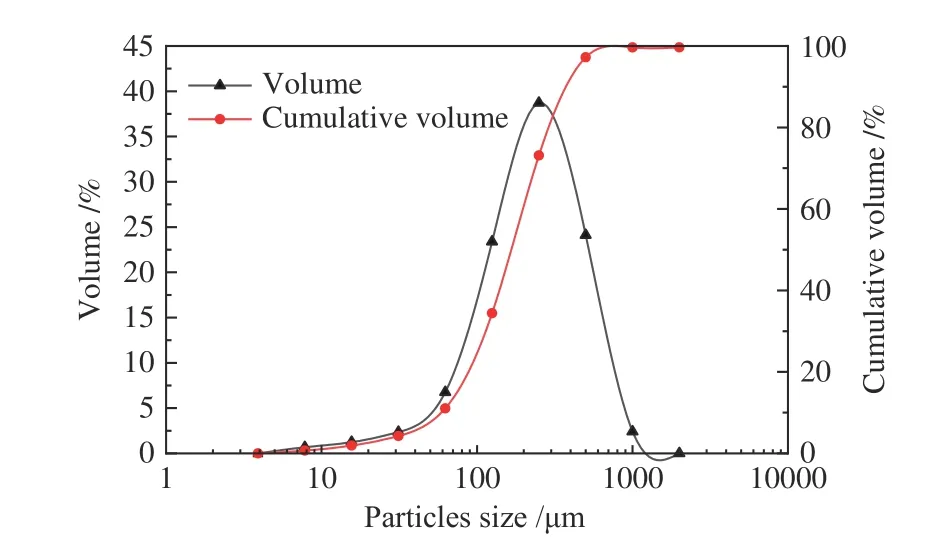
Figure 2 Particle size distribution of the RA sample
The particles with large size tended to have greater quality,which led to the deposition in the incinerator or the pipes of flue treatment system.On the contrary,the small sized particles with less quality were able to reach bag filter with flue gas and then be captured[19].When particles arrived at filter bags,the pressure and water gas resulted in agglomeration.Moreover,the flocculent matter on the sample was also a factor in this phenomenon[20].
2.1.2 Elements constitute of the RA and WA samples
Some element constitutes of raw fly ash (RA) and sample treated after water-washing process (WA) are shown in Table 1.Incineration was an oxidation process[21].It generated inorganic oxide with a factor of 37.62% and 39.98% O in RA and WA samples,respectively.In addition,the injection of lime in MSWI flue gas treatment system after incineration process for removing the acidic gas evidently enhanced O element in samples[16].The relatively abundant Ca in fly ash was due to lime injecting treatment system.As for Cl,it was directly related to the citizens’ life and eating habits.During incineration process,the polyvinyl chloride (PVC) in plastics and NaCl in kitchen garbage was released in flue gas because of high temperature[1].The high levels of chlorine resulted in the formation of HCl.Water cooling process enhanced the absorption sites of chloride as well[20].Evaporable Cl was captured by fly ash and reacted with CaO.These factors accounted for high proportion of Cl in fly ash.

Table 1 Main elements constitute of the RA and WA samples
Other elements in RA such as Si,Na,S,Ca and K resulted from their attribution and composition of original municipal solid waste.It was accepted that Ca and K was easily oxidized at room temperature and more violent in incineration with much higher temperature.The reaction products were accumulated into fly ash particles and preserved in them.The oxidization state of Si had stable chemical properties and thus became the matrix of fly ash particles.Water washing process was unable to leach them out or change their chemical formation.As a result,these kinds of elements presented increasing inclination after some soluble compounds being leached out by water washing treatment.
Chlorine compounds in fly ash was easily extracted out by water washing[22]and significantly reduced Cl content from 10.78% to 0.74%.The decline content of element Na,Ca and K from 2.2%,36.82%and 2.27% to 1.29%,27.68% and 1.17% demonstrated that water washing process removed NaCl,CaCl2and KCl in fly ash.However,most of substances in fly ash were in inorganic oxidation states and insoluble or slightly soluble compounds such as CaSO4and CaCO3.Therefore,element Ca displayed slightly reduction.
2.1.3 Mineral phases characterization
Loss on ignition of RA sample was 5.2%.After burning in municipal solid incineration systems,most organic matters in municipal solid waste were transformed into inorganic substances.Therefore,the unburned carbon and some chemical reactions factored into this result.
We recorded the FT-IR spectra range from 400 to 4000 cm-1of RA in Figure 3.The FT-IR curve indicated the presence of inorganic chemical groups in raw fly ash including carbonate (1415,680 and 879 cm-1) and sulfate (1105 and 611 cm-1).Some other minor chemical bonds such as Si-O (1150 cm-1) and O-Si-O (594 cm-1) were also detected in RA sample.It indicated that there were silicone-containing compounds such as SiO2andin fly ash[23].The XRD results are shown in Figure 4.The complex mineralogy in fly ash was due to substance variation in fly ash like condensation,vitrification,precipitation and melting during incineration operation and flue gas treatment[24].
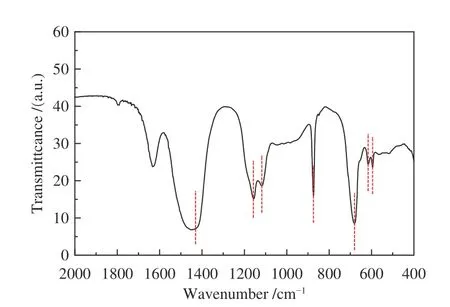
Figure 3 FT-IR spectrum of RA sample

Figure 4 XRD patterns of RA and WA sample
XRD analysis results of WA and RA indicated the mineralogical composition of the two samples were different.The main mineralogical phases in RA sample were calcium carbonate,gypsum,potassium chloride and sodium chloride.This result of metal species analysis was consistent with the EDS and FT-IR(Figure 3) results.It is believed that the raw fly ash contained various compounds containing calcium,chlorine,sodium,potassium and silicon.The formation of these elements in RA sample was affected by incinerator temperature and gas treatment technique.The silicon dioxide or silicone-containing matrix became fly ash core.Calcium crystal phase and chlorine compounds in RA agglomerated together to form fly ash surface.And element Ca in fly ash came from lime injection process.The chemical reactions are shown as follows[12,25]:
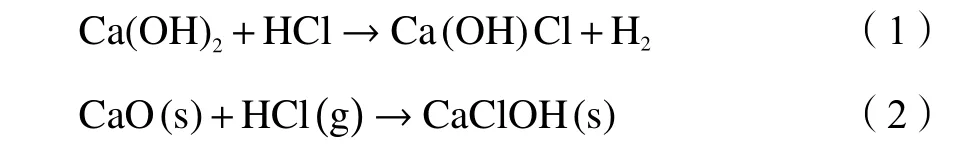
The chlorine-containing compounds in fly ash was due to volatilization of KCl and NaCl at high temperature and the accumulation on the surface of particles[26].Other compounds were not detected due to their trace quantity or the signal peaks overlaps.
The XRD patterns of WA indicated that there was a significant change in composition of sample after water washing process.Compared with RA sample,there were less types of compounds in WA.In particular,it is believed that the disappearance of NaCl,KCl and Ca(OH)Cl was due to the fact that these compounds were dissolved in water and removed by pre-treatment.Some materials of them were able to consume acidity and reduce the heavy metal removal efficiency during the leaching process.The water washing pre-treatment,obviously,was necessary before leaching to achieve a high metal removal efficiency.And it was plausible that there were some chemical actions happened[18].However,CaCO3,CaSO4and SiO2still existed because they were poorly soluble and even insoluble in water.
2.1.4 Surface characteristic of fly ash
The SEM images of RA are presented in Figure 5((a) and (b)).It indicates that the surface structure of fly ash was complex and there were some irregular substances existing.It is plausible to conclude that the diversity and complexity of garbage components together with violent reactions resulted from high temperature in incinerator led to the complex surface structure and material composition[22].
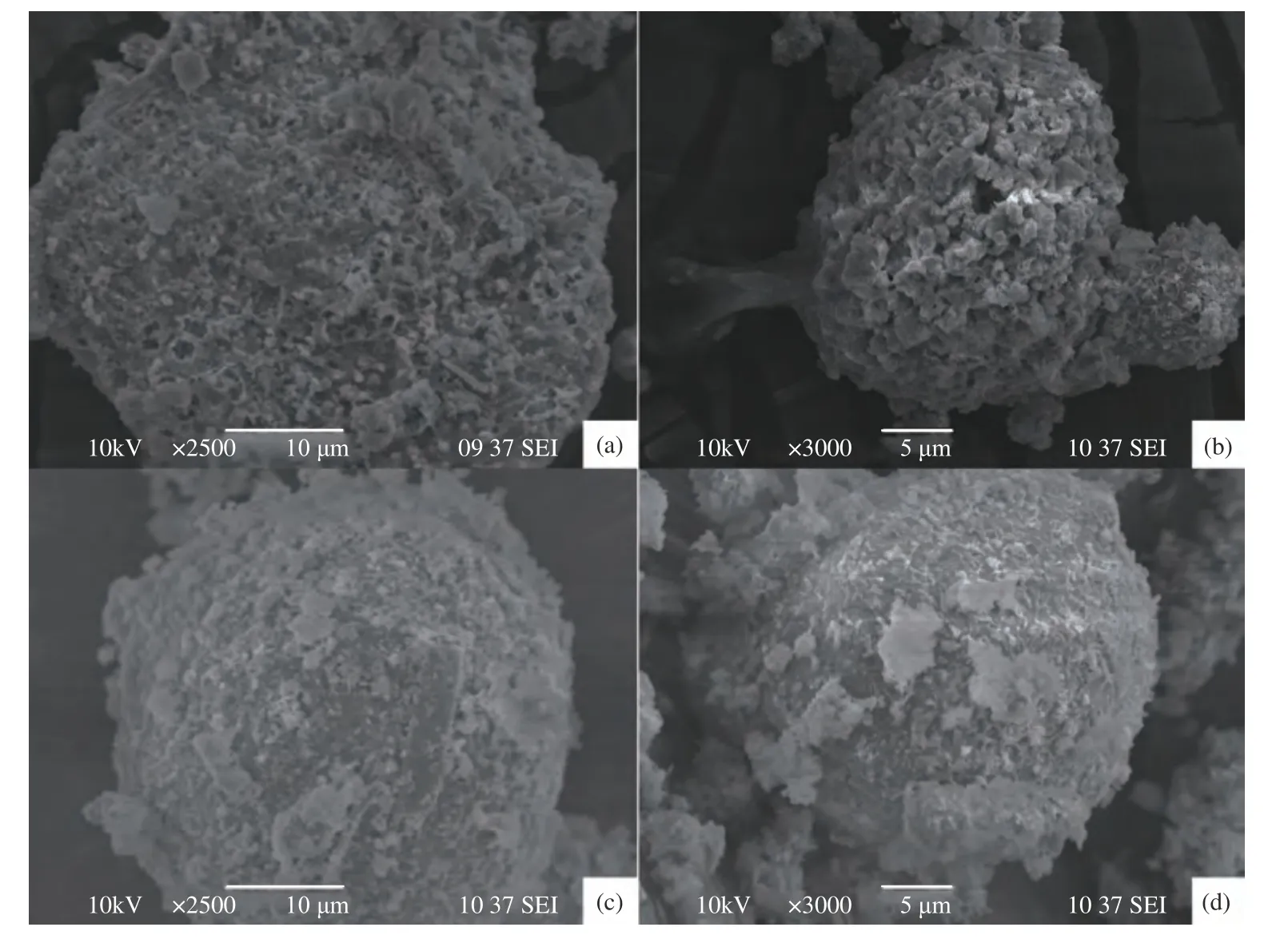
Figure 5 Scanning electron micrographs of RA ((a),(b)) and WA ((c),(d))
The substances on RA surface combined in random and made the shapes of fly ash particles become irregular.The size of fly ash particles varied greatly from a single sphere to multiple combining spheres[27].According to SEM images,we can see that fly ash surface was dominated by needle crystal,spherical particles and many irregular agglomerates,which resulted in an increase of particle size.The different crystals had various shapes and were determined as different minerals[28].
Figure 5 ((c) and (d)) showed the surface morphologies of WA sample.Compared with the SEM images of RA,there were less spherical particles existing.It indicates that spherical particles might be composed of complex compound salts of calcium,magnesium,aluminum,sodium and potassium,which were mainly soluble species.These substances were efficiently removed during process.On the other hand,the acicular crystal might be metal salt hydrates or combination of amorphous glassy and polycrystalline substances which were not soluble or slightly soluble in water.
2.1.5 N2 adsorption
The specific surface area of RA was 6.84 m2/g,the total-pore volume was 0.02 cm3/g and the average pore size was 12.21 nm.The N2adsorption-desorption isotherm and pore-size distribution of RA sample are shown in Figure 6.The pores of RA were classified as mesoporous pore whose distribution is between 10-30 nm.The hysteresis loop appeared atp/p0=0.4-1 was resulted from capillary condensation effect.By this way,nitrogen molecules condensed and blocked mesoporous channel under ordinary pressure[29].The well closure of curve indicated that there were no organic functional groups causing irreversible adsorption or the so-called chemical adsorption.

Figure 6 Nitrogen adsorption/desorption isotherms and the corresponding pore-size distribution of RA sample
The low temperature nitrogen adsorption curve was categorized in type IV isotherm and H3 hysteresis loop.It represented that the mesoporous pores of fly ash surface were considered as slot pores formed by flake particles accumulation under high temperature or pressure.The properties of adsorption equilibrium and adsorption dynamics may affect pore size distribution of material.The uneven structure and pore size distribution of material were mostly determined by this factor[30].The pore size distribution of RA sample demonstrates that the diameters of most pores on the fly ash surface were nearly 2 and 50 nm.Although the large pores also accounted for fairly high proportion the pore volume,the number of them was not very high.
2.2 Chemical leaching
2.2.1 Leaching behavior of heavy metals
The TCLP results of RA sample with 15,30,60,120,240 min leaching time are presented in Figure 7 ((a),(b),(c)).The amount of heavy metal leached out from fly ash tended to be stable with treatment time increasing.The TCLP concentrations of As,Pb and Cr approached minimum concentration when the leaching time was more than 60 min.After 60 min,the TCLP concentration was no longer significantly reduced.However,the better removal efficiency was obtained with setting treatment time as 120 min for Zn,Cd and Cu.It may be because that longer treatment time was needed for reaction equilibrium of Zn,Cd and Cu.The TCLP concentration of Zn showed a slightly increase at leaching time of 240 min.It may result from amphoteric attribution[31].The solubility of Zn as pH value presented a U-shaped curve and reached a minimum with pH value ranging from 8 to 10.With leaching time increasing,the acidity of solution became weaker due to the consumption of hydrochloric and sulfuric acid in reacting system.And it is possible to result in rising of TCLP concentration of Zn[32].The dissolution of Zn was also affected by the surface pore structure of the fly ash and mutual interaction with other ions.These factors led to the dissolved Zn entering the fly ash again and the increase of its TCLP concentration.
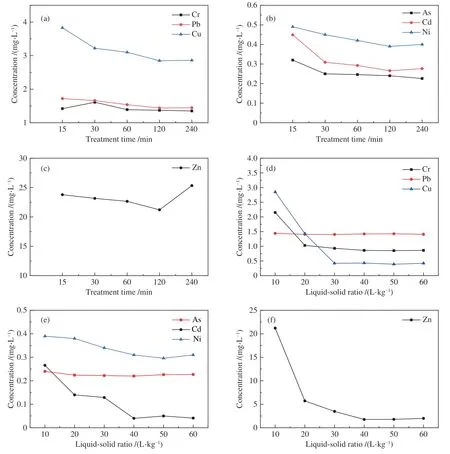
Figure 7 Change in toxicity characteristic leaching procedure concentrations of samples as treatment time and leaching ratio during leaching process
2.2.2 Effect of leaching ratio
The TCLP results of RA sample with 10∶1,20∶1,30∶1,40∶1,50∶1 and 60∶1(L/kg) leaching ratio are presented in Figure 7((d),(e),(f)).The curve showed that TCLP concentrations of Cd,Cr,Cu,Ni decreased consistently as increasing of liquid to solid ratio.It indicated that Cd,Cr,Cu,Ni could be leached out more with low pH condition and expressed typical cationic pattern.As is categorized as oxidational metal and the amount of it leached out varied little under acidic condition[33].
Zn is amphoteric metal which has lower removal efficiency approaching neutral condition.Although Pb is classified in cationic metal,the amount of it leached out was determined byand fairly amount of Pb remained in fly ash after leaching process.The chemical reactions are shown as follows:
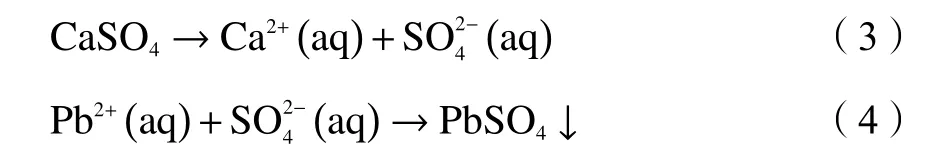
There were different types of alkaline substance and carbonate were removed during water washing process.But the acid neutralization capacity of fly ash still existed.The leaching ratio variation changed the acidity of the reacting system and it determined the amount of heavy metal leached out from fly ash.Without enough liquid-solid (L/kg) ratio,the pH value of the reacting system was not substantially changed.Hence,the TCLP concentrations were not affected significantly.And the consumption of acid led to the neutral environment of solution,which made heavy metals remain in fly ash.On the contrary,reacting system with higher liquid-solid (L/kg) ratio converted the stable form of heavy metals into exchangeable fraction.With the ratio increase,the mass of hydrochloric and sulfuric acid in mixture increased.In this way,the pH value of reaction system was remarkably changed.Then the heavy metal was removed from fly ash efficiently.
2.3 Toxicity characteristic leaching tests of samples
The TCLP concentrations of targeted heavy metals for RA,LA and WLA sample are shown in Table 2.
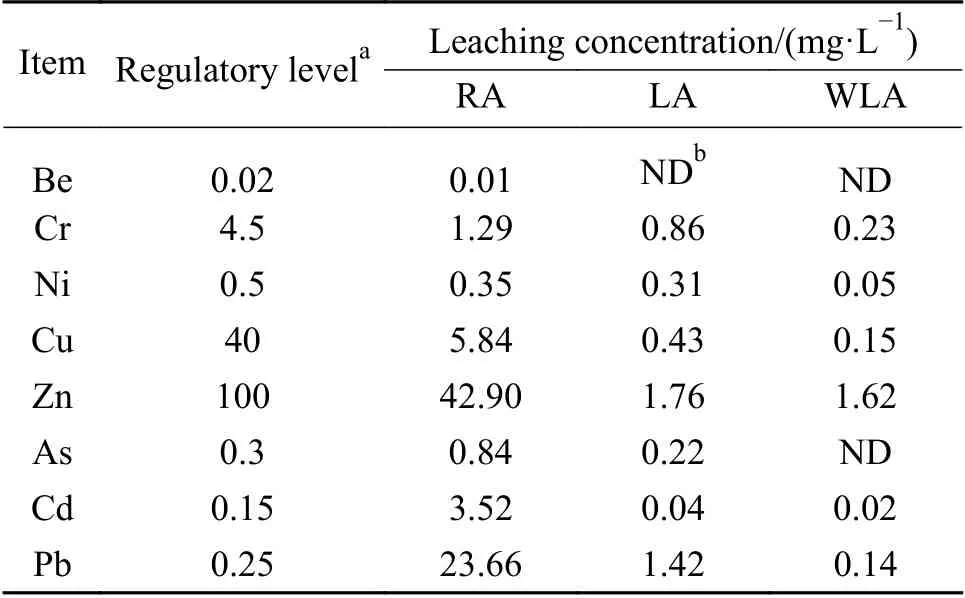
Table 2 TCPL concentration of targeted metals detected in RA,LA and WLA samples by HJ/T300-2007
Fly ash surface was uneven and it was easily for the heavy metal to accumulate.The harmful materials were adsorbed during the process of burning and transportation with flue gas in the incineration systems.Therefore,As,Cd and Pb presented higher TCLP concentrations in RA than regulatory level.Compared with RA sample,the TCLP concentrations dropping of LA sample was significant.Leaching process showed relatively high dropping yields of TCLP concentration for Cu,Zn Cd and Pb (80%-100%),moderate dropping yields for As (30%-80%) and relatively low dropping yields of Ni (< 30%).This result indicated that the leaching process with suitable leaching time and liquidsolid (L/kg) ratio efficiently removed heavy metal in fly ash and remarkably reduced fly ash environmental risk.
On a comparative basis,it was found that the higher TCLP concentrations dropping yields of heavy metals were achieved in twice water washing and chemical leaching.In the case of Pb and Zn,it arrived the higher TCLP concentrations dropping yields.By this treatment,about 99% and 96% TCLP concentrations dropping yield of Pb and Zn were achieved.Especially,TCLP concentration of Pb exceeding the current government regulatory threshold descended to a regulatory limit range.
The pre-treatment was essential for improving heavy metal removal efficiency.The strong acid buffering capacity and high pH value of MSWI fly ash resulted from the presence of carbonate and alkaline oxides[34].Therefore,we created an alkaline condition by controlling water washing ratio.Some heavy metals were washed out easily in alkaline condition such as Pb whose leaching process was affected by.And 2∶1 liquid-solid (L/kg) ratio for 10 min during waterwashing treatment created a strong alkaline condition with pH=13.24.It was able to remove partial heavy metal in fly ash.In addition,calcium sulfate was hard to dissolve in liquid during pre-treatment process.As a result,there was little PbSO4generating.
By using alkali leaching,a part of Pb and some Zn in fly ash was leached out[35].In the solution with pH value more than 12,the removal of Pb showed an increasing tendency.It was plausible to illustrate that the Pb in fly ash transformed into Pb(OH)nwhich was soluble under strong alkaline condition.In addition,some other heavy metals in fly ash might transform from oxidation form into carbonate form or exchangeable form in strong alkaline solution[36].This process promoted removal efficiency during leaching process.Therefore,WLA performed a better heavy metal removal efficiency and lower environmental risk than LA sample.
3 Conclusions
The fly ash sample from incinerator of municipal solid waste in Fujian Province,China was investigated.Sample’ s determination of the proportions and morphological characteristics were analyzed by combination of analytical methods.It was treated by leaching in this research.The results can be illustrated as following:
The raw fly ash was characterized by different elements of O,Ca,Cl,Si,K,Na and S.It was caused by burning process and ingredients in MSW.The main mineralogical phases of the raw fly ash were SiO2,CaCO3,CaSO4,NaCl and KCl.They were considered as the rapid formation and cooling processes taking place during incineration.Raw fly ash had small size and strong acid neutralization ability.
Water washing could effectively remove chlorides and some heavy metals such as Pb and Zn from MSWI fly ash.In this way,Ca,Na,K and Cl were removed by 24.8%-93.1% because most compounds containing these elements are freely soluble.Water washing at L/S=2∶1 remarkably changed the chemical components of fly ash which transformed some metal from oxidation form into exchangeable form.But it was difficult to remove Si,S,Ca and O in fly ash by water.
It was found that leaching process combined with water washing is an effective way to reduce MSWI fly ash environmental risk.The results indicated that the TCLP concentrations of most heavy metals in the fly ash decreased with the increasing leaching ratio or time.The low TCLP concentrations factors for heavy metal were due to a combination of more acid attack at a higher L/S ratio,a more extensive reaction due to a higher equilibrium during leaching process.The extraction of Pb was affected byin reaction system.And higher TCLP concentration dropping of heavy metal (99% for Pb,96% for Zn) was achieved by combination of alkaline and acid leaching.This treatment could remarkably reduce fly ash environmental risk and could be considered as a pretreatment of fly ash reusage.
Acknowledgements
Authors are thankful to Xiamen Environmental Monitoring Station for analytical equipment support.

