基于PI3K/Akt/mTOR信号通路的三七总皂苷调控自噬抗H9c2细胞缺氧/复氧损伤机制研究
王飘,郑晴,胡婷,张海银,许言午,包怡敏
基于PI3K/Akt/mTOR信号通路的三七总皂苷调控自噬抗H9c2细胞缺氧/复氧损伤机制研究
王飘,郑晴,胡婷,张海银,许言午,包怡敏
上海中医药大学基础医学院,上海 201203
探讨三七总皂苷(PNS)能否通过PI3K/Akt/mTOR信号通路调控自噬从而减轻H9c2细胞缺氧/复氧损伤机制。将H9c2细胞分为正常组、缺氧/复氧(A/R)组、A/R+PNS组、A/R+PNS+雷帕霉素(Rapa)组、A/R+Rapa组、A/R+羟氯喹(HCQ)组、A/R+3-甲基腺嘌呤(3-MA)组。通过缺氧6 h、复氧2 h建立H9c2细胞A/R模型。CCK-8法检测细胞存活率,乳酸脱氢酶(LDH)检测试剂盒检测细胞损伤,Western blot检测自噬相关蛋白Atg5、p62、Beclin-1、LC3Ⅱ/LC3Ⅰ及PI3K/Akt/mTOR信号通路相关蛋白PI3K、Akt、mTOR的表达,荧光法观察细胞自噬流。与正常组比较,A/R组H9c2细胞存活率明显下降,细胞损伤率明显升高(<0.05),自噬相关蛋白Atg5、Beclin-1表达和LC3Ⅱ/LC3Ⅰ比值明显升高(<0.05),自噬体和自噬溶酶体数量显著增加,自噬流显著增强(<0.05);与A/R组比较,用PNS预处理后,H9c2细胞存活率明显上升,细胞损伤率明显降低(<0.05),自噬相关蛋白Atg5、Beclin-1表达和LC3Ⅱ/LC3Ⅰ比值明显降低(<0.05),p62蛋白表达明显升高,与自噬抑制剂HCQ、3-MA作用相似,荧光结果显示,PNS对自噬流有明显抑制作用(<0.05);PNS预处理后p-PI3K、p-Akt、p-mTOR蛋白表达明显升高(<0.05)。PNS能有效减轻H9c2细胞A/R损伤,其机制与激活PI3K/Akt/mTOR信号通路从而抑制自噬流相关。
三七总皂苷;缺氧/复氧损伤;自噬;PI3K/Akt/mTOR信号通路;H9c2细胞
缺血性心脏病发病率逐年上升,迄今已成为发病率及致死率最高的疾病之一[1]。虽通过药物或手术等手段可达到恢复血流灌注的目的[2],但恢复过程中可引起不可逆的心肌组织损伤,即心肌缺血再灌注损伤(myocardial ischemia-reperfusion injury,MIRI)[3]。目前研究表明,细胞自噬作为内源性调节机制参与到MIRI过程中[4],抑制过度自噬可减轻MIRI[5]。研究发现,激活PI3K/Akt/mTOR信号通路可抑制自噬并减少细胞氧化应激的发生[6-7]。中药毒副作用小、安全性高,对MIRI防治效果较好。现代药理研究发现,三七主要成分三七总皂苷(Panax notoginseng saponins,PNS)能通过抗氧化应激、抗凋亡等途径减轻MIRI[8-9],但其能否通过调节自噬减轻MIRI仍不清楚。因此,本研究通过观察PNS对缺氧/复氧H9c2细胞的影响,运用自噬激动剂及自噬抑制剂等工具药,观察PNS对自噬流的作用,探讨PNS能否通过PI3K/Akt/mTOR信号通路调控自噬流,从而达到减轻MIRI的目的。
1 材料与方法
1.1 细胞株和药物
H9c2细胞,中国科学院上海细胞库。血栓通注射液(成分PNS),昆明制药集团股份有限公司,批号13HK03;自噬抑制剂3-甲基腺嘌呤(3-MA),MCE,批号HY-19312;自噬抑制剂羟氯喹(HCQ),Selleck,批号S4430;自噬激动剂雷帕霉素(Rapa),MCE,批号HY-10219。
1.2 主要试剂与仪器
CCK-8,上海翊圣生物科技有限公司,批号40203ES76;乳酸脱氢酶(LDH)检测试剂盒,同仁化学研究所,批号CK12;DAPRed,同仁化学研究所,批号D677;DALGreen,同仁化学研究所,批号D675;SQSTM1/p62(CST,货号5114s)、Beclin-1(CST,货号3495s)、LC3B/MAP1LC3B(Novus,货号NB100-2220)、Atg 5(CST,货号12994s)、Akt(CST,货号4691s)、p-Akt(CST,货号4060s)、PI3K(CST,货号4257S)、p-PI3K(Abcam,货号ab182651)、mTOR(CST,货号2983S)、p-mTOR(CST,货号5536S)抗体。全自动凝胶成像系统,上海天能公司,型号Tanon-2500;激光共聚焦显微镜,德国徕卡,型号TCS SP8;细胞培养箱,美国Thermo Scientific,型号class100;超净台,苏州净化设备公司,型号SW-CS-IFD。
1.3 分组、造模及给药
将H9c2细胞分为正常组、缺氧/复氧(A/R)组、A/R+PNS组、A/R+PNS+Rapa组、A/R+Rapa组、A/R+HCQ组、A/R+3-MA组。造模前H9c2细胞分别用含PNS(100 μg/mL)、Rapa(50 nmol/L)、HCQ(50 μmol/L)、3-MA(5 mmol/L)的培养液处理2 h。A/R模型制备时,细胞培养液更换为含/不含药物的无血清培养液,将培养皿置于缺氧条件下(95%N2和5%CO2)培养6 h,之后培养液更换为含/不含药物的正常培养液,置于37 ℃、5%CO2培养箱中继续培养2 h。正常组细胞正常条件下培养箱中培养8 h。
1.4 细胞存活率检测
将细胞按1×105个/孔铺于96孔板,另设只添加培养液的空白组。造模前PNS组根据药物浓度梯度给药2 h;缺氧前PNS组和A/R组分别更换为含/不含药物的无血清培养液;缺氧6 h后,更换为含/不含药物的正常培养液,37 ℃培养箱中培养2 h;2 h后每孔加入10 μL CCK-8,培养1 h,于酶标仪波长450 nm处检测OD值,计算细胞存活率。细胞存活率(%)=(模型组OD值-空白组OD值)÷(正常组OD值-空白组OD值)×100%。
1.5 细胞损伤率检测
根据LDH试剂盒说明书配制工作液。造模完成后,每孔加100 μL工作液,用铝箔纸包裹96孔板,避光室温反应30 min;反应结束后每孔加50 μL终止液,并于酶标仪波长490 nm处检测OD值,计算细胞损伤率。细胞损伤率(%)=(模型组OD值-空白组OD值)÷(正常组OD值-空白组OD值)×100%。
1.6 细胞自噬荧光检测
将细胞按2×105个/皿铺于激光共聚焦专用皿中,培养24 h;依次使用1 µmol/L红色荧光(DAPRed)和0.5 µmol/L绿色荧光(DALGreen)在37 ℃培养箱中孵育30 min[10];随后进行A/R造模,最后在相同条件下使用激光共聚焦显微镜进行荧光图片拍摄。自噬体被DAPRed染色,自噬溶酶体被DALGreen染色。根据红绿荧光强度判断细胞自噬体、自噬溶酶体数量和自噬流的变化。
1.7 Western blot检测
造模结束后收集细胞,RIPA裂解液裂解细胞,4 ℃、12 000 r/min离心15 min,取上清得细胞总蛋白;BCA法检测蛋白浓度。蛋白经SDS-PAGE凝胶电泳,转膜,5%BSA封闭1 h,加入稀释的一抗,4 ℃孵育过夜,二抗室温孵育1 h,ECL发光试剂显影蛋白条带,Image J软件测定蛋白条带灰度值。
1.8 统计学方法
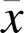
2 结果
2.1 三七总皂苷对模型细胞存活率及损伤率的影响
与正常组比较,A/R组H9c2细胞存活率明显降低(<0.05),细胞损伤率明显升高(<0.05);与A/R组比较,PNS浓度为100、200 μg/mL时,H9c2细胞存活率明显升高(<0.05);PNS浓度为50、100、200 μg/mL时,H9c2细胞损伤率明显降低(<0.05)。见图1、图2。综合细胞存活率及损伤率结果可以看出,浓度为100 μg/mL PNS减轻A/R损伤效果最显著。

注:与正常组比较,*P<0.05;与A/R组比较,#P<0.05
注:与正常组比较,*P<0.05;与A/R组比较,#P<0.05
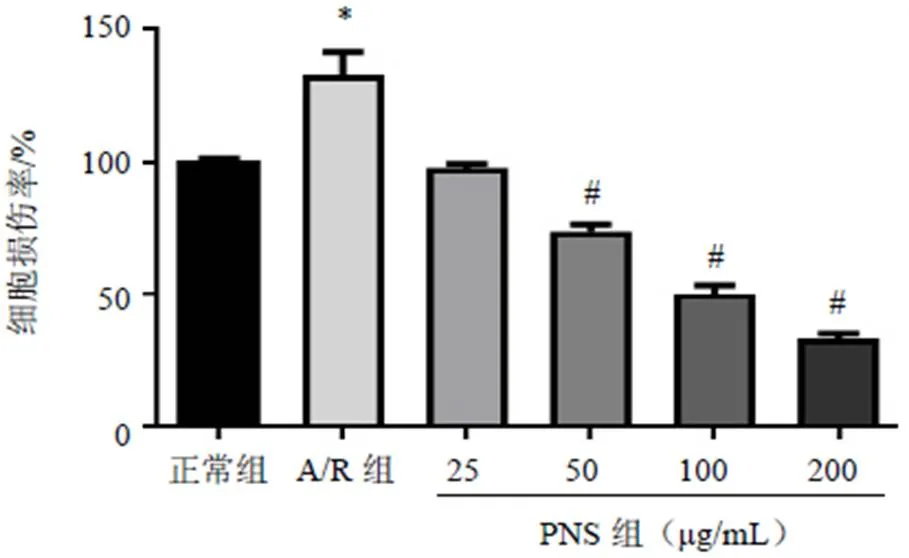
2.2 三七总皂苷对模型细胞PI3K/Akt/mTOR信号通路相关蛋白表达的影响
与正常组比较,A/R组H9c2细胞p-PI3K、p-Akt和p-mTOR蛋白表达差异均无统计学意义(>0.05);与A/R组比较,PNS预处理后,H9c2细胞p-PI3K、p-Akt和p-mTOR蛋白表达明显升高,差异有统计学意义(<0.05),见图3。
注:A.正常组;B. A/R组;C. A/R+PNS组;与A/R组比较;#P<0.05
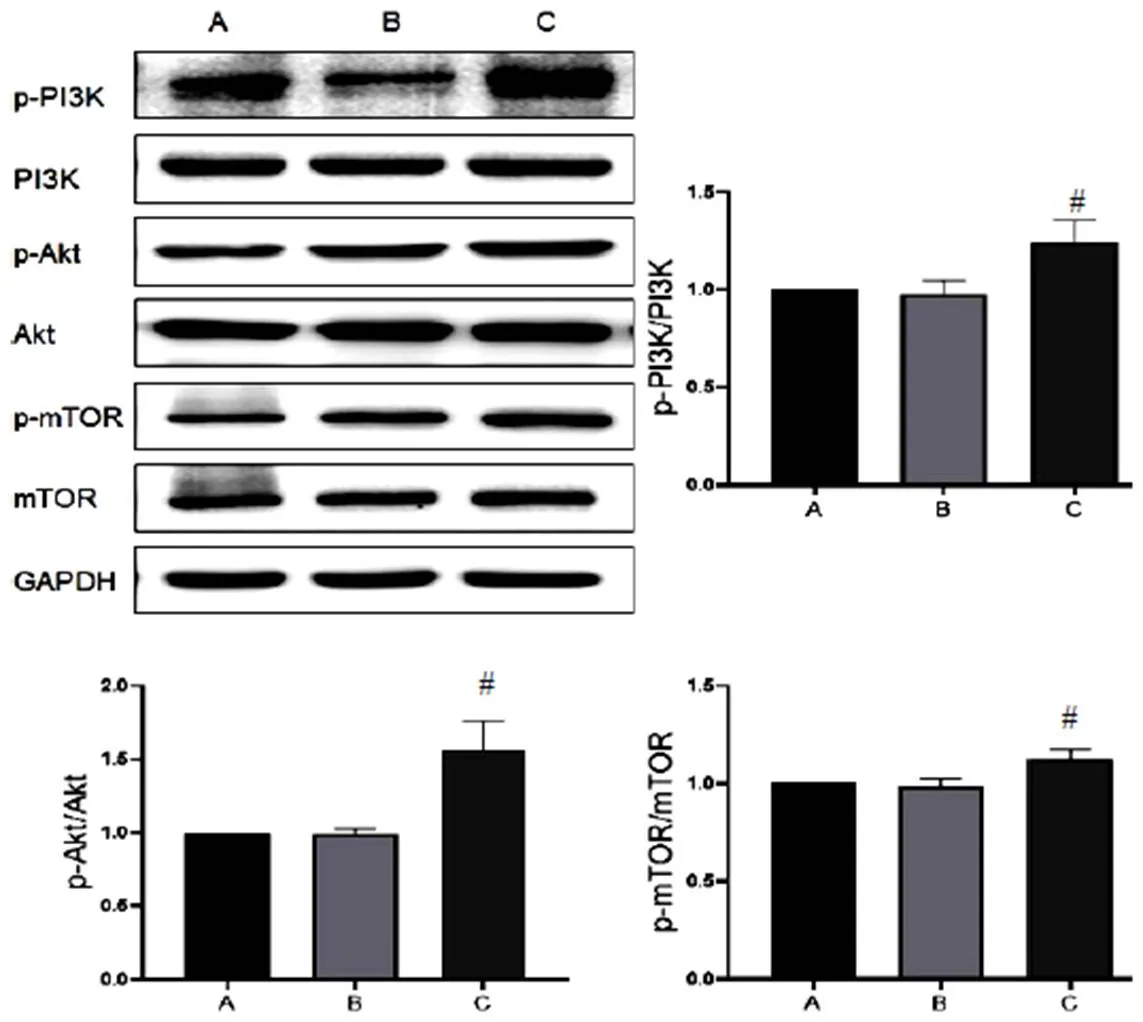
2.3 三七总皂苷对模型细胞自噬相关蛋白表达的影响
与正常组比较,A/R组H9c2细胞Atg5、Beclin-1蛋白表达明显升高,LC3Ⅱ/LC3Ⅰ比值显著增加(<0.05),p62蛋白表达无明显变化;与A/R组比较,A/R+PNS组H9c2细胞自噬相关蛋白Atg5、Beclin-1表达及LC3Ⅱ/LC3Ⅰ比值显著降低(<0.05),p62蛋白表达明显升高(<0.05),见图4。表明PNS对自噬有明显抑制作用,且可能抑制自噬流,其作用与自噬抑制剂HCQ和3-MA一致。此外,A/R+PNS+Rapa组H9c2细胞Atg5、Beclin-1蛋白表达及LC3Ⅱ/LC3Ⅰ比值明显低于A/R+Rapa组,提示PNS逆转了Rapa增强自噬的作用。
2.4 三七总皂苷对模型细胞自噬流的影响
使用自噬荧光染料标记H9c2细胞,DAPRed代表自噬体,DALGreen代表自噬溶酶体。结果显示,正常组DAPRed和DALGreen荧光强度较弱,表明此时自噬程度较低。经A/R造模后各组DAPRed和DALGreen荧光强度均明显增强,见图5a,表明A/R后自噬增强。结果显示,A/R组和A/R+Rapa组DAPRed和DALGreen荧光强度明显增强(<0.05),表明自噬流水平显著增加;与A/R组比较,A/R+PNS组和A/R+3-MA组DAPRed荧光强度明显降低(<0.05),见图5b,表明此时自噬体形成较少,但PNS无3-MA作用强。A/R+PNS组和A/R+HCQ组DALGreen荧光强度明显低于A/R组(<0.05),见图5c,表明此时自噬溶酶体数量减少,其可能与前期对自噬体生成抑制有关;也可能是PNS与HCQ作用效果一致,即对自噬溶酶体形成产生抑制作用。此外,A/R+PNS+Rapa组DALGreen荧光强度明显低于A/R+Rapa组(<0.05),表明PNS可能通过抑制自噬溶酶体形成逆转Rapa的作用。综合自噬蛋白表达与荧光结果发现,PNS可通过抑制自噬体及自噬溶酶体的形成抑制自噬流。
A.正常组;B. A/R组;C. A/R+PNS组;D. A/R+PNS+Rapa组;E. A/R+Rapa组;F. A/R+HCQ组;G. A/R+3-MA组;与正常组比较,*P<0.05;与A/R组比较,#P<0.05;与A/R+Rapa组比较,▲P<0.05
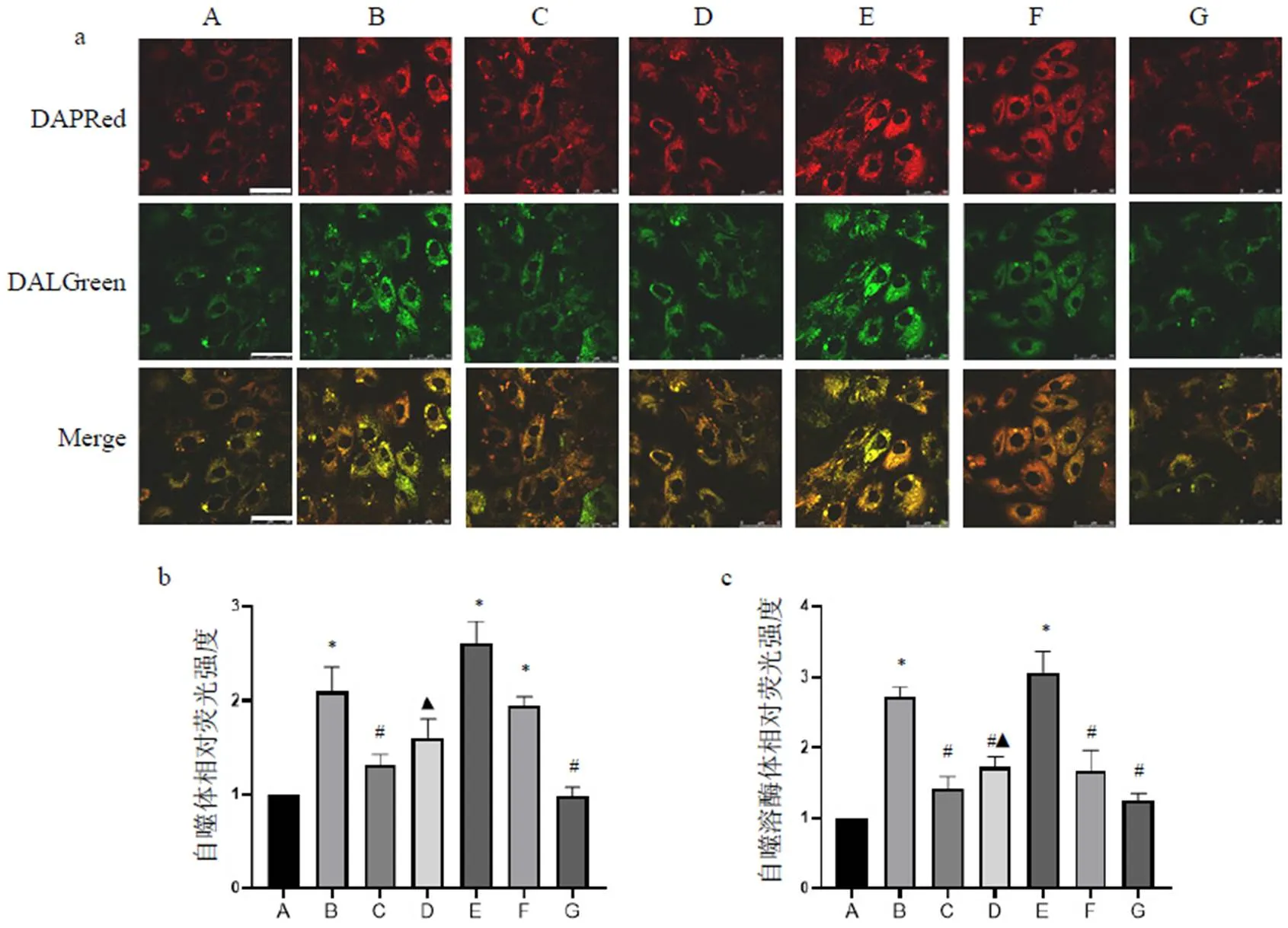
注:A.正常组;B. A/R组;C. A/R+PNS组;D. A/R+PNS+Rapa组;E. A/R+Rapa组;F. A/R+HCQ组;G. A/R+3-MA组;与正常组比较,*P<0.05;与A/R组比较,#P<0.05;与A/R+Rapa组比较,▲P<0.05
3 讨论
心肌缺血再灌注可引起心肌细胞损伤甚至死亡,表现为心功能下降、心律失常等。本研究采用H9c2细胞制备A/R模型,模拟在体心肌缺血/再灌注模型,观察PNS对A/R损伤细胞的影响。本实验结果显示,A/R后H9c2细胞存活率明显下降,100、200 μg/mL PNS处理后细胞存活率明显升高。除细胞活力检测外,细胞培养液LDH含量检测也能部分反映细胞损伤情况。LDH在细胞受损或死亡时被释放到细胞外环境。本实验中,不同浓度PNS处理细胞后,其培养液中LDH含量呈剂量依赖性降低,且浓度为50、100、200 μg/mL时较A/R组差异有统计学意义,表明此浓度PNS对H9c2细胞A/R损伤有一定的改善作用。
现有研究表明,PI3K/Akt/mTOR信号通路可能因损伤、氧化应激等刺激而被激活[11],是保护心肌减轻MIRI的有效手段之一。此外,PI3K/Akt/mTOR信号通路的激活与自噬的发生密切相关[12]。当发生MIRI时,PI3K被激活并可上调自噬的关键调节因子,包括Akt,PI3K可磷酸化Akt的Thr308和Ser473位点使Akt活化[13-14],而活化的Akt可磷酸化mTOR[15]。mTOR是细胞生长和代谢的关键调节剂,其mTORC1亚型也是自噬过程中发挥调节作用的上游活性因子之一[16]。磷酸化的mTOR可通过PI3K/Akt/mTOR信号通路抑制由局部缺血诱导的瞬时自噬,从而降低MIRI[17-18]。本实验结果表明,A/R组p-PI3K、p-Akt和p-mTOR表达无明显变化,PNS组p-PI3K、p-Akt和p-mTOR表达显著升高,表明PI3K/Akt/mTOR信号通路被激活。
为探讨PNS能否通过PI3K/Akt/mTOR信号通路调控自噬,本研究进行了自噬相关实验。心肌缺血时自噬增强,以清除受损的细胞器和细胞;再灌注时过高水平的自噬则加速心肌细胞死亡,导致MIRI[19]。自噬相关蛋白Atg5、Beclin-1表达水平可反映细胞自噬起始阶段的变化情况[20-21]。LC3存在于自噬小体,同时也对自噬体形成具有重要作用[22],当自噬发生时,LC3Ⅰ大量转化为LC3Ⅱ,LC3Ⅱ/LC3Ⅰ比值增加[23]。本研究中,A/R组H9c2细胞Atg5、Beclin-1蛋白表达和LC3Ⅱ/LC3Ⅰ比值明显升高,表明此时自噬水平上升;而PNS处理后H9c2细胞Atg5、Beclin-1表达和LC3Ⅱ/LC3Ⅰ比值明显降低,表明PNS逆转了A/R所致的自噬相关蛋白表达升高的趋势,对自噬有一定的抑制作用。p62作为自噬下游阶段的经典标志物之一,也可反映自噬的变化,其可与自噬体内膜上的泛素化底物和LC3结合,并通过在溶酶体系统中形成自噬溶酶体而被降解[24]。当自噬被抑制时,p62蛋白表达升高[25]。本研究结果显示,PNS预处理后,H9c2细胞p62蛋白表达明显升高,结合LC3Ⅱ/LC3Ⅰ的变化,推测自噬体与溶酶体结合受阻,自噬流处于被抑制状态。
为探明PNS对自噬流的作用,我们通过自噬荧光检测自噬体和自噬溶酶体的形成情况,观察PNS对自噬流上下游环节的作用。细胞自噬荧光检测试剂可观察自噬流的整个过程,以DAPRed标记自噬体,其荧光强度较弱时,表明自噬体数量较少;以DALGreen标记自噬溶酶体,其荧光强度弱时,表明自噬溶酶体数量较少,其生成可能受阻或被过度降解。本实验结果显示,正常组DAPRed和DALGreen荧光强度较弱,表明自噬程度较低;A/R组H9c2细胞DAPRed和DALGreen荧光强度均明显增强,表明A/R可显著增强自噬流。与A/R组比较,A/R+PNS组和A/R+3-MA组DAPRed荧光强度明显降低,表明PNS与3-MA作用相似,即可抑制自噬体形成和发展[26];由于PNS不能完全抑制自噬体的形成,因此使用自噬抑制剂HCQ,结果显示,A/R+PNS组和A/R+HCQ组DALGreen荧光强度明显降低,表明PNS与HCQ作用相似,可能抑制自噬溶酶体形成[27]。此外,A/R+PNS+Rapa组DALGreen荧光强度低于A/R+Rapa组,表明PNS可通过抑制自噬溶酶体发挥逆转Rapa的作用。综合自噬蛋白表达及荧光结果,表明PNS影响自噬流的上下游环节,抑制自噬体及自噬溶酶体的形成,从而抑制自噬流。
综上,PNS可通过激活PI3K/Akt/mTOR信号通路抑制自噬流,发挥对A/R H9c2细胞的保护作用,从而达到减轻MIRI保护心脏的作用。
[1] 中国心血管健康与疾病报告编写组.中国心血管健康与疾病报告2019概要[J].心脑血管病防治,2020,20(5):437-450.
[2] 王斌,李毅,韩雅玲.稳定性冠心病诊断与治疗指南[J].中华心血管病杂志,2018,46(9):680-694.
[3] FERDINANDY P, SCHULZ R, BAXTER G F. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning[J]. Pharmacol Rev, 2007,59(4):418-458.
[4] MA S, WANG Y, CHEN Y, et al. The role of the autophagy in myocardial ischemia/reperfusion injury[J]. BBA-Biomembranes, 2015,52(2):271-276.
[5] LIU L, JIN X, HU C F, et al. Exosomes derived from mesenchymal stem cells rescue myocardial ischaemia/reperfusion injury by inducing cardiomyocyte autophagy via AMPK and Akt pathways[J]. Cell Physiol Biochem,2017,43(1):52-68.
[6] LIU M W, SU M X, TANG D Y, et al. Ligustrazin increases lung cell autophagy and ameliorates paraquat-induced pulmonary fibrosis by inhibiting PI3K/Akt/mTOR and hedgehog signalling via increasing miR-193a expression[J]. BMC Pulm Med,2019,19(1):35.
[7] HAN D, WU X, LIU L, et al. Sodium tanshinone ⅡA sulfonate protects ARPE-19 cells against oxidative stress by inhibiting autophagy and apoptosis[J]. Sci Rep,2018,8(1):15137.
[8] WANG L, CHEN X, WANG Y, et al. MiR-30c-5p mediates the effects ofsaponins in myocardial ischemia reperfusion injury by inhibiting oxidative stress-induced cell damage[J]. Biomed Pharmacother,2020,125:109963.
[9] CHEN S, LIU J, LIU X, et al. Panax notoginseng saponins inhibit ischemia-induced apoptosis by activating PI3K/Akt pathway in cardiomyocytes[J]. J Ethnopharmacol,2011,137(1):263-270.
[10] SAKATA T, SAITO A, SUGIMOTO H. In situ measurement of autophagy under nutrient starvation based on interfacial pH sensing[J]. Sci Rep,2018,8(1):8282.
[11] LI X, HU X, WANG J, et al. Inhibition of autophagy via activation of PI3K/Akt/mTOR pathway contributes to the protection of hesperidin against myocardial ischemia/reperfusion injury[J]. Int J Mol Med,2018,42(4):1917-1924.
[12] LI Y, GUO Y, FAN Y, et al. Melatonin enhances autophagy and reduces apoptosis to promote locomotor recovery in spinal cord injury via the PI3K/AKT/mTOR signaling pathway[J]. Neurochem Res, 2019,44(8):2007-2019.
[13] ABEYRATHNA P, SU Y. The critical role of Akt in cardiovascular function[J]. Vascul Pharmacol,2015,74:38-48.
[14] GUO Y, PEI X. Tetrandrine-induced autophagy in MDA-MB-231 triple-negative breast cancer cell through the inhibition of PI3K/AKT/mTOR signaling[J]. Evid Based Complement Alternat Med, 2019,2019:7517431.
[15] KIM J, JUNG K H, RYU H W, et al. Apoptotic effects of xanthium strumariumvia PI3K/AKT/mTOR pathway in hepatocellular carcinoma[J]. Evid Based Complement Alternat Med,2019,2019:2176701.
[16] DUNLOP E A, TEE A R. mTOR and autophagy:a dynamic relationship governed by nutrients and energy[J]. Semin Cell Dev Biol,2014, 36:121-129.
[17] SHI B, MA M, ZHENG Y, et al. mTOR and Beclin1:Two key autophagy-related molecules and their roles in myocardial ischemia/reperfusion injury[J]. J Cell Physiol,2019,234(8):12562-12568.
[18] NICKLIN P, BERGMAN P, ZHANG B, et al. Bidirectional transport of amino acids regulates mTOR and autophagy[J]. Cell,2009,136(3):521-534.
[19] GUSTAFSSON A B, GOTTLIEB R A. Autophagy in ischemic heart disease[J]. Circ Res,2009,104(2):150-158.
[20] WANG M, QIU S, QIN J. Baicalein induced apoptosis and autophagy of undifferentiated thyroid cancer cells by the ERK/PI3K/Akt pathway[J]. Am J Transl Res,2019,11(6):3341-3352.
[21] LI Y, ZHAO Y, SU M, et al. Structural insights into the interaction of the conserved mammalian proteins GAPR-1 and Beclin 1, a key autophagy protein[J]. Acta Crystallogr D Struct Biol, 2017,73(Pt 9):775-792.
[22] MONASTYRSKA I, ULASLI M, ROTTIER P J, et al. An autophagy-independent role for LC3 in equine arteritis virus replication[J]. Autophagy,2013,9(2):164-174.
[23] 刘娜娜,贾学昭,王茎,等.艾灸对慢性心力衰竭大鼠心肌细胞自噬功能的影响[J].针刺研究,2019,44(1):25-30.
[24] BITTO A, LERNER C A, NACARELLI T, et al. P62/SQSTM1 at the interface of aging, autophagy, and disease[J]. Age (Dordr),2014, 36(3):9626.
[25] SHARIFI M N, MOWERS E E, DRAKE L E, et al. Measuring autophagy in stressed cells[J]. Methods Mol Biol,2015,1292:129-150.
[26] WU Y T, TAN H L, SHUI G, et al. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class Ⅰ and Ⅲ phosphoinositide 3-kinase[J]. J Biol Chem,2010,285(14):10850-10861.
[27] MAUTHE M, ORHON I, ROCCHI C, et al. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion[J]. Autophagy,2018,14(8):1435-1455.
Study on Panax Notoginseng Saponins Regulate Autophagy Against H9c2 Cells Anoxia/reoxygenation Injury via PI3K/Akt/mTOR Signaling Pathway
WANG Piao, ZHENG Qing, HU Ting, ZHANG Haiyin, XU Yanwu, BAO Yimin
To explore mechanism whether Panax notoginseng saponins (PNS) can regulate autophagy through the PI3K/Akt/mTOR signaling pathway to reduce anoxia/reoxygenation (A/R) injury of H9c2 cells.H9c2 cells were divided into seven groups: control, anoxia-reoxygenation (A/R), A/R+PNS, A/R+PNS+ Rapamycin (Rapa), A/R+Rapa, A/R+hydroxychloroquine (HCQ), and A/R+3-methyladenine (3-MA). The A/R model of H9c2 cells was established by anoxia for 6 h and reoxygenation for 2 h. Cell survival rate was detected by CCk-8 method; cell damage was detected by LDH detection kit; autophagy-related proteins of Atg5, p62, Beclin-1, LC3Ⅱ/LC3Ⅰ and PI3K/Akt/mTOR signaling pathway related proteins PI3K, Akt, mTOR expression were detected by Western blot; autophagy flow was observed by fluorescence.Compared with the control group, the cell viability of A/R group was decreased significantly while the cell damage rate was increased (<0.05). The expressions of autophagy-related proteins Atg5, Beclin-1 and LC3Ⅱ/LC3Ⅰ ratio were increased significantly (<0.05); The numbers of autophagosome and autolysosome increased significantly, and the level of autophagic flow increased significantly (<0.05). Compared with the A/R group, PNS preconditioning increased cell viability, reduced cell damage rate (<0.05); The expressions of Atg5, Beclin-1, and LC3Ⅱ/LC3Ⅰ ratio were reduced significantly (<0.05), and the expression of p62 was increased significantly, which were similar to the autophagy inhibitors HCQ and 3-MA; Autophagy fluorescence detection also revealed an inhibition of autophagic flow (<0.05). The expressions of p-PI3K, p-Akt and p-mTOR protein were increased after PNS preconditioning (<0.05).PNS can effectively reduce A/R damage in H9c2 cells, and its mechanism is related to the inhibition of autophagic flow by activating the PI3K/Akt/mTOR signaling pathway.
Panax notoginseng saponins; anoxia/reoxygenation injury; autophagy; PI3K/Akt/mTOR signaling pathway; H9c2 cells
R285.5
A
1005-5304(2021)08-0087-06
10.19879/j.cnki.1005-5304.202012070

国家自然科学基金(81303256)
包怡敏,E-mail:yiminbao@163.com
(收稿日期:2020-12-04)
(修回日期:2021-01-05;编辑:华强)

