Expression and clinicopathologic significance of RASSF1A and WT1 in recurrent epithelial ovarian cancer
Yan Wang,Na Han,Ya-Juan Tang,Ping Han,Li-Fen Zhang,Su-Xin Han,and Yan-Fang He
Abstract—Objective To explore the expression and clinicopathological significance of RASSF1A and WT1 in recurrent epithelial ovarian cancer. Methods Sixty-three cases of patients with pathologically confirmed epithelial ovarian cancer were collected in the department of Gynaecology and Obsterics of the North China University of Technology from January 2013 to December 2018. The expression of RASSF1A and WT1 were measured by IHC staining. The relation of these proteins with ovarian cancer was also analyzed. Results Compared with non-recurrent group (46.4%), the positive expression rate of RASSF1A was 17.1%in recurrent group.The positive expression rate of WT1 was 74.3% and higher than the rate of 42.8% in non-recurrent group. The reducing expression of RASSF1A was related to clinical stage,differentiation,and with ascites(P<0.05).The increasing expression of WT1 was related to pathological type, clinical stage, histological differentiation, with ascites, and lymph node metastasis (P<0.05). Conclusion Low expression of RASSF1A and high expression of WT1 may be related to recurrent epithelial ovarian cancer.
Keywords—epithelial ovarian cancer; recurrence; Ras association domain family member 1A; Wilms'tumor 1; Immunohistochemical staining
I. INTRODUCTION
Ovarian cancer is a common malignant tumor of female reproductive system in China, which is mainly epithelioid tumor, accounting for the fifth death of female cancer in the world. It can occur at any age, and the recurrence rate and mortality rate are higher than other malignant tumors of reproductive system[1]. At present, the standard treatment for ovarian cancer is still cytoreductive surgery plus postoperative platinum based chemotherapy, in which primary tumor reduction and chemotherapy resistance are the important factors for ovarian cancer recurrence[2,3]Although a variety of treatment methods are used at present,there are not still breakthrough progress in the overall recurrence rate and survival rate of ovarian cancer patients, which seriously affects the quality of life of patients and brings the great pain to patients and their families. Therefore, it is of great significance for the diagnosis and treatment of ovarian cancer to study the molecular biological mechanism of recurrence and analyze the possible factors of chemotherapy resistance. Recent studies had found that the promoter methylation level of RAS association domain family member 1A(RASSF1A)was increased and the protein expression level was decreased in ovarian cancer and other malignant tumors[3,4], while Wilms' tumor 1 (WT1) acted as an oncogene to participate in the regulation of tumor cell proliferation[5,6]. Therefore, the purpose of this study was to detect the differential expression of RASSF1A and WT1 in recurrent epithelial ovarian cancer by immunohistochemical staining and analyze their clinicopathological significance in order to provide further theoretical and experimental basis for the diagnosis and treatment of ovarian cancer.
II. MATERIAL AND METHODS
A. General information
A total of 63 patients with epithelial ovarian cancer in the Department of Obstetrics and Gynecology(Ethics number is 2019021), Affiliated Hospital of North China University of Scienceand Technology from January 2013 toDecember2018 were selected as the research objects.35 patients with recurrence after clinical remission by cytoreductive surgery and standard chemotherapy were selected as the recurrence group,including 24 patients with platinum sensitive recurrenceand 11 patients with platinum resistant recurrence.Patients without recurrence were selected as Non-recurrence group (Control group),a total of 28 patients.The inclusive criteria were as follows: ①The standardized operation and chemotherapy were performed in the hospital with complete case data;② The diagnosis was confirmed by the pathology department of the hospital; ③The first operation and treatment after recurrence were completed in the hospital.The exclusion criteria were as follows: ①The operation,chemotherapy and pathological diagnosis were performed inotherhospitals;②Patientswith refractory ovarian cancer,metastatic ovarian cancer and non- epithelial ovarian cancer were confirmed; ③Patients were confirmed with severe heart, lung, liver, kidney, blood system diseases and other organ malignancies. All procedures performed in the study involving human participants were approved by the ethics committee of Affiliated Hospital of North China University of Science and Technology, and the informedconsentwassignedbypatientsandtheirfamilies.
B. Method
The localization and expression of RASSF1A and WT1:The paraffin sections were treated with conventional dewaxing and hydration, high-pressure repair and H2O2blocking endogenous peroxidase.The first antibodies of RASSF1A(eb114-10h1, ebioscience company, USA) and WT1 (sc-393498, Santa Cruz company, USA) were treated overnight at 4°C, and the second antibodies (pv6000, Beijing zhongshanjinqiao Biotechnology Co., Ltd.) were treated for 20 min at 37°C, then DBA staining, the slight hematoxylin restaining, the dehydrated transparent, and the neutral gum seal were treated. RASSF1A was located in the cytoplasm and WT1 in the nucleus. Under the high power microscope, 5 different visual fields were chosen and scored according to the percentage of positive cells and staining intensity. The score to the percentage of positive cells: 0 score for <5%, 1 score for 5%-24%,2 score for 25%-49%,3 score for 50%- 75%,4 score for >75%; Dyeing intensity score: No staining score was 0 score, light yellow was 1 score, brown yellow was 2 score, chocolate brown was 3 score, and the resultsof the two criterion scores was the final result. Among them,<2 score was defined as negative, and≥ 2 was defined as positive[4,5]. The above results were determined by at least two pathologists.
C. Statistical methods
SPSS17.0 software was used for statistical analysis. Data were expressed as mean± SD. T test was used for measurement data. Chi square test and Fisher exact test method were used for counting data. Differences were considered statistically significant atP<0.05.
III. RESULTS
A. The comparison of basic data between non-recurrence group and recurrence group
There was no significant difference between non recurrence group and recurrence group in menostasis, age, differentiation degree and histological type (P>0.05), however there was significant difference in clinical stage, ascites, lymph node metastasis and complete operation(P<0.05,Table 1).
B. The expression of RASSF1A and WT1 in recurrent epithelial ovarian cancer
In epithelial ovarian cancer, RASSFIA protein was expressed in 6 patients in recurrence group, the proportion was 17.1%, significantly lower than that of the non-recurrence group(46.4%),and there was statistical difference between the two groups (P<0.05). There were 26 cases of WT1 positive expression in the recurrence group, the proportion was 74.3%,which was significantly higher than that of the non-recurrence group(42.9%),and the difference between the two groups was statistically significant(P<0.05,Fig.1, Table 2).
C. Clinicopathological significance of RASSF1A and WT1 expression in recurrent epithelial ovarian cancer
RASSF1A was associated with clinical stage, histological differentiation and ascites (P<0.05), meanwhile WT1 was associated with clinical stage, degree of differentiation, pathological type, ascites and lymph node metastasis (P<0.05,Table 3).
2.4 The expression of RASSF1A and WT1 in recurrent epithelial ovarian cancer and its relationship with recurrence
The median recurrence time of RASSFIA positive patients was 9.5 months, and there was no significant difference by non-parametric test(P>0.05,Table 4).The median recurrence time of WT-1 positive patients was 13 months, which was longer than that of WT-1 negative patients. There was no significant difference between the two groups (P> 0.05).The expression of RASSF1A and WT1 in recurrent epithelial ovarian cancer was not related to the recurrence times and platinum sensitive/ platinum resistant recurrence (P>0.05,Table 5 and Table 6). There was no correlation between the protein expression of RASSFIA and WT-1 in recurrent epithelial ovarian cancer(Table 7).
IV. DISCUSSION
The incidence rate of ovarian cancer is the second, and the mortality rate is the highest in gynecologic malignancies.Over 70% of patients with ovarian cancer are at the advanced stage, the cure rate is low, and the long-term prognosis is not still good[1,2]. For recurrent ovarian cancer, it is generally believed that patients who have achieved clinical remission after standard treatment have the following conditions, including the elevated tumor marker CA125, pleural effusion and ascites, the mass found by physical examination or image, and the intestinal obstruction of unknown cause,in which one or more positive results indicate ovarian cancer recurrence,and the pathological diagnosis is the gold standard.In addition, the National Comprehensive Cancer Network guidelines of the United States further were divided into: ①Platinum sensitive relapse: patients who achieved complete remission after initial treatment and the interval between the chemotherapy termination and relapse was more than 6 months; ②Recurrence of platinum resistance: the complete remission was achieved after the initial treatment, and the interval between chemotherapy termination and recurrence was less than 6 months; ③Refractory ovarian cancer: the partial remission is not achieved by the initial treatment,including patients with stable or progressive disease or relapse within three months. After the neoadjuvant chemotherapy, the cytoreductive surgery,platinum chemotherapy and other treatments, the recurrence rate of patients with ovarian cancer was not significantly decreased,seriously affecting the reproductive health of women[7]. However, the molecular mechanism of ovarian cancer recurrence was still unclear. Most patients had short-term or long-term recurrence after completing the standard treatment and reaching clinical remission, which was closely related to multidrug resistance. In this study, a total of 63 patients with epithelial ovarian cancer were included.Among them, 35 patients showed the signs of recurrence after clinical remission by cytoreductive surgery and standard chemotherapy (including 24 patients with platinum sensitive recurrence and 11 patients with platinum resistant recurrence),and 28 patients showed no sign of recurrence. There was no significant difference between the two groups in menopausal history, age, degree of differentiation and histological type,but there was significant difference in clinical stage, ascitesand lymph node metastasis, suggesting that patients with recurrence may be associated with the change of malignant biological typing and poor prognosis.

Table 1 Comparison of basic data between non-recurrent group and recurrent

Figure 1 Expression of RASSF1A and WT1 in recurrent epithelial ovarian cancer(×200)
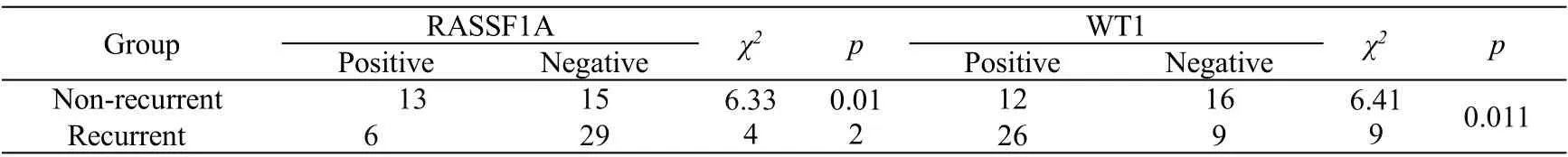
Table 2 Expression of RASSF1A and WT1 in recurrent epithelial ovarian cancer
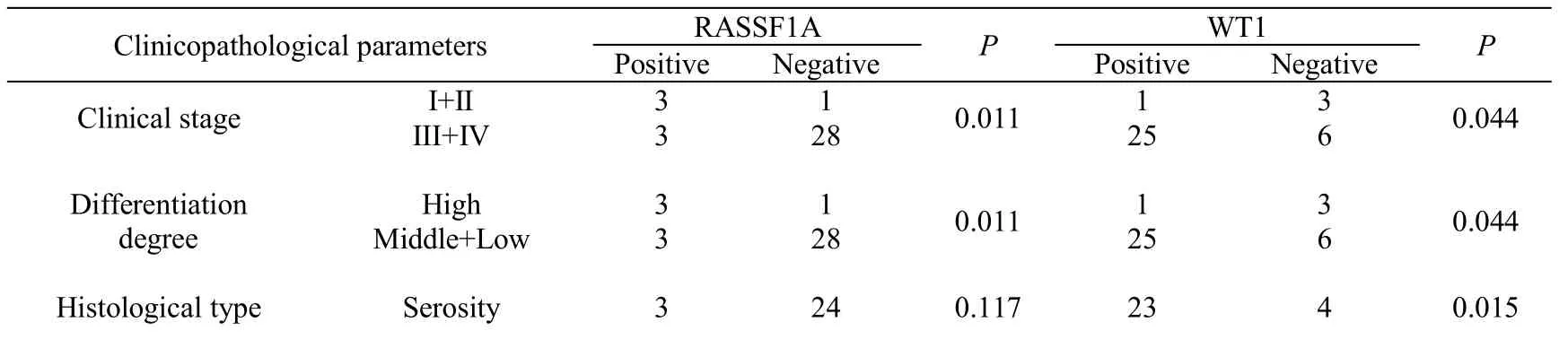
Table 3 Clinicopathological significance of RASSF1A and WT1 expression in recurrent epithelial ovarian cancer tissues*
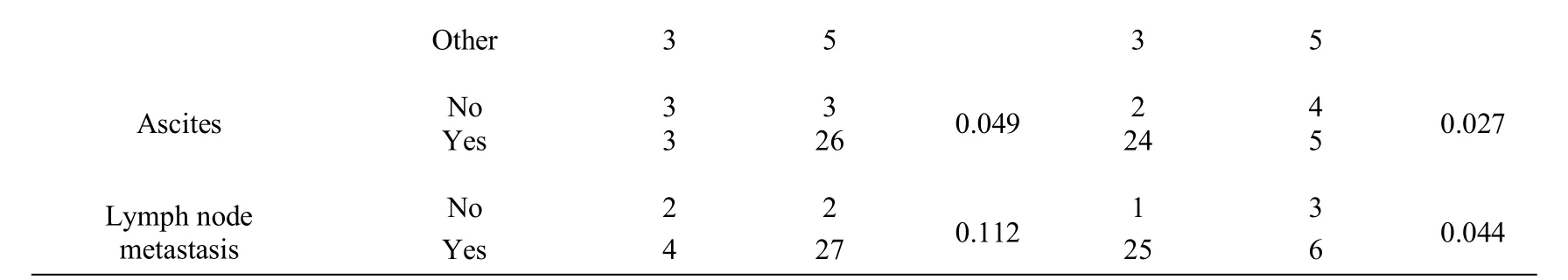
*Fisher exact test
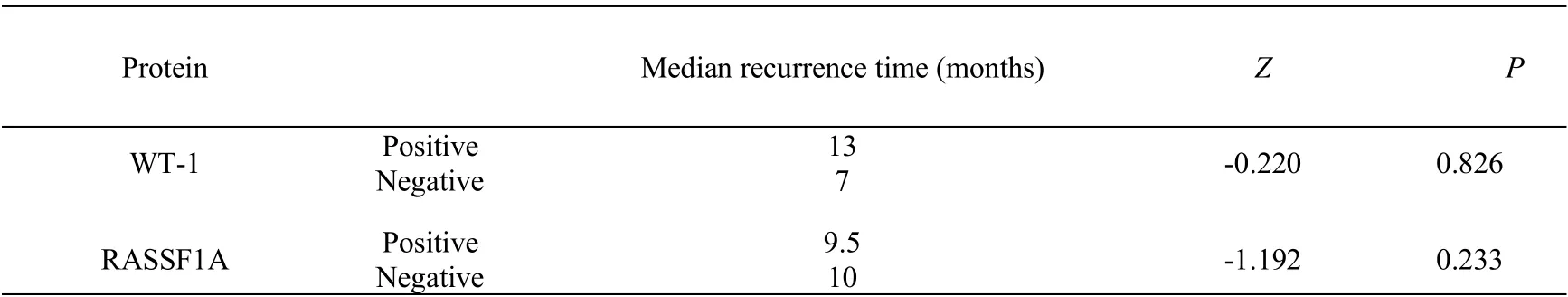
Table 4 Relationship between the expression of RASSFIA and WT-1 and the time of recurrence
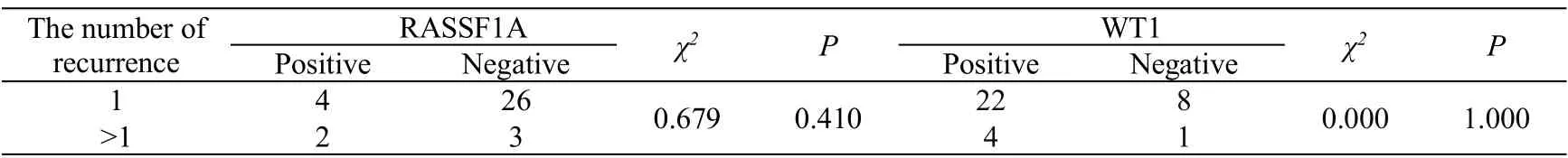
Table 5 Relationship between RASSFIA and WT-1 protein expression and the number of recurrence
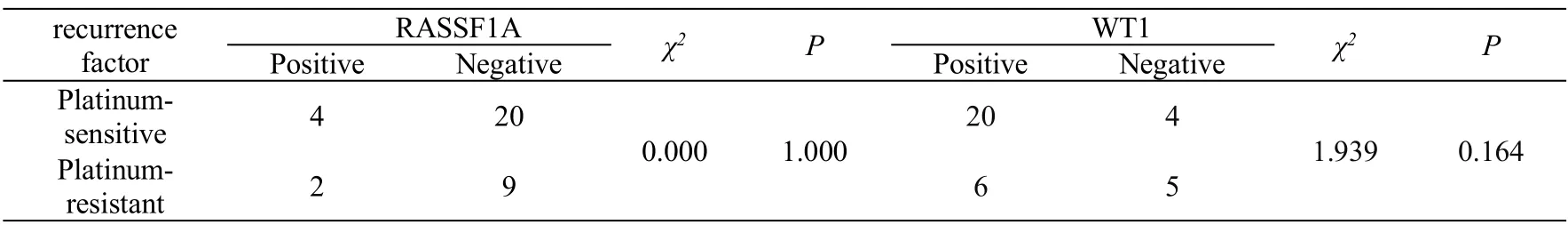
recurrence factor RASSF1Aχ2PWT1χ2P PositiveNegativePositiveNegative Platinumsensitive420 0.0001.000 204 1.9390.164 Platinumresistant2965
As a tumor suppressor gene, RASSF1A is a microtubule binding associated protein, which inhibits abnormal mitosis by stabilizing microtubule protein,causes the decline of tumor cell proliferation,invasion and metastasis,and promotes tumor cell apoptosis[8]. A Meta-analysis of ovarian cancer showed that RASSF1A promoter methylation level was increased in ovarian cancer tissues with low malignant potential, but it was not correlated with other clinicopathological parameters, and its hypomethylation level was not correlated with the survival rate of ovarian cancer patients[9]. Other studies also showed that the methylation level of RASSF1A gene in 112 patients with ovarian cancer was as high as 49.1%, which might be one of the indicators for the early diagnosis of ovarian cancer[10]. In vitro studies also found that overexpression of RASSF1A could promote apoptosis and inhibit proliferationof ovarian cancer cells[11]. Most studies believed that RASSF1A methylation was one of the sensitive indicators of poor tumor prognosis[12], and the low expression of RASSF1A protein was also closely related to the progress, recurrence and malignant transformation of some tumors[4]. In this study, the immunohistochemistry method was used to detect the in situ expression of RASSF1A in ovarian cancer.The results showed that the positive expression rate of RASSF1A in recurrent epithelial ovarian cancer was lower than that in non-recurrent epithelial ovarian cancer, which was related to clinical stage,histological differentiation and ascites. It further confirmed that the low expression of RASSF1A might be related to the recurrence of ovarian cancer.
The expression of WT-1 is limited, and the expression is very low in normal tissues, such as heart, kidney, spleen and gonad, but it is overexpressed in solid tumors and hematological malignancies, which may be a diagnostic marker of some tumors[13]. Combined with cytokeratin 7, it can be used for the diagnosis of ovarian cancer[14]. Overexpression of WT1-177AA/-KTS can significantly promote the malignant biological effect of ovarian cancer cells through vascular endothelial growth factor signaling[15]. The high expression of WT1 mRNA is closely related to the grade, lymph node metastasis and ascites of epithelial ovarian cancer, but is not related to recurrence. The median disease-free survival time of patients with low expression of WT1 is higher than that of patients with high expression of WT1, and the overexpression of WT1 can significantly promote the proliferation and invasion of ovarian cancer cells[16]. Recent studies had also shown that the high expression of WT1 was related to the pathological type,grade,FIGO stage and survival rate of ovarian cancer. Combining with p53 protein indicated the poor prognosis of ovarian cancer, which had important diagnostic value for diagnosis and treatment[5]. In this study, the immunohistochemical staining showed that the positive expression rate of WT1 in recurrent epithelial ovarian cancer was higher than that in nonrecurrent epithelial ovarian cancer. The expression of WT1 was related to the pathological type,clinical stage,histological differentiation, ascites and lymph node metastasis of recurrent epithelial ovarian cancer.
In this study,we found that the low expression ofRASFF1A and the high expression of WT1 might be related to the recurrence of ovarian cancer, and closely related to the malignant biological effect of recurrent ovarian cancer. Although the further analysis did not find a correlation between the differential expression of RASSF1A and WT1, and the relationship between the expression changes of RASSF1A and WT1 and recurrence time, recurrence frequency and drug resistance recurrence, which might be due to that the case study itself was a retrospective study, the sample size was small, and there was a certain bias. Therefore, the specific mechanism of RASSF1A and WT1 involved in the regulation of epithelial ovarian cancer recurrence still needs to be further verified in vitro in the future.
Acknowledgments:
The author did not receive any funding for this study.
Competing interests:
The authors declare no conflicts of interest.
Citation:
Wang Y,Han N, Li XX, Tang YJ, et al.Expression and clinicopathologic significance of RASSF1A and WT1 in recur- rent epithelial ovarian cancer.Prec Med Res. 2021;3(2):10.doi:12032/PMR20210608005
Executive editor:Na Liu.
Submitted:12 Marth 2021,Accepted:3 June 2021,Online:4 June 2021.©2021 By Authors.Published by TMR Publishing Group Limited.This is an open access article under the CC-BY license(http://creativecommons.org/licenses/BY/4.0/)
 Precision Medicine Research2021年2期
Precision Medicine Research2021年2期
- Precision Medicine Research的其它文章
- Myeloid-derived suppressor cells as a therapeutic target in lung cancer
- Targeting mitochondrial protein transport system:a promising treatment strategy for heart failure?
- Expression and molecular mechanism of PCNA,Caspase-3,IL-6 and Survivin proteins in chorionic villi and decidual tissue of early embryo damage
- The predictive value of preoperative albumin-to-globulin ratio in patients with hilar cholangiocarcinoma
