Targeting mitochondrial protein transport system:a promising treatment strategy for heart failure?
Luan Yi and Yang Yang
Heart failure(HF)is a severe condition in which the heart is unable to pump enough blood to maintain the normal demand of body for oxygen and nutrients [1]. Heart failure is also the common end result of several heart diseases and the leading cause of death of cardiovascular diseases(CVDs)[2].There is still no effective method for the diagnosis of heart failure, and its diagnosis is made by a comprehensive history, symptoms and objective examination [3]. The therapeutic drugs for heart failure mainly include diuretics, RAAS inhibitors, β-receptor antagonists and antiplatelet drugs, etc., which can improve the symptoms of heart failure patients by increasing urination,reducing myocardial remodeling, inhibiting sympathetic nerve activity and relieving blood embolism, delaying the disease progression and reducing mortality [4]. However,there is still a lack of effective early diagnostic markers and therapeutic drugs for heart failure.
Interestingly, heart failure is also known as a metabolic disease, mainly caused by the abnormality of myocardial metabolism and the disorder of the energy balance [5].Therefore, using the animal model of heart failure caused by energy metabolism disorder, carefully analyzing its regulation mechanism and intervention strategies, and further providing targeted treatment in clinical practice, is a powerful means to combat this disease[6].
Mitochondria are the core organelles that supply energy to cells, and the maintenance of mitochondria function is vital to the homeostasis of bodies [7]. Abnormal mitochondria can cause disorders in ATP synthesis,which disorders the function of multiple organs and leads to a range of human diseases,the most common of which is heart-related disease [8]. The heart is one of the most important organs,providing the continuous power for the flow of blood to the rest of the body [9]. As a result, myocardial cells are particularly sensitive to a lack of energy[10].Cardiomyocyte mitochondria need to produce around 6 kg of ATP a day to keep the heart functioning [11].
Myocardial cells typically contain hundreds of mitochondria to meet their high energy demands. Abnormal mitochondrial function is an important pathological index of heart failure.Mitochondrial genomic mutations,ATP reduction, mitochondrial Ca2+increase, and ROS increase all play important roles in heart failure [12]. Once mitochondrial ATP synthesis is reduced, it can lead to abnormal function, damage and even death of cardiomyocytes. Severe ATP reduction can lead to heart failure. A decrease in the activity of enzymes involved in the mitochondrial respiratory chain leads to heart failure [12]. For an example, the NDUFS4 protein involved in the assembly of mitochondrial respiratory chain complex I was mutated in mice with symptoms of heart failure [13].Therefore,improving the function of myocardial mitochondria is one of the effective methods for the treatment of heart failure.
In mitochondria,approximately 99%of proteins encoded by nuclear genes enter through mitochondrial membrane channels after synthesis in the cytoplasm and are processed into mature mitochondrial proteins inside the mitochondria. Several different mechanisms mediate the entry of proteins into the mitochondria. The most important of these is that proteins with leading peptides enter the mitochondria through the TOM Complex in the outer membrane of mitochondria [14].
In addition,some hydrophobic proteins and proteins without signal peptides enter the mitochondrial intima through carrier proteins [15]. These proteins often have mitochondrial localization sequences in the middle segment, and with the help of molecular chaperone, Hsp70 or Hsp90, promote the binding to the mitochondrial outer membrane protein TOM70, and further mediate its passage through the TOM40 channel.Some cysteine-rich proteins still enter the mitochondria through the TOM complex after synthesized in the cytoplasm. Meanwhile,these proteins are in a reduced state and bind with MIA40 protein to form disulfide bonds,which contribute to the structural stability of these proteins[16].When the mitochondrialprotein transport system is abnormal, the mitochondrial proteins could not enter mitochondria, therefore leading to a variety of heart diseases. Cardiomyocytes, as energy-intensive cells, are very sensitive to abnormalities in the transport of mitochondrial proteins into the system [17]. Studies have confirmed that the abnormal mitochondrial protein transport system is closely related to the mechanism of heart-related diseases [18]. The key proteins involved in the regulation of heart development can enter the mitochondria through the mitochondrial protein transport system, so as to maintain the normalization of mitochondrial function. For an example, as an important mitochondrial protein, OPA1 enters the mitochondria dependent on the TOM Complex, and is involved in the regulation of mitochondrial dynamic changes, and its mutation will lead to congenital heart disease(CHD) [19].
Abnormalities in the transport of mitochondrial proteins into the system often lead to mitochondrial dysfunction and accumulation of proteins in the cytoplasm to form aggregates[20]. In yeast cells, abnormal mitochondrial protein transport system causes excessive accumulation of mitochondrial proteins in cytoplasm and cell death, and the aggregation of proteins in cardiomyocytes often induces autophagy [21]. So far, the role of autophagy in heart disease is complex and multifaceted. In an animal model of cardiac hypertrophy, autophagy activity was significantly increased incardiomyocytes.Overexpression of Beclin 1 enhanced autophagy and resulted in decreased cardiac function, while inhibition of autophagy by 3-MA alleviated myocardial fibrosis [22]. There are also studies suggesting that autophagy plays a protective role in cardiomyocytes: ATG5 knockout can accelerate the decline of heart function and ventricular enlargement induced by stress load in mice; Rapamycin, a drug that activates autophagy,inhibits cardiac hypertrophy induced by thyroid hormones or aortic constriction [23].Although defects in the mitochondrial protein transport system can lead to protein aggregation in the cytoplasm and further promote autophagy, whether this further promotes the pathogenesis of heart failure remains to be further investigated.
Interestingly, there is a lot of evidence that suggests this is a possibility.A previous study demonstrated that decreased expression of TOM40 is associated with decreased cytoplasmic proteasome function,accompanied by abnormalities in autophagy,suggesting that the accumulation of protein aggregates common may be related to abnormal mitochondrial proteint transport and inhibition of autophagy [21]. Therefore,it is worth to speculate that in cardiomyocytes, the functional defects of mitochondrial protein transport into the complex would lead to the inhibition of autophagy and the accumulation of mitochondrial protein,which would further lead to the inhibition of cardiomyocyte function and induce heart failure.In view of the pathogenesis of heart failure, Mitochondrial function as a therapeutic target in heart in recent years [24].Mitochondria are the core organelles of energy metabolism in cardiomyocytes [25]. Interfering with the disorder of energy metabolism caused by abnormal mitochondria may be an effective strategy for the treatment of heart failure. The mitochondrial protein transport system is crucial for maintaining the normal function of mitochondria[26].Defects in the mitochondrial protein transport system will lead to the aggregation of mitochondrial proteins in the cytoplasm and further damage to cells, which may also be an important cause of abnormal function of cardiomyocytes and heart failure (Figure 1) [27].Of course, the detailed molecular mechanism still needs to be further studied to determine whether the relevant regulatory proteins can be used as potential therapeutic targets.
Acknowledgments:
This research was funded by the National Natural Science Foundation of China(Nos.31900502).
Competing interests:
The authors declare that they have no conflict of interest.
Citation:
Luan Y,Yang Y.Targeting Mitochondrial Protein Transport System: A Promising Treatment Strategy for Heart Failure?.Prec Med Res.2021;3(2):8.doi:10.12032/PMR2021060803.
Executive editor:Na Liu.
Submitted:18 May 2021,Accepted:20 May 2021,Online:21 May 2021.
© 2021 By Authors. Published by TMR Publishing Group Limited. This is an open access article under the CC-BY license(http://creativecommons.org/licenses/BY/4.0/).
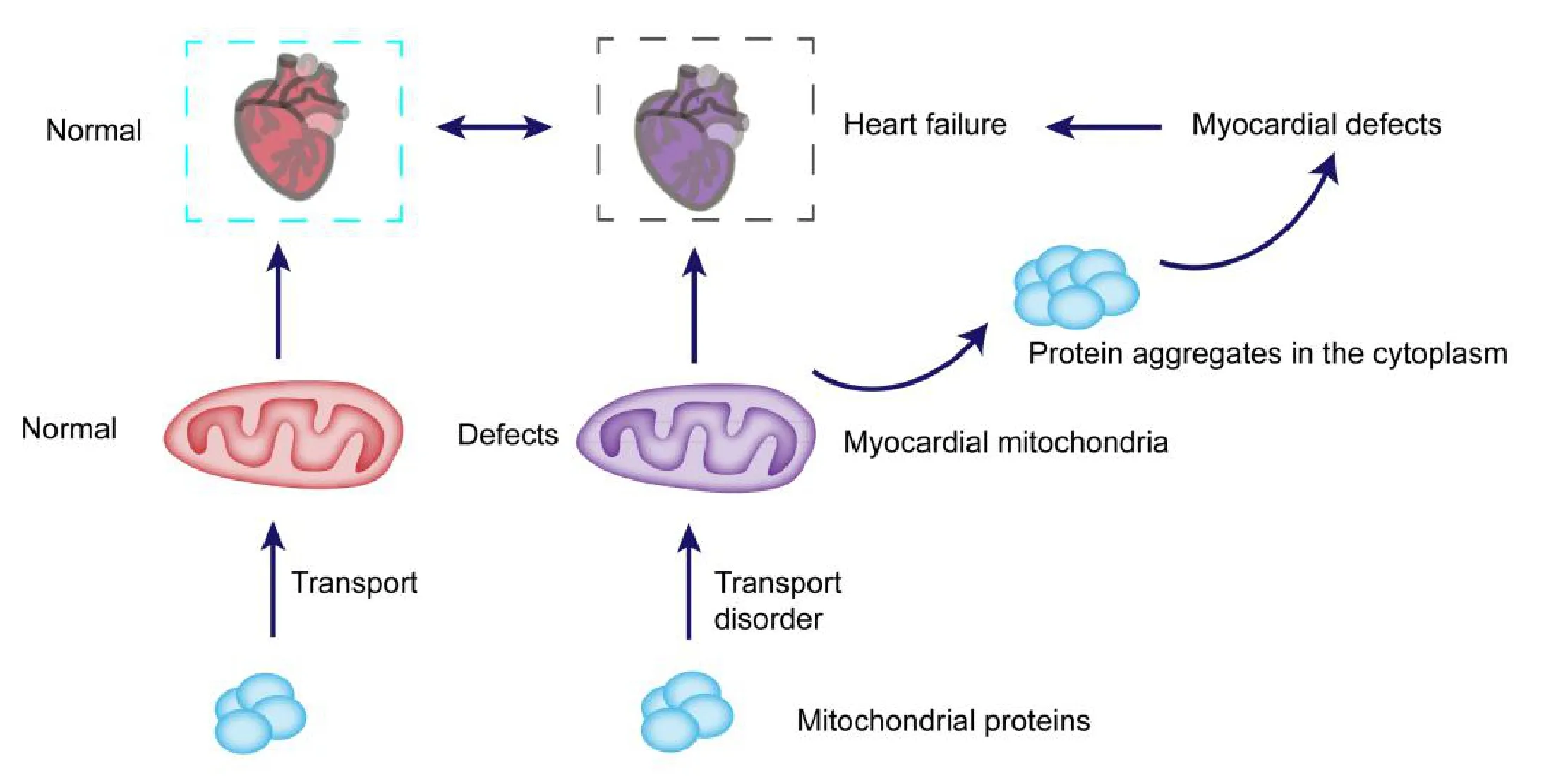
Fig. 1. Hypothesized diagram of the mechanism underlying abnormal mitochondrial protein transport system leading to heart failure. Normal mitochondrial protein transport system is crucial for the transport of mitochondrial proteins to the mitochondria,the defects of this system can lead to proteins are unable to tranport to the mitochondria,and formed protein aggregates in cytoplasm,further inhibiting myocardial cell function and leading to heart failure.
 Precision Medicine Research2021年2期
Precision Medicine Research2021年2期
- Precision Medicine Research的其它文章
- Myeloid-derived suppressor cells as a therapeutic target in lung cancer
- Expression and molecular mechanism of PCNA,Caspase-3,IL-6 and Survivin proteins in chorionic villi and decidual tissue of early embryo damage
- Expression and clinicopathologic significance of RASSF1A and WT1 in recurrent epithelial ovarian cancer
- The predictive value of preoperative albumin-to-globulin ratio in patients with hilar cholangiocarcinoma
