Hydrothermal synthesis of hydroxyapatite coating on the surface of medical magnesium alloy and its corrosion resistance
Shifeng Wen,Xianglei Liu,Jiahui Ding,Yulai Liu,Ziting Lan,Zimeng Zhang,Guangmeng Chen
School of Mechanics and Civil &Architecture Northwestern Polytechnical University,Xi’an,710129,China
Keywords:Medical magnesium alloy Hydroxyapatite coating Corrosion property Electrochemistry
ABSTRACT The early corrosion control of biomedical magnesium alloy is an important measure to determine its good performance during implantation into human body.The deposition of calcium phosphate biological coating is the most effective solution at present.In this paper,hydroxyapatite (HAP)coating was hydrothermal synthesized on the surface of AZ31B magnesium alloy,and the influence mechanism of hydrothermal synthesis temperature,time and solution concentration was investigated.The synthesis conditions and deposition mechanism of hydroxyapatite coating without DCPA(CaHPO4)were proposed.The surface morphology of the coating was observed by field emission electron scanning microscope (FESEM).The types and contents of microelements in the material were analyzed by energy disperse spectroscopy(EDS).Fourier transform infrared spectroscopy(FTIR)was used to analyze the functional group information of the coating surface.The corrosion resistance of different experimental groups was studied by electrochemical test.The results showed that when the calcium phosphate solution concentration was 0.1 mol/L and the calcium/phosphorus ratio was 1.67,the coating had better morphology structure and corrosion resistance,and the calcium/phosphorus ratio of HAP crystals reached 1.58,the epit of the prepared AZ31B-HAP coating by bare metal increased from -1.51 V to -1.18 V,the impedance value reached 1.0 × 105 Ω▪cm2,and the early corrosion of magnesium alloy substrate was effectively delayed.
1.Introduction
In recent years,biodegradable magnesium alloys have set off a new wave of applications in the field of biomaterials [1],but they also have the problem of fast corrosion rate in human body,and the deposition of hydroxyapatite coating on the surface of magnesium alloys is still an effective solution [2].Physical deposition techniques in the case of low temperature and wet-chemical techniques[3,4]are more suitable for the deposition and application of biological coating [5].At lower temperature,the deposition of calcium phosphates(CaP)on magnesium substrate can reduce the adverse effect on the mechanical properties of the matrix during the preparation process.Therefore,it is necessary to choose a suitable low-temperature preparation method to prepare hydroxyapatite coating on the surface of magnesium alloy.
Calcium phosphate biological coating includes many types,there are mainly DCPA (CaHPO4),DCPD (CaHPO4▪2H2O),OCP (octacalcium phosphate,Ca8H2(PO4)6▪5H2O) and α-TCP (Ca3(PO4)2),β-TCP(Ca3(PO4)2),HAP(hydroxyapatite),etc.These coatings having different properties can be applied to the surfaces of metal implant with different functional requirements to improve the surface properties of the contact between the implants and human tissues[6].Hydroxyapatite is the main component of human bone,accounting for 60%–70%of the bone,which has excellent biocompatibility and is widely used as tissue replacement material and coating material for metal implants.Hydrothermal method is mainly used for the synthesis of crystals,zeolites and ceramic powders[7].The basic principle is to create a high-temperature and high-pressure reaction environment by heating up in a closed kettle,and use water or organic solvents as reaction medium to prepare nanomaterials.Therefore,hydrothermal method is usually used to synthesize hydroxyapatite powder in the chemical industry [8],but hydrothermal deposition of hydroxyapatite coating on the surface of magnesium alloy is a newly proposed synthesis process.Sachiko Hiromoto et al.[9]successfully synthesized the hydroxyapatite coating by changing different pH values on the metal matrix of pure magnesium,AZ31 and AZ61,and studied the effect of Ca2+concentration on the surface morphology and composition of the coating.Li KaiKai et al.[10]synthesized the protective calcium phosphate composite coating on ZK60 magnesium alloy matrix by hydrothermal method,and the inner and outer layers were composed of calcium-deficient hydroxyapatite (Ca-def HA) and dicalcium phosphate(DCPA),respectively.However,the influence mechanism ofhydrothermal method on the preparation of hydroxyapatite coating is not well revealed and the degradation mechanism of prepared coating is unknown,which needs further research.Asif Ali et al.[11]deposited monetite(CaHPO4)coating on the surface of AZ91-3Ca magnesium alloy with diammonium hydrogen phosphate ((NH4)2HPO4) and calcium nitrate (Ca(NO3)2⋅4H2O) as the starting reagent,and the result of potentiodynamic polarization scan showed that CaP coating reduced the corrosion rate of magnesium alloy by 80%compared with bare Mg-alloy.Sachiko Hiromoto et al.[12]conducted in vivo implantation experiments,found that the corrosion rate of HAP-AZ31 coating decreased by about 20% compared with OCP-AZ31 coating.This indicates that the corrosion resistance of the composite coating containing other phases is inferior to that of pure HAP coating,so pure HAP coating deposition is the experimental direction to further reduce the corrosion rate[13–15].Under certain conditions,OCP and DCPD with low crystallinity can be converted into HAP with low solubility as precursors [16].So,it is of great significance to study the growth mechanism of HAP crystal and the precipitation behavior of different calcium phosphate phases.
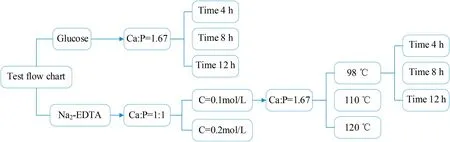
Fig.1.Flow chart of experimental scheme.
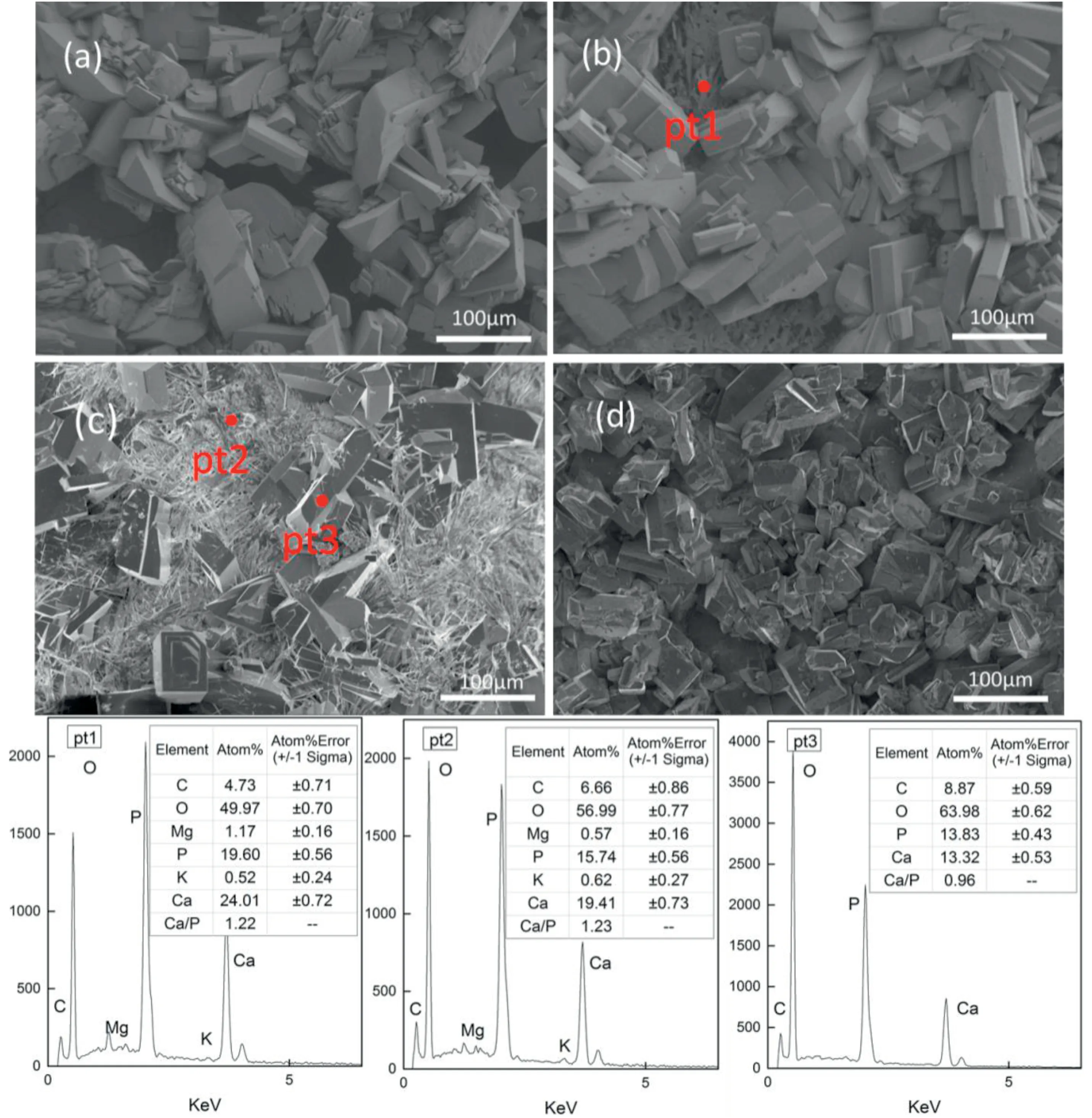
Fig.2.SEM diagram and energy spectrum analysis of glucose hydrothermal synthesis of calcium phosphate coating,Ca/P=1.67.(a)4 h;(b)8 h;(c)12 h;(d)control group:8 h without C6H12O6.
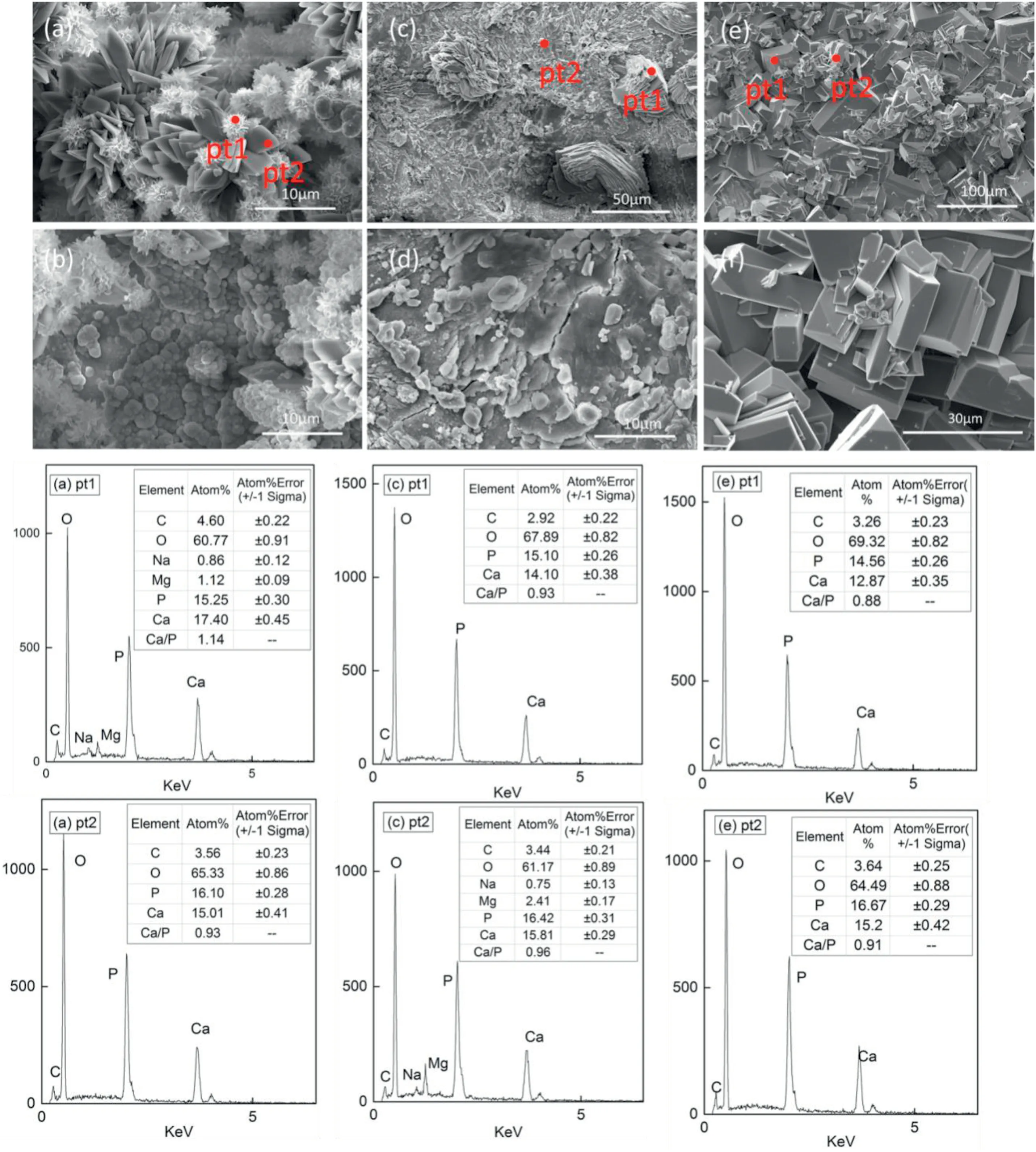
Fig.3.SEM diagram and energy spectrum analysis of Na2-EDTA hydrothermal synthesis of calcium phosphate coating,Ca/P=1:1(a) (b) 0.1 mol/L;(c) (d) 0.2 mol/L;(e) (f) control group:without Na2-EDTA.
This paper,the hydroxyapatite coating with petal structure on the surface of magnesium alloy was prepared using hydrothermal synthesis method,the influence mechanism of the chelating agent,reactant concentration,Ca–P ratio,hydrothermal synthesis time and synthetic temperature on the coating were studied,and the deposition mechanism of hydrothermal synthesis coating was put forward.The results showed that the prepared HAP coating hindered the pitting corrosion of magnesium alloys and improved the corrosion resistance of the magnesium alloy.
2.Test material and methods
The substrate material was AZ31B which was cut into square specimens(10 mm× 10 mm× 2 mm).The specific components of the alloy are shown in Table 1.The samples were mechanically polished up to 2000# by SiC sandpaper,then ultrasonic cleaned with ethanol and deionized water respectively,each for 5 min,and then dried in the air.The Ca(NO3)2and KH2PO4reagents were selected as the sources of calcium and phosphorus ions.Glucose (C6H12O6) and disodium ethylene diamine tetraacetic acid(Na2-EDTA)were selected as additives.Glucose can coordinate with metal ions in aqueous solution[17,18].When pH is adjusted to 6,EDTA ionizes H2Y2-ion [19],which can be used for chelating with magnesium.In addition,H2Y2-ion also has strong chelating effect on Ca2+,helping to improve the Ca2+concentration on the substrate.

Table 1 Chemical composition of AZ31B Magnesium alloy.
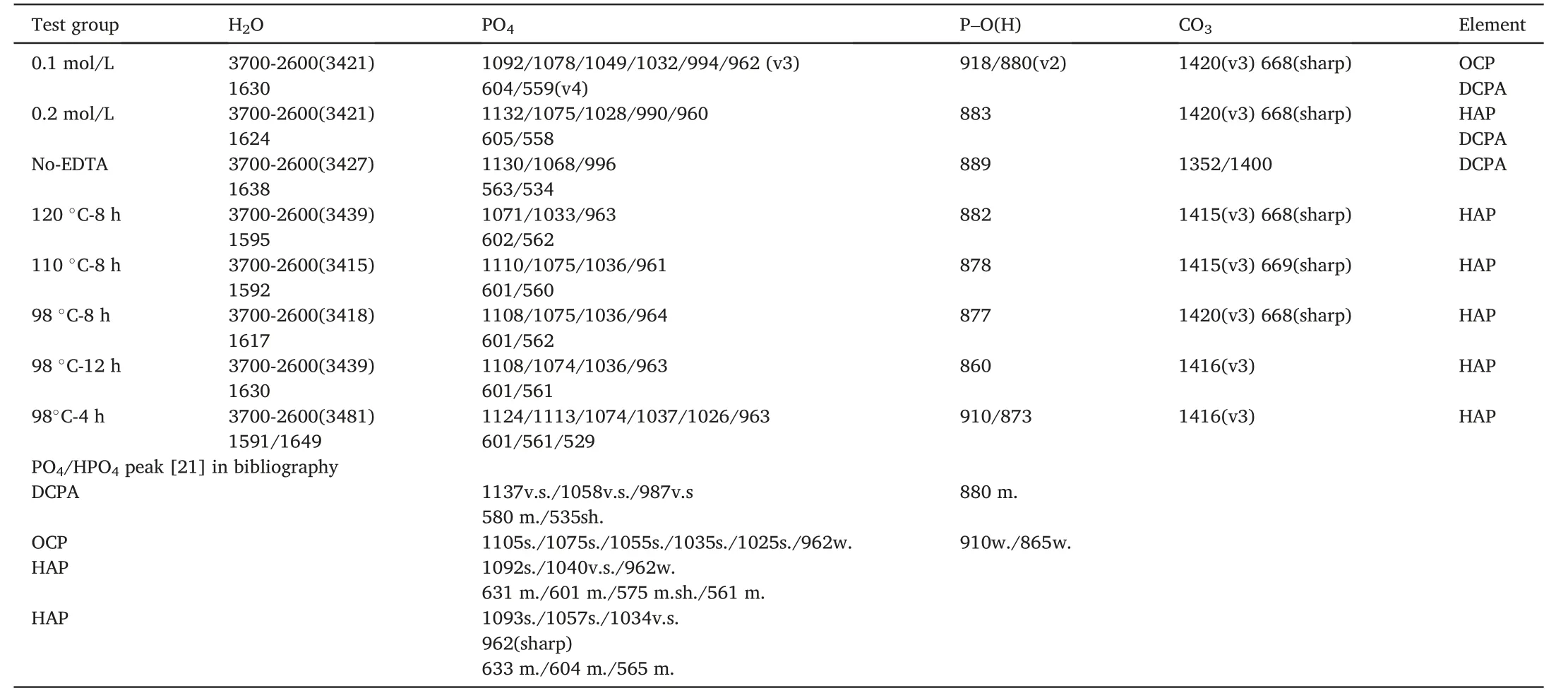
Table 2 Location of infrared absorption peak of calcium phosphate coating hydrothermal synthesized by Na2-EDTA under different conditions (unit:cm-1).
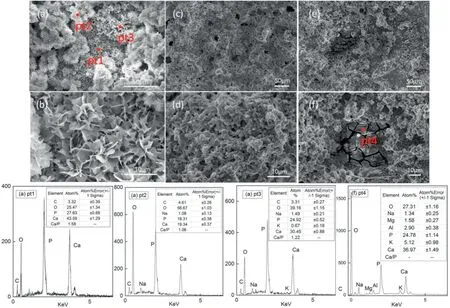
Fig.4.SEM diagram and energy spectrum analysis of Na2-EDTA hydrothermal synthesis hydroxyapatite coating under different hydrothermal temperature,Ca/P=1.67:1.
In the test scheme,the effects of chelating agents,the concentration of calcium and phosphorus,the solution treat temperature,the hydrothermal time and the Ca–P ratio on the properties of synthetic coating in reaction kettle were investigated.The hierarchical optimization method was adopted to control the single variable exploration,such as selecting the best concentration and exploring the influence of other factors under this concentration.
Fig.1 is the flow chart of experimental scheme.The glucose was first added as chelating agent in the design process.When the Ca–P ratio in solution was set at 1.67:1,the coating was obtained after 4 h,8 h and 12 h hydrothermal treatment in the solution.Secondly,Na2-EDTA was chosen as additive which can directly chelate Ca2+on the surface of the substrate to form a layer of monolayer.The effect of Ca2+concentration,temperatures,synthesis time and Ca/P were studied.The temperature setting should be lower than 200°C to ensure the mechanical properties of the matrix.The hydrothermal synthesis reaction was carried out in the lining of tailor-made ptfe reactor.The high-low temperature test chamber was selected to ensure stable heating rate of 5°C/min,and the equipment can meet the temperature range error of±0.1°C.
The surface morphology and element composition of the samples were measured by field emission scanning electron microscopy (ZEISS,GeminiSEM 300) and EDS energy spectrum (Oxford,MAX 80).FTIR(Thermo Fisher Scientific,IS10 AKX1401250),used to distinguish the functional groups of these phosphate ions,was applied to distinguish the surface composition and bonding relationship of the coating.The test piece(working electrode)was tested by the electrochemical workstation(CorrTest,CS350),and the electrochemical impedance spectroscopy test.The coated samples were immersed in a 37°C water bath with 100 ml of phosphate buffer solution (PBS) which can maintain a stable pH 7.4 as human body fluid.The composition of the PBS is 8 g/L NaCl,0.2 g/L KCl,1.44 g/L Na2HPO4and 0.24 g/L KH2PO4.
3.Test results
3.1.Microstructure and composition of hydrothermal composite coating
The surface morphology of the composite coating deposited with glucose in different time is shown in Fig.2.The control group is shown in Fig.2 (d).(a) (b) (c) are microstructures of the samples subjected the hydrothermal treatment with glucose for 4 h,8 h and 12 h respectively.There was a DCPA layer with a single structure formed on the surface in the first 4 h.And when the hydrothermal time prolonged for 8 h,the spicule overlayer apatite formed.The final composite coating after 12 h formed spicule structures that reduced the surface micro-voids,forming a coating structure to prevent from the direct contact between the corrosive solution and the matrix surface,as shown in (c).
Changing the type of the additives for metal chelating agents and the monolayer formed on the metal surface to continue the increasing concentration of Ca2+at the reaction interface.Fig.3 shows SEM diagram and energy spectrum analysis of Na2-EDTA hydrothermal synthesis of calcium phosphate coating.Ca–P ratio of solution was all set at 1:1.Fig.3(e) is the control group without Na2-EDTA.A large number of cuboid crystals whose Ca–P ratio was close to 1 grew on the surface.Obviously,CaHPO4deposited on the substrate surface.The composition of apatite often reduces the Ca–P ratio due to the introduction of Na+and K+plasma by cation substitution.Adding Na2-EDTA in hydrothermal treatment solution,as shown in Fig.3 (a) (b),not only the single CaHPO4crystal but also the underlying dense apatite coating and globular calcium phosphate generated.According to (a) pt2,(c)pt1,(e)pt1-2,the radiation petal shaped and block liked crystals are all DCPA.In conclusion,dicalcium phosphate is easy to grow on the surface of the matrix and presents different crystal morphology.
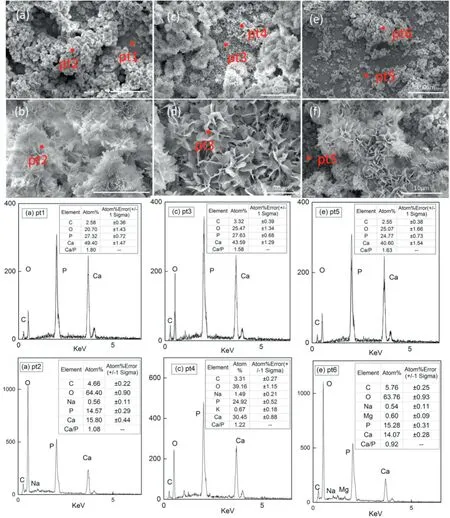
Fig.5.SEM diagram and energy spectrum analysis of Na2-EDTA hydrothermal synthesis hydroxyapatite coating at different hydrothermal time.(a)(b)4 h;(c)(d)8 h;(e) (f) 12 h;(b) (d) (f) enlarged versions of (a) (c) (e),respectively.
From the above experimental results,it can be seen that changing Ca–P ratio or chelating agents will affect the phases composition in the coating.DCPA crystals were easy to precipitate and deposit in the coating structure,no matter which additive was chosen.As mentioned above,when Ca/P=1:1,under the action of Na2-EDTA,the mixed coating structure formed.DCPA crystal rapidly grew into the radial pattern crystal morphology in several sites,instead of cuboid shape,which is different from the deposition with glucose.It may be due to the different growth order of DCPA and HAP under the action of different chelating agents.Na2-EDTA is a strong chelator due to its four lone pair electrons.It rapidly chelates in solution and deposites on the surface,meanwhile,DCPA grows on the surface with heterogeneous nucleation,and the two reactions occur simultaneously to produce HAP-DCPA coating.As seen from the experimental phenomena,glucose only reacts with magnesium to form carboxyl group and participates in the hydrothermal deposition of hydroxyapatite [17],and the reaction occurred in the later sedimentary,while additive Na2-EDTA can provide better nucleation conditions for HAP crystal.The calcium/phosphorus molar ratio and chelating agent play a key role in promoting the nucleation of HAP.
3.2.Microstructure and composition of hydrothermal HAP coating
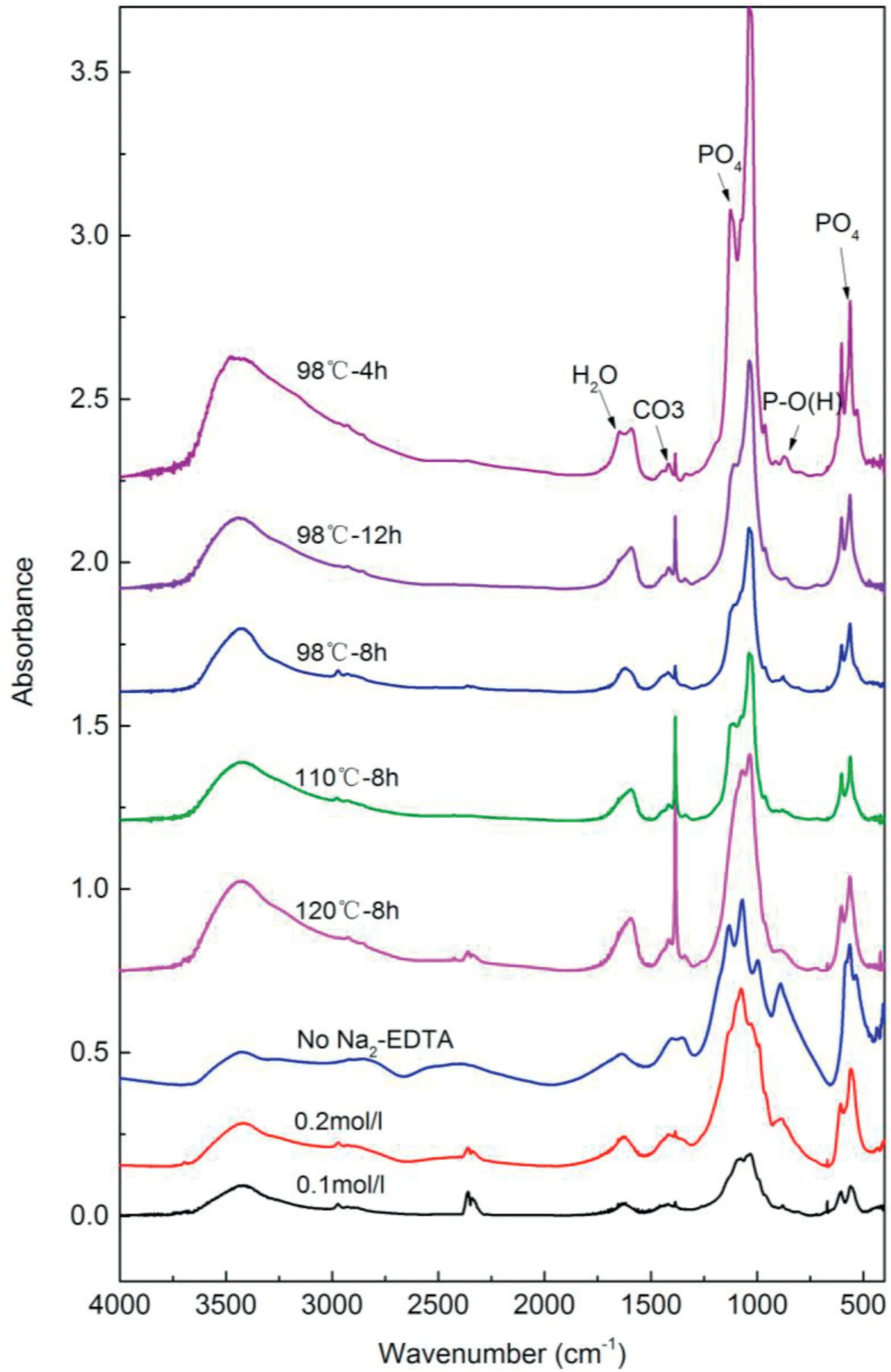
Fig.6.FTIR Spectrogram of calcium phosphate coating hydrothermal synthesized by Na2-EDTA under different experimental conditions.
In order to further synthesize HAP coating,the Ca–P ratio of the solution was adjusted to 1.67.The Na2-EDTA hydrothermal deposition coating at different temperatures is shown in Fig.4.The deposited coating was divided into two layers.According to (a) pt1,the bottom layer developed into a stable flake hydroxyapatite structure,with a Ca–P ratio up to 1.58.The crystal morphology of HAP coating was consistent with that of the relevant HAP coating in the bibliography [3,20].The Ca–P ratio plays an important role in the growth of hydroxyapatite coating.The increased Ca–P ratio inhibits the growth of dicalcium phosphate and promotes the growth of HAP during the nucleation process.It was found that the morphology of HAP coating was affected by the experimental temperature in hydrothermal solution.At the synthesis temperature of 110°C and 120°C,pores and undeposited areas appeared on the surface of the coating.At 120°C,even the surface of the substrate was covered by corrosion products,which contained magnesium and aluminum.It shows that in hydrothermal process,overly high temperature affected the heterogeneous nucleation and crystal growth of HAP crystals,which is considered to be due to that large amount of Mg2+generated the corrosion process of the matrix,which inhibits HAP nucleation on the interface.And the homogeneous HAP coating layer depends on well combination between the coating and magnesium matrix,making sure that the initial crystal growth bonding surface was intact.When the temperature was 98°C in the hydrothermal synthesis environment,1 μm of flake HAP coating could be deposited on the substrate surface of AZ31B magnesium alloy.
In order to explore the growth of flake HAP,the experimental temperature was controlled at 98°C.Fig.5(a)(c)and(e)show the coating deposited under the action of Na2-EDTA for 4 h,8 h and 12 h,respectively.The surface porous layer and the underlying compact layer formed from the initial stage and run through the whole process.According to the energy spectrum of pt1,pt3 and pt5,the underlying Ca/P was 1.80,1.58 and 1.63,respectively.The elements were only C,O,P and Ca,andthe Ca–P ratio of bottom deposition crystal was close to that of HAP,so the composition of bottom coating is considered as HAP.It can be observed that the underlying morphologies (d) and (f) are wellcrystallized flake HAP crystal structures.EDS spectrogram of pt2,pt4 and pt6 have reduced Ca–P ratio due to the introduction of sodium ions in the base,and the reduction of these cations concentration in the solution was more conducive to the deposition of high-purity hydroxyapatite.As the hydrothermal time increased the tectorium with poor surface binding degree decreased,as shown in Figure (c) (e).However,the prolonging of hydrothermal time increased the thickness of the underlying dense HAP layer,which is conducive to the growth of flake HAP crystals.
There are many factors to obtain high purity flake HAP crystal and dense layer structure.From the perspective of reaction kinetics,increasing the concentration of Ca2+at the interface can adsorb a large number of anions such as PO43-,which can create a good HAP crystallization environment [21].Substrate corrosion will also affect the entire deposition process,therefore it is necessary to avoid pits,cracks,voids and uncovered areas forming on the surface due to excessive concentration,temperature or deposition time.
3.3.Infrared spectroscopic analysis
Fig.6 shows the infrared spectrogram analysis of apatite coating deposited with Na2-EDTA under different conditions.When the ratio of calcium to phosphorus was 1:1 and the concentration of solution was 0.1 mol/L,The peaks of PO43-and CO3appeared,and according to the peak absorption of PO43-in OCP [21],as shown in Table 2,that the weak spectral band at 910 cm-1and 865 cm-1is the out-of-plane bending mode of POH,it is generally considered to be a typical feature of OCP.the hair globule and the underlying apatite composition contain OCP as shown in Fig.3 (a).Under the condition of a solution concentration of 0.2 mol/L and Ca/P=1:1,the absorption peaks of PO43-(v3 and v4)appeared in the infrared spectrogram.There were two absorption peaks of water molecules in 3400 cm-1and 1624 cm-1.In addition,a CO3absorption peak also appeared.According to the comparison and analysis of the corresponding infrared spectrogram the structure composition of the synthetic coating was a lamellar mixture of DCPA and calcium-deficient HAP.The control group without Na2-EDTA showed that the absorption peaks ofwhich were consistent with the absorption peaks of DCPA in Table 2.The sharp absorption peak at 1384 cm-1in some groups was due to the retention of nitrate ions in the solution of hydroxyapatite samples during the synthesis process.
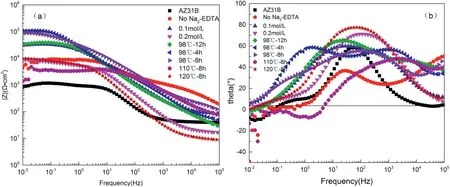
Fig.7.Bode diagram of Na2-EDTA hydrothermal synthesis of calcium and phosphate coating under different experimental conditions (a) Impedance modulus-Frequency (b)Phase angle -Frequency.
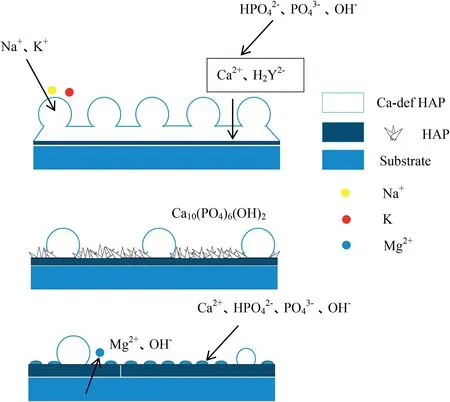
Fig.8.Deposition mechanism of hydrothermal synthesis of hydroxyapatite coating.
The calcium/phosphorus ratio of solution was adjusted at 1.67:1 in groups of 120°C-8 h,110°C-8 h,98°C-8 h,and 98°C-12 h.The characteristics of infrared absorption spectra were similar at different temperatures,and the synthesized coatings were all hydroxyapatite coatings,as compared with the PO4absorption peak characteristics of bibliography[22].At the same time,corresponding to the conclusion of energy spectrum scanning in Fig.5,HAP coating with intact structure deposited under the condition of Ca/P=1.67.What’s more,the frequent ion exchange of porous layers can effectively delay or postpone the dissolution of the underlying coating,which plays a positive role in the deposition process and corrosion stage.
3.4.EIS test
Fig.7 shows the impedance Bode diagram of Na2-EDTA hydrothermal coating.According to the phase angle-frequency diagram,the appearance of negative phase angle constant below line 0 is the induced reactance region [23].In an erosive environment,there are permeable channels between DCPA block crystals in the control group,so the solution in direct contact with the matrix produces inductive resistance and pitting [24].As the experimental conditions set with the Ca/P of 1:1,calcium phosphate solution concentration at 0.1 mol/L,impedance value reached 3.3 × 104Ω▪cm2,which is sharply increased compared to the value of the substrate 8.5 × 102Ω▪cm2.The impedance value of experimental group with 0.2 mol/L solution concentration decreased to 8.8×103Ω▪cm2.The characteristics of the coating morphology indicate that the excessive nucleation rate of the crystal resulted in the disordered lamellar growth of the coating and the generation of micron-scale pits and cracks,which caused the decrease of corrosion resistance of the coating.It is indicated that overly high solution concentration is unfavorable to the coating deposition.
In the phase angle -frequency diagram of hydroxyapatite coating deposited at different temperatures,the induced reactance also appeared at 110°C.When the temperature was too high,the corrosion medium corroded through the surface cavity by the contact with the matrix,and the corrosion resistance could not be improved.When the temperature set to 98°C,the impedance value of the coating increased obviously compared to the matrix impedance,reaching the value of 1.0 × 105Ω▪cm2.The coating surface of 8 h depositing hydroxyapatite had good crystallinity and could resist the corrosion of chloride ion solution.
3.5.Mechanism of coating deposition
According to the influence of hydrothermal synthesis time,the deposition mechanism model of hydrothermal synthesis coating with time was proposed in this paper,as shown in Fig.8.Thin hydroxyapatite grew on the initial substrate,and the apatite porously accumulated in the upper layer indicates cations exchanged in the hydration layer channel of the apatite crystal structures to form calcium-deficient apatite layer.The initial deposition process determines whether the coating can grow coating structure with good performance in later stage.The ionized HY2-ion of EDTA chelated Ca2+on the surface of the magnesium alloy matrix.The Ca2+concentration increases rapidly and continues to be adsorbed.The anions,OH-are further adsorbed,HAP and OCP converted to HAP are reacted at the interface.The possible reaction equations are shown in Eqs.(1)and(2).Then the upper coating further falls off,and the bottom hydroxyapatite deposits thicken.However,in the later hydrothermal stage,the growth morphology of HAP changes with the decrease of reactant ion concentration,and magnesium appears in the surface composition of the coating[8,25].
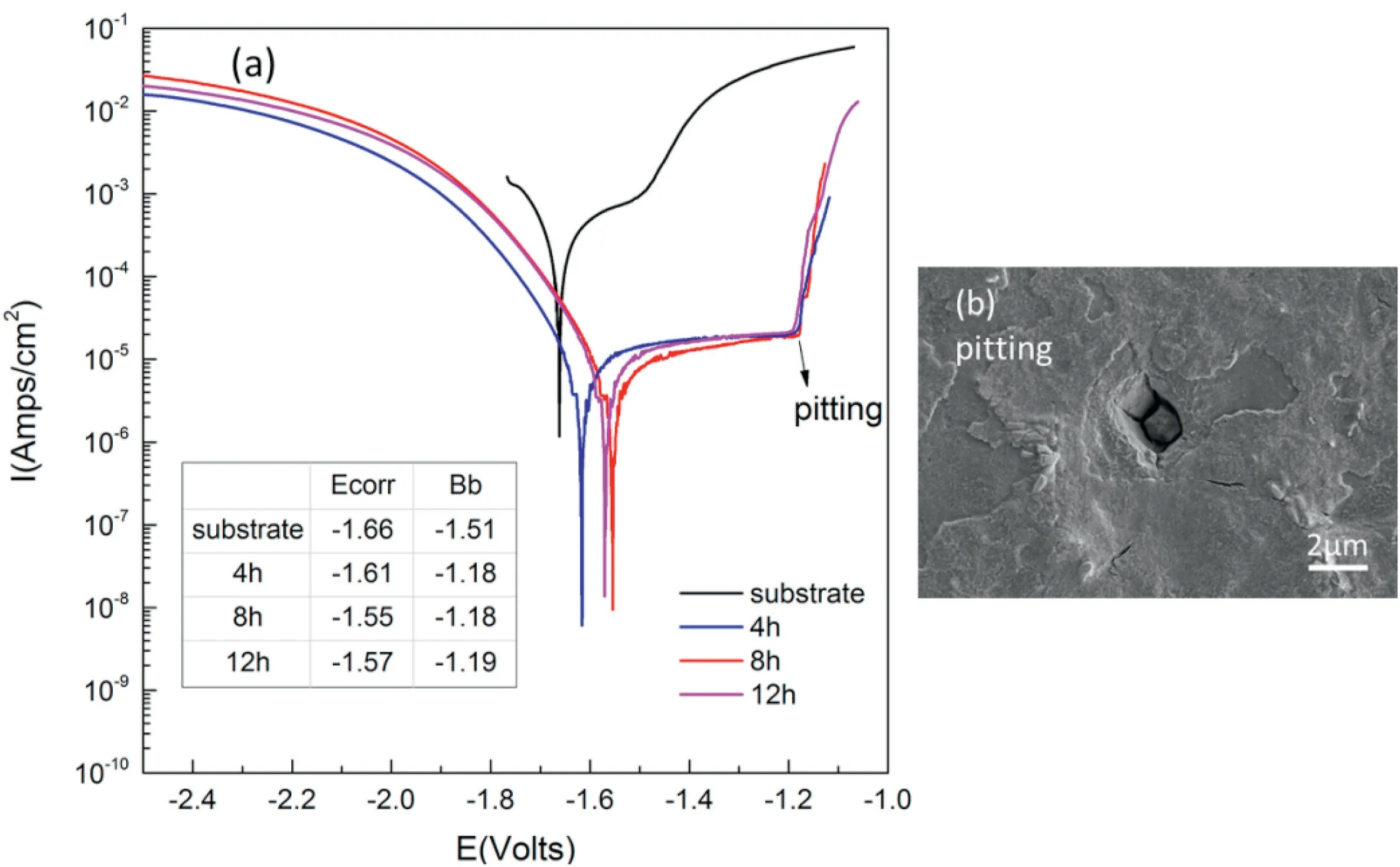
Fig.9.Polarization curves of AZ31B magnesium alloy and HAP coating(a)polarization curves(b)SEM image of pitting corrosion of AZ31B after soaking in SBF for 1 h.
In the hydrothermal reaction process,the crystal nucleation speed of the loose layer structure was fast,but it was precisely because of overly rapid growth that leaded to poor adhesion,incompletion and agglomeration phenomenon.In comparison,the crystal growth speed of the bottom substrate surface was moderate and formed a good structure.That is to say,with the passage of time,the hydroxyapatite coating can be completely deposited on the magnesium alloy substrate surface.
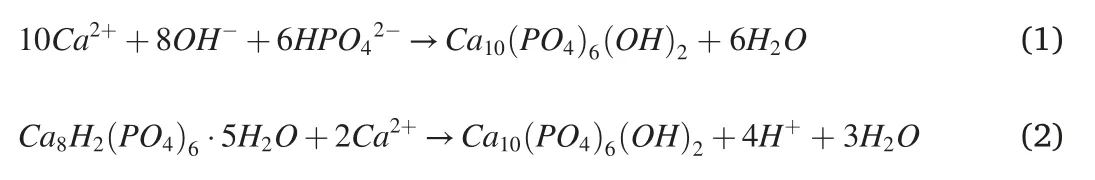
The effect of hydrothermal time on the corrosion resistance of HAP coating was studied by conducting potentiodynamic polarization test,and the test used PBS as the corrosion solution.PBS can stabilize pH of the solution with contains NaCl,KCl,Na2HPO4,and KH2PO4.Fig.9 shows the polarization curve of corrosion system.The corrosion potential Ecorr of HAP coating increases from -1.51 v to -1.18 v compared with bare metal.The increase of corrosion potential means the increase of corrosion resistance tendency.The initial corrosion of magnesium alloy is characterized by pitting corrosion where Bb represents the pitting potential.In the aspect of delaying corrosion,the HAP coating deposited at 98°C has a low corrosion tendency,and the corrosion potential of the coating is decreased in the hydrothermal environment for 12 h,corrosion channels begin to appear.Magnesium ions corroded from the substrate was observed to enter the coating structure under the hydrothermal environment for a long time,which results in the further corrosion of the magnesium matrix,and the possible coating defects in the coating reduced the corrosion resistance.
4.Conclusion
In this paper,calcium and phosphorus biological coatings were deposited on the surface of magnesium alloys by chelation of additives,and the coating type,composition and morphology were changed by controlling the change of experimental conditions,so as to obtain HAP coatings that can delay the corrosion rate of magnesium alloys.The main experimental conclusions are as follows:(1) control the solution concentration at 0.1 mol/L and the calcium/phosphorus ratio is 1.67.Under the action of Na2-EDTA chelating agent,the growth of calcium hydrophosphate can be avoided and the petal-like hydroxyapatite coating structure can be prepared on the surface of AZ31.The key influencing factors were chelation and the ratio of calcium to phosphorus,and the excessive temperature and solution concentration had adverse effects on the coating.(2)A small amount of CO3in aqueous solution can easily be substituted within HAP.However,Na+,K+and Mg2+were replaced with the superstructure HAP by cations,and Ca2+deficiency reduced the coating calcium-phosphorus ratio.(3) In the proposed coating deposition mechanism model,HAP coating is a two-layer structure model.The crystallization growth rate of the underlying HAP is slow,and it requires sufficient hydrothermal synthesis time for the formation of flake structure crystals.However,too long time is not conducive to the improvement of coating corrosion resistance.(4) In this experiment,1 μm of flake HAP coating was deposited on the substrate of AZ31B magnesium alloy,and the prepared AZ31B-HAP coating achieved good corrosion resistance.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors gratefully acknowledge the support for this work from the National Natural Science Foundation of China (No.51872237,51801159,21301161 and12072273),Natural Science Basic Research Plan in Shaanxi Province of China (No.2017JM5098),Aeronautical Science Foundation of China (No.2017ZF53069),Filed Foundation of Equipment Pre-research (No.61409220304),Provincial Nature Science Foundation of Shaanxi(No.2018JM5032).
 Progress in Natural Science:Materials International2021年2期
Progress in Natural Science:Materials International2021年2期
- Progress in Natural Science:Materials International的其它文章
- Introduction to Progress in Natural Science:Materials International
- Effect of superplastic deformation on precipitation behavior of sigma phase in 3207 duplex stainless steel
- Reversible hydrogenation of AB2-type Zr–Mg–Ni–V based hydrogen storage alloys
- Formation mechanism of interfacial microstructures and mechanical properties of Ti2AlNb/Ni-based superalloy joints brazed with NiCrFeSiB filler metal
- Influence of γ’ precipitate on deformation and fracture during creep in PM nickel-based superalloy
- Tuning microstructure,transformation behavior,mechanical/functional properties of Ti–V–Al shape memory alloy by doping quaternary rare earth Y
