Reversible hydrogenation of AB2-type Zr–Mg–Ni–V based hydrogen storage alloys
Yong Wu ,Yunting Peng ,Xiojing Jing ,Hui Zeng ,Zeyun Wng ,Jie Zheng,* ,Xingguo Li,**
a Beijing National Laboratory for Molecular Sciences (BNLMS),College of Chemistry and Molecular Engineering,Peking University,Beijing,100871,China
b Wuhan Institute of Marine Electric Propulsion,Wuhan,430064,China
Keywords:Laves phase Hydrogen storage Zr–Mg–Ni–V alloy Reversible hydrogenation Vanadium substitution
ABSTRACT The development of hydrogen energy is hindered by the lack of high-efficiency hydrogen storage materials.To explore new high-capacity hydrogen storage alloys,reversible hydrogen storage in AB2-type alloy is realized by using A or B-side elemental substitution.The substitution of small atomic-radius element Zr and Mg on A-side of YNi2 and partial substitution of large atomic-radius element V on B-side of YNi2 alloy was investigated in this study.The obtained ZrMgNi4,ZrMgNi3V,and ZrMgNi2V2 alloys remained single Laves phase structure at asannealed,hydrogenated and dehydrogenated states,indicating that the hydrogen-induced amorphization and disproportionation was eliminated.From ZrMgNi4 to ZrMgNi2V2 with the increase of the degree of vanadium substitution,the reversible hydrogen storage capacity increased from 0.6 wt%(0.35H/M) to 1.8 wt% (1.0H/M),meanwhile the lattice stability gradually increased.The ZrMgNi2V2 alloy could absorb 1.8 wt%hydrogen in about 2 h at 300 K under 4 MPa H2 pressure and reversibly desorb the absorbed hydrogen in approximately 30 min at 473 K without complicated activation process.The prominent properties of ZrMgNi2V2 elucidate its high potential for hydrogen storage application.
1.Introduction
Hydrogen energy is a promising alternative to fossil for its abundance,high energy density and cleanliness.However,considerable difficulties associated with its liquefaction,inflammable and explosive property.Therefore,safe and efficient hydrogen storage technology is the most difficult technical bottleneck in the large-scale application of hydrogen energy [1–5].Hydrogen storage alloy is the most classic hydrogen storage materials and has been successfully used in nickel metal hydride(Ni-MH) batteries,which features high volume hydrogen density,excellent reversibility and kinetics for hydrogen absorption and desorption.These alloys consist of A-side hydride-forming elements and B-side non-hydride-forming elements with different stoichiometry and crystal structure,such as AB5-type LaNi5,AB-type TiFe,AB2-type YNi2,A2B-type Mg2Ni and AB3-type LaCo3,etc.[6–15]Nevertheless,the low hydrogen storage density restricts the application of hydrogen storage alloy.Currently,lithium ion batteries gradually replace Ni-MH batteries for its higher energy storage density in various application fields [16,17].Therefore,it is of great significance to explore new high-density hydrogen storage alloys.
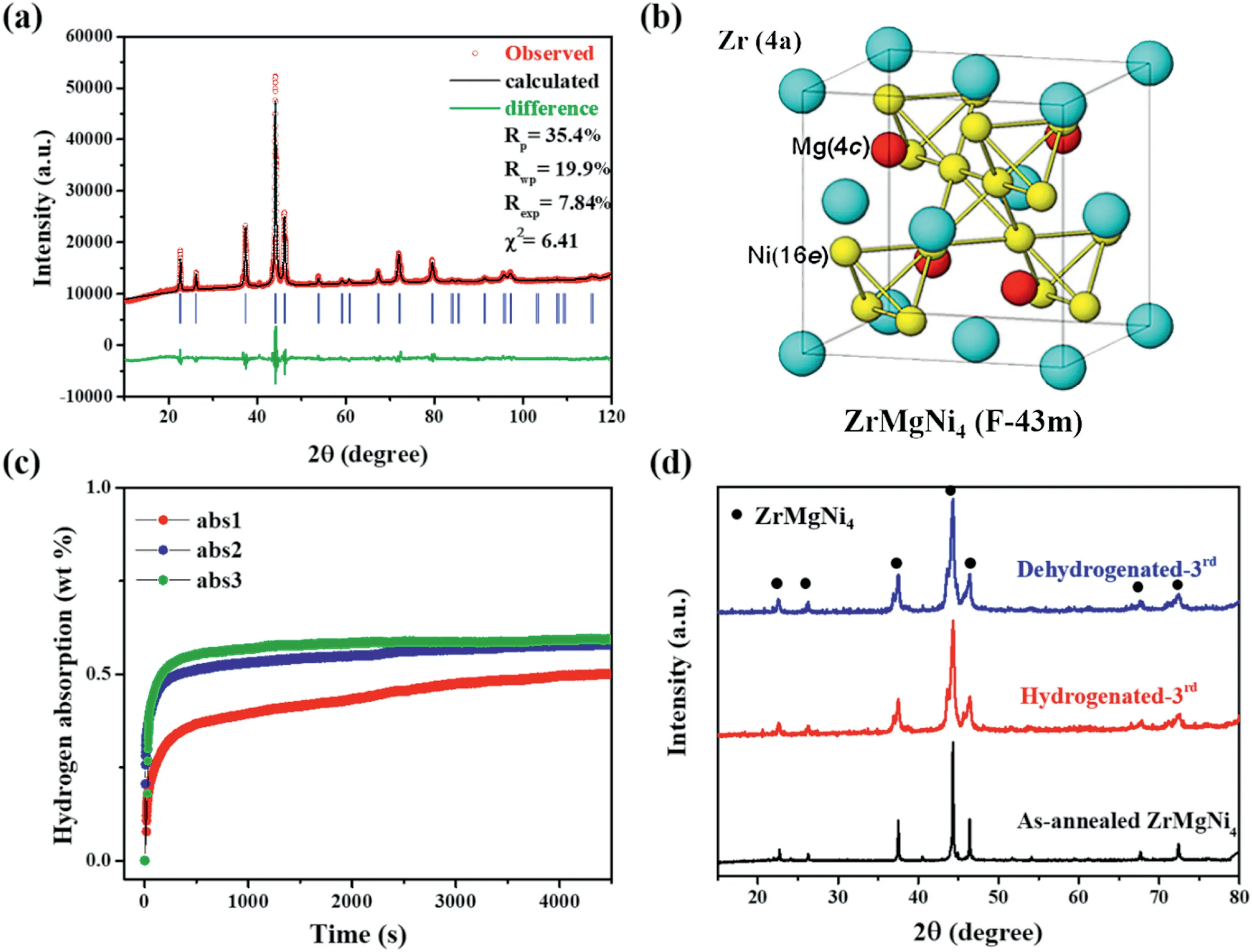
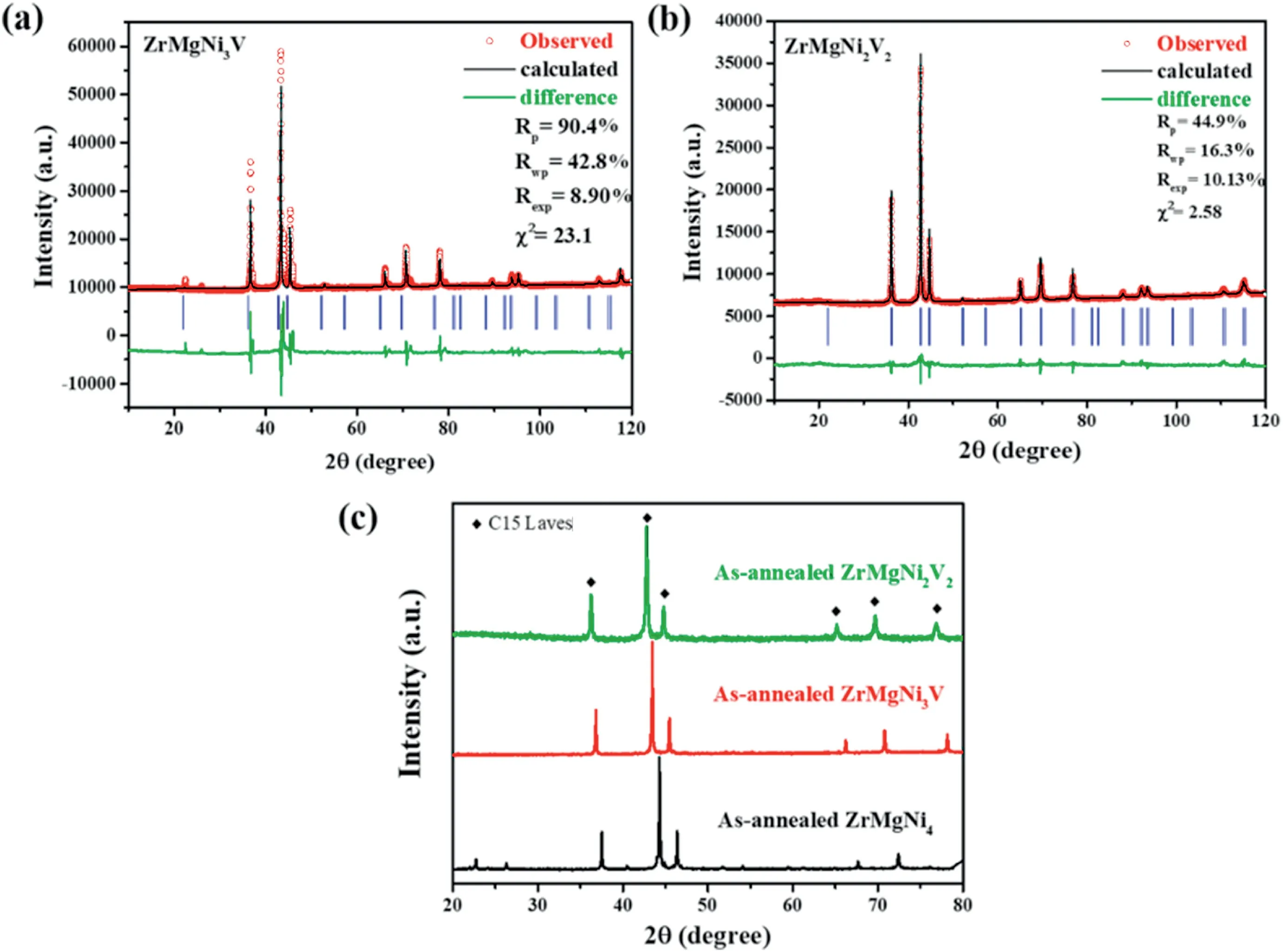
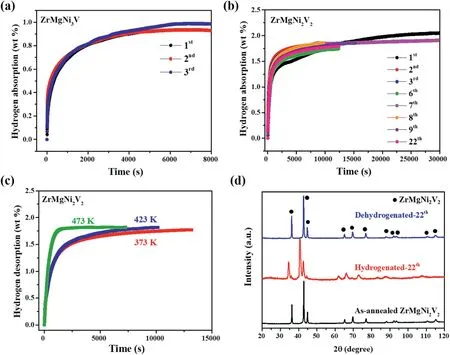
Among the enormous hydrogen storage alloys,Laves-phase-structure AB2-type alloys with the A-elements of Ti,Zr,Y,La etc.and the B-elements of V,Cr,Mn,Fe,Ni etc.transition element (TM) receive great attention due to their relatively high hydrogen storage capacities.TiCrMn and ZrFe2etc.based alloys are extensively investigated for the application in high-pressure hydrogen tank and thermally driven hydrogen compressor due to their high equilibrium pressures [18–20].(Ti1-yZry)TM(0 However,most of rare earth based AB2-type alloys such as YFe2and YNi2are not regard as suitable hydrogen storage materials since they suffer hydrogen-induced amorphization and disproportionation during the hydrogenation and dehydrogenation process.Taking YFe2as the example,its hydride tends to decompose to Fe and YH3at elevated temperature,breaking the hydrogen storage cycle owing to the high thermostability of YH3and YH2[23–27].K.Aoki et al.found that the AB2-type compounds with the ratio of the radius of A to the radius of B(rA/rB) above 1.37 are tend to amorphized by hydrogenation [28].As a consequence,the hydrogen-induced amorphization and disproportionation of AB2-type alloys can be prevented by the decreasing of rAor increasing of rB.Indeed,Zhu et al.achieved the hydriding reversibility by partial Y substitution with Zr,Ti and partial Al substitution with Fe in YFe2,while their desorption capacities are less than 0.9 wt% at 473 K[29–31].I.Yu.Zavaliy et al.achieved the reversible hydrogen storage by half Y substitution with Mg in YNi2,merely the hydride (YMgNi4H4) is thermodynamically unstable[32].The radii of Y,Fe,Ni,Zr,Ti,Al,Mg,V are 1.81 Å,1.241 Å,1.246 Å,1.60 Å,1.448 Å,1.431 Å,1.60 Å,1.31 Å,respectively[33]. To obtain novel high-capacity hydrogen storage alloys,we partially substituted Y with Zr in Y0.95Ni2and successfully suppressed the hydrogen-induced amorphization and disproportionation when more than half of Y was substituted (Fig.S1).Despite all that,the absorption capacity of the obtained Y0.45Zr0.5Ni2was only 0.9 wt% at 373 K(Fig.S2). In order to increase the capacity,we further substituted the remaining Y with Mg and partially substituted Ni with V.Here we report the novel AB2-typed reversible hydrogen storage alloy ZrMgNi4-xVx(Zr0.5Mg0.5Ni2-x/2Vx/2,x=0,1,2) without hydrogen-induced amorphization and disproportionation.The reversible hydrogen storage capacity of ZrMgNi2V2,the alloy with the best performance,was 1.8 wt% at 300 K–373 K.The capacity was over that of the AB5-type alloys,making it a great promising hydrogen storage material. The ZrMgNi4-xVx(x=0,1,2) alloys were prepared from Zr (99.9%purity,Trillion Metals Co.,Ltd.),Mg (99.9% purity,Beijing General Research Institute for Nonferrous Metals),Ni (99.9% purity,Trillion Metals Co.,Ltd.) and V (99.9% purity,Trillion Metals Co.,Ltd.) by induction melting at 1073 K for 20–30 min in a high purity argon atmosphere.10 wt%excess Mg was used considering its evaporation,and the induction melting was conducted for 3–4 times to evaporate all of the extra Mg.The obtained ingots were homogenized at 873 K in a sealed quartz tube(~10 Pa)for 3 days to obtain a single phase,and then were polished and pulverized into powder with the size less than 300 mesh in an argon filled glove box for further measurement. The material was characterized by X-ray diffraction (XRD,Rikagu PANalytical X’Pert3Powder,Cu Kα) and scanning electron microscopy(SEM,Zeiss,Merlin Compact).The hydrogen storage properties were measured in a home-made Sievert apparatus [34].About 0.2 g sample was sealed into the sample holder and activated in dynamic vacuum at 473 K for 2 h before every hydrogen absorption kinetic measurement.The hydrogen absorption kinetic was measured under 4.0 MPa hydrogen pressure at 300 K.The hydrogen desorption kinetic was measured using the hydrogenated samples under static vacuum by heating from room temperature to the designed temperature(373–473 K),and then holding the temperature. Fig.1.(a)The observed(circles),calculated(line)and difference(bottom line)X-ray powder diffraction patterns,(b)the unit cell,(c)the hydrogen absorption kinetic curves (300 K,4 MPa H2) and (d) the X-ray powder diffraction patterns after hydrogenation and dehydrogenation for the ZrMgNi4 alloy. The observed,calculated and differential XRD patterns of the asannealed ZrMgNi4samples are shown in Fig.1(a).All the diffraction peaks can be indexed as an MgSnCu4type cubic structure with a space group of F-43m (No.216),which can be considered also as a derivative for the structure of cubic Laves phase(MgCu2type,C15 Laves phase,Fd-3m) [35,36].No binary phases with analogous structure were found to exist.Based on the Rietveld refinement data,the lattice constants were calculated to be a=b=c=6.81756 Å [37].ZrMgNi4is isostructural with REMgNi4(RE=Sc,Y,La,Ce,Pr,Nd,Sm,Gd,Tb,Dy,Ho,Er,Tm,Yb,Lu),YMgCo4,YMgCu4,YMgCo2Ni2,CeMgNi2Co2,CeMgNi2Cu2,Nd0.5Ca0.5MgNi4,Er0.5Ca0.5MgNi4alloys [32,38–40].Compared to the structure of YNi2,the coordination number of each atom in ZrMgNi4remained unchanged,yet the position of Ni at the site of 16e shifted slightly(Fig.1(b))[32]. As shown in Fig.1(c) and (d),the reversible hydrogenation of ZrMgNi4was achieved without hydrogen-induced amorphization and disproportionation.After two hydrogen absorption and desorption cycles,ZrMgNi4could absorb 0.6 wt% hydrogen within 20 min under 4.0 MPa H2at 300 K.The component of the hydrogenated alloy is ZrMgNi4H2.The hydrogenation of YMgNi4compound leads to a distortion of the cubic structure,forming YMgNi4H4with an orthorhombic structure[32].Since the radius of Zr is smaller than that of Y,ZrMgNi4H2remains the cubic structure.In spite of that,the crystallinity becomes poor after hydrogenation and dehydrogenation as the X-ray diffraction peaks obviously broaden for the ZrMgNi4alloy(Fig.1(d)).Moreover,the hydrogen storage capacity of ZrMgNi4is too low.To increase the capacity and stability,the Ni was partially substituted by V with a larger atomic radius on the basis of ZrMgNi4. The XRD patterns of the ZrMgNi3V and ZrMgNi2V2alloys were refined,as shown in Fig.2(a) and (b).ZrMgNi4,ZrMgNi3V and ZrMgNi2V2are isostructural,while the lattice constants were calculated to be a=b=c=6.92071 Å and 7.01692 Å for ZrMgNi3V and ZrMgNi2V2,respectively.The unit cell became bigger along with the increase of the amount of V,which corresponds with the left shift of X-ray diffraction peaks from ZrMgNi4to ZrMgNi2V2(Fig.2(c)). With regard to most AB2-type alloys,hydrogen preferably occupies A2B2and/or AB3position[41].On the one hand,large-volume tetrahedral interstices constructed by large-radius atoms are favorable for the hydrogen occupation.On the other hand,more outer electrons of the alloy would cause stronger repulsive interaction to the hydrogen atoms,and thus hindering the hydrogen atoms into the lattice according to Wagner’s theory[30].Therefore,V is often used as a modifying element in AB2-type hydrogen storage alloys since it helps increase the hydrogen storage capacity due to its relatively large atomic radius and fewer outer shell electrons [42].Furthermore,V substitution in ZrMgNi4would increase the lattice stability by decreasing the rA/rBvalue to prevent the hydrogen-induced amorphization and disproportionation. The SEM images of the ZrMgNi3V and ZrMgNi2V2alloys(Fig.3(a)and(b))show that the alloy particles exhibit irregular morphology with uneven size.The corresponding elemental mapping images(Fig.S3 and S4)reveal that the compositional distributions of the four elements(Zr,Mg,Ni and V)in the ZrMgNi3V and ZrMgNi2V2samples were uniform,further confirming the alloy structure. According to the cyclic hydrogen absorption kinetic curves,the reversible hydrogen storage capacities were 0.9 wt%and 1.8 wt%for the ZrMgNi3V alloy and the ZrMgNi2V2alloy,respectively(Fig.4(a)and(b)).The corresponding hydrides were ZrMgNi3VH3and ZrMgNi2V2H6,respectively.Compared to ZrMgNi4,ZrMgNi3V and ZrMgNi2V2were easier to be activated as the difference of hydrogen absorption rates was small in the first three cycles.The hydrogen storage capacities increased along with the increase of the amount of V in ZrMgNi4-xVxas expected. For the high-capacity alloy ZrMgNi2V2,twenty-two hydrogen absorption and desorption cycles were achieved without remarkable decrease of capacity and reaction rates.ZrMgNi2V2could absorb 1.8 wt%hydrogen in about 2 h at 300 K under 4 MPa H2pressure (Fig.4(b)).ZrMgNi2V2H6could release the hydrogen,it stores in about 3 h at 373–423 K,and in approximately 30 min at 473 K (Fig.4(c)).ZrMgNi2V2H6remained the cubic structure and the crystallinity of ZrMgNi2V2remained high even after twenty-two hydrogen storage cycles since the XRD patterns of the hydrogenated ZrMgNi2V2remained unchanged in the relative location of signal peaks,and the XRD patterns of the dehydrogenated ZrMgNi2V2had no obvious peak broadening phenomenon compared with the XRD patterns of the as-annealed ZrMgNi2V2(Fig.4(d)). Fig.2.Observed(circles),calculated(line)and difference(bottom line)X-ray powder diffraction patterns for(a)the ZrMgNi3V alloy and(b)the ZrMgNi2V2 alloy;(c)the X-ray powder diffraction patterns of ZrMgNi4-xVx (x=0,1,2). Fig.3.The SEM images of (a) the ZrMgNi3V alloy and (b) the ZrMgNi2V2 alloy. Fig.4.Hydrogen absorption kinetic curves(300 K,4 MPa H2)for(a)the ZrMgNi3V alloy and(b)the ZrMgNi2V2 alloy;(c)the hydrogen desorption kinetic curves at different temperatures and (d) the X-ray powder diffraction patterns after hydrogenation and dehydrogenation for the ZrMgNi2V2 alloy. ZrMgNi4-xVx(x=0,1,2) alloys composed of a single Laves-phase MgSnCu4type cubic structure with a space group of F-43m (No.216)have been prepared by induction melting.Based on the strategy of decreasing the value of rA/rBin AB2-type hydrogen storage alloys,ZrMgNi4-xVxalloys successfully avoid the hydrogen-induced amorphization and disproportionation and achieve reversible hydrogenation.The capacity and thermostability gradually increase with the increasing amount of V in ZrMgNi4-xVxsince V can expand the tetrahedral interstices and reduce the repulsive interaction to hydrogen atoms.The reversible hydrogen storage capacities are 0.6 wt%,0.9 wt%and 1.8 wt%for ZrMgNi4,ZrMgNi3V,and ZrMgNi2V2,respectively.The crystallinity of ZrMgNi4becomes poor after only three hydrogen absorption and desorption cycles.On the contrary,the crystallinity of ZrMgNi2V2keeps almost unchanged after twenty-two hydrogen absorption and desorption cycles.Hence,the high hydrogen storage capacity and lattice stability of ZrMgNi2V2make it become an excellent candidate for hydrogen storage application. Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Acknowledgments This work is supported by Ministry of Science and Technology(MOST) of China (No.2018YFB1502102) and Equipment Development Department of People’s Republic of China Central Military Commission(Pre-research Project of the thirteenth Five-Year plan,Contract No.170441421075).2.Material and experimental methods
3.Results and discussion
3.1.The properties of ZrMgNi4
3.2.The properties of ZrMgNi3V and ZrMgNi2V2

4.Conclusion
 Progress in Natural Science:Materials International2021年2期
Progress in Natural Science:Materials International2021年2期
- Progress in Natural Science:Materials International的其它文章
- Introduction to Progress in Natural Science:Materials International
- Effect of superplastic deformation on precipitation behavior of sigma phase in 3207 duplex stainless steel
- Hydrothermal synthesis of hydroxyapatite coating on the surface of medical magnesium alloy and its corrosion resistance
- Formation mechanism of interfacial microstructures and mechanical properties of Ti2AlNb/Ni-based superalloy joints brazed with NiCrFeSiB filler metal
- Influence of γ’ precipitate on deformation and fracture during creep in PM nickel-based superalloy
- Tuning microstructure,transformation behavior,mechanical/functional properties of Ti–V–Al shape memory alloy by doping quaternary rare earth Y
