Glycated haemoglobin reduction and fixed ratio combinations of analogue basal insulin and glucagon-like peptide-1 receptor agonists: A systematic review
Poobalan Naidoo, Celia Bouharati, Virendra Rambiritch, Sumanth Karamchand, Barbara A Tafuto, Rory F Leisegang
Poobalan Naidoo, Department of Nephrology, Inkosi Albert Luthuli Central Hospital, Durban 4092, KwaZulu-Natal, South Africa
Celia Bouharati, Department of Medical Research, Independent Researcher, Paris 75000, France
Virendra Rambiritch, Department of Pharmacology, University of KwaZulu-Natal, Durban 3629, KwaZulu-Natal, South Africa
Sumanth Karamchand, Department of Internal Medicine, Stellenbosch University and Tygerberg Hospital, Cape Town 7600, South Africa
Barbara A Tafuto, Department of Health Informatics, Rutgers University, Piscataway, NJ 08854, United States
Rory F Leisegang, Department of Pharmaceutical Biosciences, Uppsala University, Uppsala 75236, Sweden
Abstract BACKGROUND Fixed ratio combinations (FRCs) of analogue basal insulin and glucagon-like peptide-1 receptor agonists are a newer addition to the therapeutic armamentarium for the management of type 2 diabetes mellitus.They reduce treatment complexity by combining two injectables in a single daily injectable, thus potentially improving adherence and persistence.Clinicians wanting to use FRCs would need to choose between members of the class.AIM To describe and contrast the glycated haemoglobin reduction of two FRCs of analogue basal insulin and glucagon like peptide-1 receptor agonist in adults with type 2 diabetes mellitus.METHODS The following Population, Intervention, Comparison, Outcome question was used for the primary analysis: Among adult patients with type 2 diabetes mellitus [P], what is the effect of iGlarLixi [I] compared to IDegLira [C] for bringing about glycaemic control (as measured by reduction in glycosylated haemoglobin) [O]? The Prisma Statement was used as a guideline for framing this systematic review.We searched PubMed, EMBASE and Cochrane library databases and Clinicaltrials.gov using various keywords and medical search headings related to type 2 diabetes mellitus, iGlarlixi, IDegLira and glycated haemoglobin A1c.RESULTS All 14 studies identified by the systematic search met the primary efficacy endpoint of reduction in glycated haemoglobin.There were no head-to-head studies between the FRCs of iGlarlixi and IDegLira, and we therefore did an indirect comparison based on a common comparator of insulin glargine U100.Both iGlarLixi and IDegLira effectively reduce glycated haemoglobin when compared to insulin glargine U100.However, using indirect comparisons, IDegLira had a greater haemoglobin A1c reducing ability (0.6% vs 0.3%).The indirect comparison is limited by the differences between the studies; the fasting blood glucose targets were slightly higher for iGlarLixi studies when compared to the IDegLira studies (4.0-5.0 mmol/L and 4.4-5.6 mmol/L), and the IDegLira study used a greater average dose of insulin glargine when compared to the iGlarLixi studies (66 U/d vs 40 U/d).CONCLUSION Both iGlarLixi and IDegLira effectively reduce glycated haemoglobin.Indirect comparisons, using insulin glargine as the common comparator, suggest that IDegLira reduces glycated haemoglobin to a greater extent than iGlarLixi.However, given the limitations of indirect comparisons, robust head to head studies and real-world data would better inform clinician choice and clinical practice guidelines.
Key Words: Diabetes mellitus; Fixed ratio combinations; Glycated haemoglobin, Glucagon like peptide-1 agonist; Analogue insulin
INTRODUCTION
Diabetes mellitus is a heterogeneous disorder of carbohydrate, protein and fat metabolism, characterized by hyperglycaemia secondary to defects in insulin secretion, insulin action or both[1].In 2019, approximately half a billion patients were living with diabetes, and this number is projected to increase to 700 million by 2045[2].Globally, diabetes is the 9thmost common cause of death[3], and 9.3% of adults aged 20-79 years have diabetes[4].The economic impact of managing diabetes and its complications are significant, with an estimated global gross domestic product cost of 2.2% by the year 2030[5].
The majority of patients with diabetes can be classified as having either type 1 (± 5%-10%) or type 2 (± 90%-95%) diabetes mellitus[1].Good glycaemic control prevents microvascular and macrovascular complications in patients with diabetes[6].Despite the vast armamentarium of therapies that include oral antidiabetic agents and injectables, attainment of glycaemic control remains suboptimal, and the World Health Organization lists diabetes mellitus as a top 10 cause of death[7,8].Managing adults with diabetes cost US$1.31 trillion globally in 2015[9].Beyond the medical complications of diabetes, patients may also be negatively impacted from an emotional, psychological and quality of life perspective[10].
The reasons for non-attainment of glycaemic goals are multifactorial and include complexity of treatment regimens and multiple injections.To reduce complexity and the number of daily injections, fixed ratio combinations (FRCs) of analogue basal insulin and glucagon-like peptide-1 (GLP-1) receptor agonists have recently been added[11].There are currently two FRCs that are marketed: IGlarLixi and IDegLira.Both have the same mode of action,i.e.the analogue basal insulin component increases cellular uptake of glucose and reduces hepatic glucose production, while the GLP-1 receptor agonist stimulates insulin release and inhibits glucagon release[11].FRCs reduce haemoglobin A1c (HbA1c) by approximately 0.5%[12], and their most common adverse effects are gastro-intestinal events (nausea, vomiting), nasopharyngitis and hypoglycaemia[13,14].Both are indicated for the management of patients with type 2 diabetes mellitus not controlled on lifestyle modification.
Given the high cost of FRCs and their recent market introduction, there are not many clinical practice guidelines that have assessed them for inclusion.For instance, the World Health Organization[15] and International Diabetes Federation[16] guidelines on diabetes mellitus do not currently include FRCs.In the diabetes field, the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) are leading the path with the publication of a joint guideline for the management of diabetes mellitus which includes FRCs[17].FRCs have been positioned for patients who are on both GLP-1 receptor agonists and basal analogue insulin.
The joint ADA/EASD consensus statement on the management of patients with type 2 diabetes mellitus does not differentiate between the two FRCs[17].Clinicians who wish to use FRCs need to consider the body of evidence before choosing between the two marketed products.However, the guideline does not differentiate between the two currently marketed FRCs, and there is no systematic review to assist clinicians decision making.The only systematic review and meta-analysis compare efficacy of FRCs with other classes of anti-diabetic treatments, but none compare iGlarLixi with IDeglira[18].Both are administeredviaa once daily subcutaneous injection and present similar adverse effects of hypoglycaemia, nasopharyngitis, nausea and vomiting[13,14].The average United States cost of a month supply of iGlarLixi and IDegLira is $851.09 and $1245.96, respectively[19,20].It is important to investigate the efficacy of iGlarLixi compared to IDegLira since it may guide the clinicians when making their decision.
The aim of this systematic review is to describe and contrast the glycated haemoglobin reduction of two FRCs of analogue basal insulin and GLP-1 receptor agonist in adults with type 2 diabetes mellitus.
MATERIALS AND METHODS
The review protocol for this systematic review has not been registered.The following Population, Intervention Comparison, Outcome (PICO) question was used for the primary analysis: Among adult patients with type 2 diabetes mellitus [P], what is the effect of iGlarLixi [I] compared to IDegLira [C] for bringing about glycaemic control (as measured by reduction in glycosylated haemoglobin) [O]?
The preferred reporting items for PRISMA Statement was used as a guideline for framing this systematic review[21].
Eligibility criteria
Clinical trials and observational studies investigating the efficacy of FRCs in patients with type 2 diabetes mellitus were identified.We included observational studies to get a sense of the real-world efficacy of FRCs.
Study inclusion criteria were: (1) Male or female, age ≥ 18 years; (2) Subjects diagnosed with type 2 diabetes mellitus; (3) Outpatients receiving treatment with FRCs of iGlarLixi or IDegLira; (4) HbA1c 7.0%-11.0% (both inclusive) (53-97 mmol/mol) by central laboratory analysis; (5) Body mass index ≥ 20 kg/m2and < 40 kg/m2; (6) Randomised clinical trial or observational study; (7) At least 10 patients per each study group; (8) Dropout rate < 20%; (9) Typically, if an author is included on more than one primary research article that is similar in content, the most recent review or article will be accepted and earlier versions will be rejected; (10) If an author is included on more than one primary research article and the content is different, then both reviews may be accepted; and (11) Studies published in English language.
Study exclusion criteria consisted of: (1) Patients < 18 years; (2) HbA1c > 11%; (3) Hospitalized; (4) History of pancreatic cancer; (5) Renal failure (estimated glomerular filtration rate < 30 mL/min); (6) Liver failure or impairment defined as alanine aminotransferase or aspartate aminotransferase ≥ 2.5 times upper limit of normal; (7) Screening calcitonin ≥ 50 ng/L; (8) Type 1 diabetes mellitus; (9) History of pancreatitis (acute or chronic); (10) Personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia type 2; (11) Subjects presently classified as being in New York Heart Association Class IV; (12) Screening calcitonin ≥ 50 ng/L; (13) Currently pregnant or breastfeeding or not using a reliable method of birth control for the duration of the trial in all females with childbearing potential; (14) < 10 subjects per intervention group; (15) Studies of less than 3-mo duration; (16) Dropout rate > 20%; and (17) Studies not reported in English.
Search strategy and study selection
Sixty-six articles were identified by searching PubMed, Embase and Cochrane library databases as well as Clinicaltrials.gov using various keywords and medical search headings (MeSH) related to type 2 diabetes mellitus, iGlarlixi, IDegLira and glycated HbA1c.The complete search syntax conducted on March 21, 2021 was as follows: (1) Patient (((((((((((((((type 2 diabetes mellitus[MeSH Terms]) OR adult-onset diabetes mellitus) OR ketosis-resistant diabetes mellitus) OR maturity-onset diabetes mellitus) OR non-insulin dependent diabetes mellitus) OR non-insulin dependent diabetes mellitus) OR noninsulin dependent diabetes mellitus) OR noninsulin-dependent diabetes mellitus) OR slow-onset diabetes mellitus) OR stable diabetes mellitus) OR type II) OR MODY) OR maturity-onset diabetes)) OR maturity onset diabetes mellitus)) OR NIDDM) OR type 2 diabetes))); (2) Outcome (((((((((((((((((((((glycated hemoglobin[MeSH Terms]) OR (a) OR glycated hemoglobin a1c)) OR OR glycated haemoglobins) OR OR glycohemoglobin a) OR OR glycosylated hemoglobin a) OR OR glycosylated hemoglobin a1c) OR OR hb A1) OR OR Hb A1a+b) OR OR Hb A1a-1) OR OR hb A1a-2) OR OR hb a1b) OR OR hb A1c) OR OR HbA1) OR OR hemoglobin A(1)) OR hemoglobin A)) OR OR glycosylated) OR OR hemoglobin, glycated A1a-2) OR OR hemoglobin, glycated A1b) OR OR hemoglobin, glycosylated) OR OR hemoglobin, glycosylated A1a-1) OR OR hemoglobin, glycosylated A1b)); (3) (1) AND (2); (4) Intervention ((((((glarlixi[MeSH Terms]) OR insulin glargine/lixisenatide) OR (insulin glargine and lixisenatide)) OR soliqua)); (5) Comparator (((((ideglira[MeSH Terms]) OR insulin degludec/Liraglutide) OR (liraglutide and insulin degludec)) OR xultrophy) OR xultrophy 100/3.6)); and (6) (4) OR (5); and (7) (3) AND (6).
Of the 66 articles that were screened (duplicaten= 0), 52 articles were excluded for the following reasons: Post-hoc analysis (n= 11), review article (n= 21), did not contain FRC (n= 9), retrospective chart review (n= 1), mathematical model (n= 1), pharmacokinetic model (n= 1), case study (n= 1), cost-effectiveness study (n= 1), type III diabetes mellitus (n= 1), study duration less than 3 mo (n= 1) and animal study (n= 1).The remaining 14 articles were used for qualitative synthesis.The PRISMA flow diagram outlining the search process used is provided in Figure 1.
Data collection
A PICO tracker was used for data extraction.We included key elements from each study,i.e.country location, clinical trial phase, patient population, intervention, comparison, outcome measure, response assessment day, time-points of study measurements and study design.The two study arms were FRCvscomparator.
Bias was assessed using the Cochrane Risk of Bias tool[22].Studies with seven or greater individual dimensions rated as “High” were assigned an overall “Poor” bias rating, studies with between three and six dimensions rated as “High” were given a “Moderate” overall bias rating and studies with less than three “High” dimensions were given a “Good” overall bias rating.
Data analysis
Since there were no head-to-head studies comparing iGlarLixi with IDeglira, we conducted an indirect comparison.We compared the FRCs if they had a common comparator to ensure that we are comparing “like with like.” From the 14 studies identified, three studies had a common comparison.

Figure 1 PRISMA flow diagram.
Outcome measures
The primary outcome measure was reduction in glycated haemoglobin after at least 6 mo of treatment, as per the aim of this systematic review.
RESULTS
Included studies and study characteristics
In total, 14 studies were identified through the systematic review process.All studies met their primary efficacy endpoint of reduction in glycated haemoglobin.The details of these studies are contained in Supplementary Tables 1 and 2.The majority of the studies were phase III studies, with one phase II study.Except for three studies conducted in Japan, the rest of the studies were multi-country clinical trials.The studies were a minimum of 24 wk and a maximum of 104 wk.
Of the 14 studies identified, none were direct comparisons between FRCs.Therefore, we focused on studies that had a common comparator; these studies totalled three and are listed in Table 1.The common comparator was insulin glargine U100.One study looked at the efficacy of IDegLira on a background of sodium glucose cotransporter inhibitors[23].We omitted this study because it was not comparable to the iGlarLixi study given the difference in background therapy.We focused on indirect comparisons in which the background therapy was similar; in this case it was the background of metformin and this included studies that were similar and relatively homogenous, thus making indirect comparison of IDeglira and iGlarLixi sensible as per Butchers method[24].
In the phase III multinational DUAL V study, Lingvayet al[25] investigated whether IDegLira was non-inferior to up-titration of glargine, with reduction in glycated haemoglobin as the primary efficacy endpoint measured at week 26.Patients had type 2 diabetes and were uncontrolled (HbA1c 7%-10%) despite the use of metformin (≥ 1500 mg/d or maximum tolerated) and insulin glargine (20-50 U/d).Patients were randomised to U100 or IDeglira in a 1:1 ratio.IDeglira was initiated at 16 dose steps (16 U of degludec/0.6 mg of liraglutide).The maximum dose of degludec and liraglutide was 50 U and 1.8 mg, respectively.Patients randomised to glargine continued with their glargine dose, with no maximum allowable dose.Both treatments were titrated to achieve a fasting blood glucose of 4.0-5.0 mmol/L.The final dose of insulin glargine and insulin degludec was 66 U and 41 U, respectively.At week 26, HbA1c had decreased by 1.81% for the IDegLira group (standard deviation 1.08%) and by 1.13% for the glargine group (standard deviation 0.98%); the estimated treatment difference was of 0.59% [95% confidence interval (CI): -0.74-0.45;P< 0.001] and was clinically and statistically significant.Further details of the DUAL V study are contained in Tables 1-3.

Table 1 Study characteristics
In a phase II, proof-of-concept, randomised, open label study, Rosenstocket al[26] investigated the safety and efficacy of iGlarLixi compared to insulin glargine U100 in insulin naïve patients with uncontrolled type 2 diabetes (HbA1c ≥ 7% to ≤ 10%) on a background of metformin (≥ 1500 mg/d for ≥ 3 mo).The primary efficacy endpoint was a reduction in HbA1c at week 24.The starting dose was of 10 U of iGlarLix and 10 U in the U100 group.iGlarlixi and U100 were titrated based on a fasting blood glucose target of 4.4-5.6 mmol/L.The maximum daily dose of iGlarLixi was 60 units U100, which corresponded to a lixisenatide dose of 30 µg.There was no upper limit for the dose of glargine U100.The mean baseline HbA1c ranged from 8.0% to 8.1%.IGlarLixi and insulin glargine U100, resulted in reduction in HbA1c of 1.82% and 1.64%, respectively.The difference between mean change from baseline for iGlarLixi and insulin glargine U100 was -0.17% (P= 0.01).The average dose of insulin glargine U100 was 39 U at week 24.
As a follow up of the above proof-of-concept study, Rosenstocket al[26] conducted a multinational, randomised, open label phase III study in which iGlarLixi was compared to its components,i.e.insulin glargine U100 and lixisenatide.The primary efficacy endpoint was change in HbA1c at week 30.Adults with type 2 diabetes who were uncontrolled on metformin (HbA1c ≥ 7.5% and ≤ 10%) or metformin in combination with other oral antidiabetic agents (HbA1c ≥ 7.0% and ≤ 9.0%) were included.Patients on metformin and second oral agent were asked to discontinue the second oral agent during the run-in phase.During the run-in phase metformin was titrated to at least 2000 mg or the maximum tolerated dose of at least 1500 mg/d.After the run in, patients with an HbA1c of ≥ 7.0% and ≤ 10.0% and fasting plasma glucose ≤ 13.9 mmol/L were randomised to one of the three arms in a 2:2:1 ratio (iGlarLixi; insulin glargine U100; lixisenatide).iGlarLixi and insulin glargine U100 was started at 10 U/d with the maximum allowed dose of 60 U/d.Lixisenatide was started at 10 µg for the first 2 wk and then 20 µg for the rest of the study period.The final mean basal insulin daily dose was 39.8 U and 40.3 U for iGlarLixi and insulin glargine U100, respectively.The baseline HbA1c was 8.1% in all three groups, and mean HbA1c at week 30 were 6.5%, 6.8% and 7.3% for iGlarLixi, insulin glargine U100 and lixisenatide, respectively.The HbA1c difference at week 30 between iGlarLix and insulin glargine U100 was -0.3% (95%CI: -0.4% to -0.2%,P< 0.0001).
Patient characteristics
All participants were adults with type 2 diabetes with a disease duration of 7-11 years.Both genders were included in the studies, and the majority of participants were Caucasian (89%-98%).Patients were obese (31-32 kg/m2) with a baseline glycated haemoglobin of approximately 8.0%-8.4%.The patient characteristics are listed in Table 2.
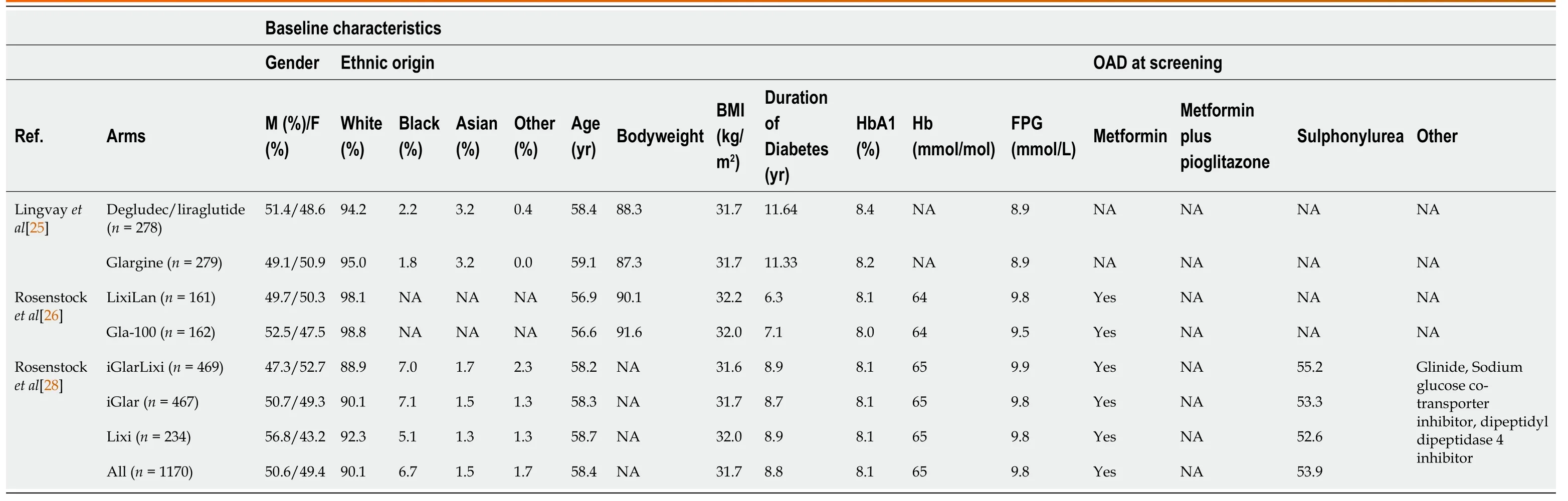
Table 2 Patient arm characteristics
Arm characteristics and interventions
Given that treatment was randomly allocated, the intervention and comparator arms were balanced with respect to baseline characteristics and glycated haemoglobin.The interventions were the FRCs compared with insulin glargine U100.
Outcomes
The primary efficacy outcome was reduction of glycated haemoglobin assessed after at least 24 wk.We did not assess secondary efficacy outcomes of reduction in fasting and postprandial glucose levels.We also did not assess safety as this was beyond the scope of this systematic review.
Risk of bias
Risk of bias and study quality were assessed for each included study using Cochrane Risk of Bias tool[22].Differences in rating were resolvedviadiscussion and consensus among all authors.The quality assessment for each study is contained in Table 3.The studies were industry sponsored randomised phase II-III studies with a moderate risk of bias.The major limitation was the open-label design.
DISCUSSION
This systematic review described the ability of FRCs to reduce glycated haemoglobin.Both iGlarLixi and IDeglira reduced HbA1c and met their primary efficacy endpoints in the 14 clinical trials identified in this systematic review.Our findings are similar to those of the systematic review and meta-analysis of Liakopoulouet al[27] who showed effective glycaemic control with FRCs when compared with each individual component alone (change in HbA1c-0.31%; 95%CI: -0.47 to -0.16;I2= 81 and -0.73%; 95%CI: -0.87 to -0.58;I2= 74% compared with basal insulin and GLP-1 RA, respectively).
The studies identified in our systematic review were well designed in general with a moderate risk of bias as assessed by the Cochrane risk of bias tool[22].The key limitation of the studies was the use of an open label design in which both study participants and investigators were not blinded to interventions.
Given the lack of head-to-head studies of iGlarLixi and IDeglira, we used an indirect comparison to get a sense if there were differences in HbA1c reducing ability of the FRCs.There were three studies in which insulin glargine U100 was the common comparator[25,26,28].IDegLira reduced glycated haemoglobin to a greater extent than iGlarLixi (approximately 0.6%vs0.3%).However, indirect comparisons have challenges as the studies do have differences that do not allow for firm conclusions.For example, although the studies were treat-to target studies, the target ranges were different with IDegLira and iGlarLixi being 4.0-5.0 mmol/L and 4.4-5.6 mmol/L, respectively[25,28].Furthermore, the IDegLira study used greater average doses of glargine when compared to the iGlarLixi studies (66 U/dvs40 U/d)[25,28].
Based on indirect comparison, the difference in HbA1c reduction may be a chance finding or due to inherent differences between the studies,e.g., different countries, different investigators and varying doses.Furthermore, there is no biological plausibility that would explain the HbA1c reducing difference between iGlarLixi and IDeglira.
Glycated haemoglobin is only a validated surrogate measure of microvascular complications (retinopathy, nephropathy, neuropathy)[29-37]; ideally one would need to have studies that determine the effect of FRCs on major adverse cardiovascular events,e.g., myocardial infarction, cerebro-vascular accident, peripheral vascular disease and ultimately mortality.
Although the studies were of reasonable duration (at least 24 wk), one would require long term studies to determine the durability of glycated haemoglobin.Ideally one would need a head to head study to determine the differences in safety, efficacy and tolerability between the two FRCs.However, it is unlikely that this would be done given the high costs of doing such a study.Perhaps real-world evidence may help differentiate between the FRCs.A network meta-analysis may assist with informing the relative efficacy and safety of the FRCs.Pharmacoeconomic considerations may also help differentiate between these agents.
Clinical trials help inform clinical practice guidelines.However, there are many factors influencing the translation of clinical practice guidelines to clinical practice.Factors include the level of evidence and the grade of recommendation, the credibility and expertise of the guideline committee, economic factors and physician and patient preference.Given the current lack of head-to-head studies between iGlarLixi and IDeglira, it is not surprising that the joint ADA/EASD guideline[15] does not differentiate between the individual FRCs.It is unlikely that this systematic review would result in a change of the clinical trial guidelines given that it only looks at differences in reduction of glycated haemoglobin and is also an indirect comparison, with its inherent limitations.
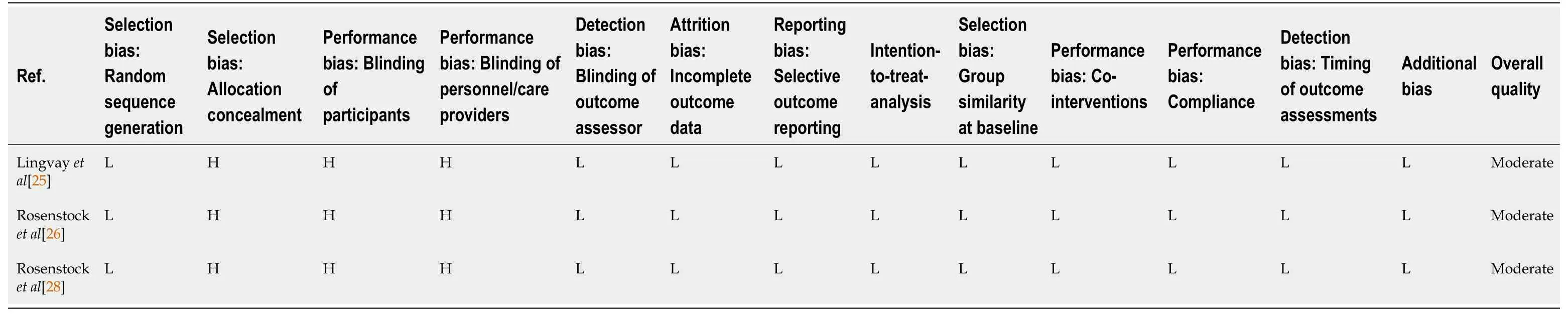
Table 3 Study bias assessment
CONCLUSION
Both iGlarLixi and IDegLira effectively reduce glycated haemoglobin.Indirect comparisons, using insulin glargine as a common comparator, indicate that IDegLira reduces glycated haemoglobin to a greater extent than does iGlarLixi.However, given the limitations of indirect comparisons, robust head to head studies and real-world data are needed to inform clinical practice guidelines.
ARTICLE HIGHLIGHTS
Research background
Fixed ratio combinations of insulin and glucagon-like peptide-1 analogues are novel therapy for the management of patients with type 2 diabetes mellitus.
Research motivation
There is minimal data comparing the fixed ratio combinations of iGlarlixi and IDegLira.
Research objectives
We aimed to compare the glucose lowering effect of iGlarLixivsIDegLira.
Research methods
We used a Population, Intervention, Comparison, Outcome (PICO) question for the primary analysis.
Research results
Both iGlarLixi and IDegLira effectively reduce glycated haemoglobin when compared to insulin glargine U100.However, using indirect comparisons, IDegLira had a greater HbA1c reducing ability (0.6%vs0.3%).
Research conclusions
Both iGlarLixi and IDegLira effectively reduce glycated haemoglobin.
Research perspectives
Head to head studies between iGlarlixi and IDegLira are required to determine if there are clinically relevant differences between the two aforementioned fixed ratio combinations.
ACKNOWLEDGEMENTS
Dr.Roopnarain C for assisting with construction of tables.
 World Journal of Meta-Analysis2021年3期
World Journal of Meta-Analysis2021年3期
- World Journal of Meta-Analysis的其它文章
- Post COVID-19 infection: Long-term effects on liver and kidneys
- Immune response to Helicobacter pylori infection and gastric cancer development
- Dengue hemorrhagic fever and cardiac involvement
- Impact of Streptococcus pyogenes infection in susceptibility to psoriasis: A systematic review and meta-analysis
- Extraintestinal infection of Listeria monocytogenes and susceptibility to spontaneous abortion during pregnancy: A systematic review and meta-analysis
