Dengue hemorrhagic fever and cardiac involvement
Wattana Leowattana, Tawithep Leowattana
Wattana Leowattana, Department of Clinical Tropical Medicine, Faculty of Tropical Medicine, Mahidol University, Bangkok 10400, Thailand
Tawithep Leowattana, Department of Medicine, Faculty of Medicine, Srinakharinwirot University, Bangkok 10110, Thailand
Abstract Dengue viral infection (DVI) is one of the world’s most significant viral infections spreading.Most of the patients have been asymptomatic, with relatively benign clinical manifestations and outcomes.However, a small number of patients have progressed to severe dengue diseases, including hemorrhage, multi-organ impairment, and increased vascular leakage causing hypovolemic shock, which can cause cardiovascular collapse and death.Numerous lines of evidence have demonstrated that DVI could also cause cardiac dysfunction, arrhythmias, and severe myocarditis.The treatment for dengue hemorrhagic fever (DHF) patients remains symptomatic and supportive, with close monitoring of hemodynamic status.The contributory role of cardiac dysfunction in DHF patients has potentially critical implications on the management.This review will address the current knowledge of cardiac involvement in DHF patients and the management strategy to reduce the fatality outcome.
Key Words: Dengue hemorrhagic fever; Dengue viral infection; Cardiac involvement; Cardiac injury; Myocarditis; Left ventricular ejection fraction; Tachycardia; Bradycardia; Electrocardiography; Echocardiography; Severe dengue disease
INTRODUCTION
Dengue viral infection (DVI) is a worldwide arthropod-borne disease that has rapidly spread throughout the tropics, with the highest risk zones in Asia and the Americas[1,2].More than 3 billion people are living in the Aedes-infested areas and at risk of DVI[3].Asia accounts for 75% of the DVI burden, followed by Latin America and Africa.The most significant vectorAedes aegyptiis a daytime mosquito, capable of stinging many people in a short period of time and breeding in several types of human-made containers with water inside[4].
Dengue virus (DENV) is an enveloped virus with a single positive-stranded RNA genome, belonging to the genusFlavivirusof the familyFlaviviridae.There are four distinct serotypes, designated as DENV-1, DENV-2, DENV-3, and DENV-4.The viral RNA encodes three structural proteins, namely capsid (C), pre-membrane, and envelope (E), and seven non-structural (NS) proteins, namely NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5[5-7].The incubation period of DVI is 3-7 d, and the symptoms start abruptly, following three sequential phases—an initial febrile phase, a critical phase, and a spontaneous recovery phase.The febrile phase's pathognomonic features include acute onset of high-grade fever with chill, severe malaise, vomiting, and constitutional symptoms.The critical phase is characterized by varying degrees of plasma leakage into the pleural and abdominal cavities, which causes intravascular volume depletion and might progress to life-threatening dengue shock syndrome (DSS)[8].Moreover, hemorrhagic manifestations commonly occur during the critical phase.The most common causes of death include bleeding into major vital organs, massive bleeding, and shock.The pathophysiology of shock is poorly understood and has been described as intravascular fluid loss due to increased capillary permeability and bleeding.Organ impairment occurs during this phase, as a hepatic dysfunction with elevated aminotransferase enzymes, cardiac dysfunction with myocardial injury, and a broad range of rhythm disturbances[9,10].The recovery phase is characterized by a sudden arrest of the plasma leaks, with concomitant reabsorption of extravasated plasma and fluids.Furthermore, DENV and the NS1 are detected in blood circulation during acute DVI, and high viral load or NS1 antigenemia have been demonstrated with severe dengue diseases (SDDs), particularly in secondary infection with different serotype from the primary infection[11,12].
Viral infection of the heart is relatively common in DVI and usually of small consequence.However, it can lead to substantial myocarditis and severe acute heart failure.Acute myocarditis can present in various clinical manifestations (Table 1).The most common viruses known to cause myocarditis are shown in Figure 1.DENV is one of the viral infections of the heart.Although the human immunodeficiency virus and coxsackie B virus are important causes of myocarditis, an extensive review including them is beyond the scope of this article.Cardiac involvement in DVI is a typical intricacy, especially in a large-scale hospital setting with many dengue hemorrhagic fever (DHF) patients, and may be a contributing factor in the pathogenesis of shock and may influence the clinical outcome.Clinical features of cardiac involvement from DENV can vary widely, from asymptomatic to severe cardiac failure, which has a high fatality rate[13,14].
In 1967, Bhamarapravatiet al[15] first reported the hearts’ pathological sections from 100 DHF autopsy cases.They found that 42 of 100 patients (42%) who died from DHF had focal areas of hemorrhage in the epicardium, sub-endocardium, interstitial tissues, or a combination of such.The most common finding was the separation of myocardial fibers with interstitial tissue edema.Hemorrhage in the myocardium was extensive, with condensation and an acidophilic pattern of myocardial fibrils.There were also a few cases of interstitial infiltration by a small number of mononuclear and lymphoid cells.Moreover, several investigators have demonstrated that cardiac involvement in DVI might cause death in DHF patients[16-20].However, clinical practice guidelines (CPGs) have only slightly addressed cardiac involvement as a mandatory concern in DHF management[21,22].Recently, the CPG from the Pan American Health Organization included myocarditis as a crucial pathophysiologic factor in SDD[23].
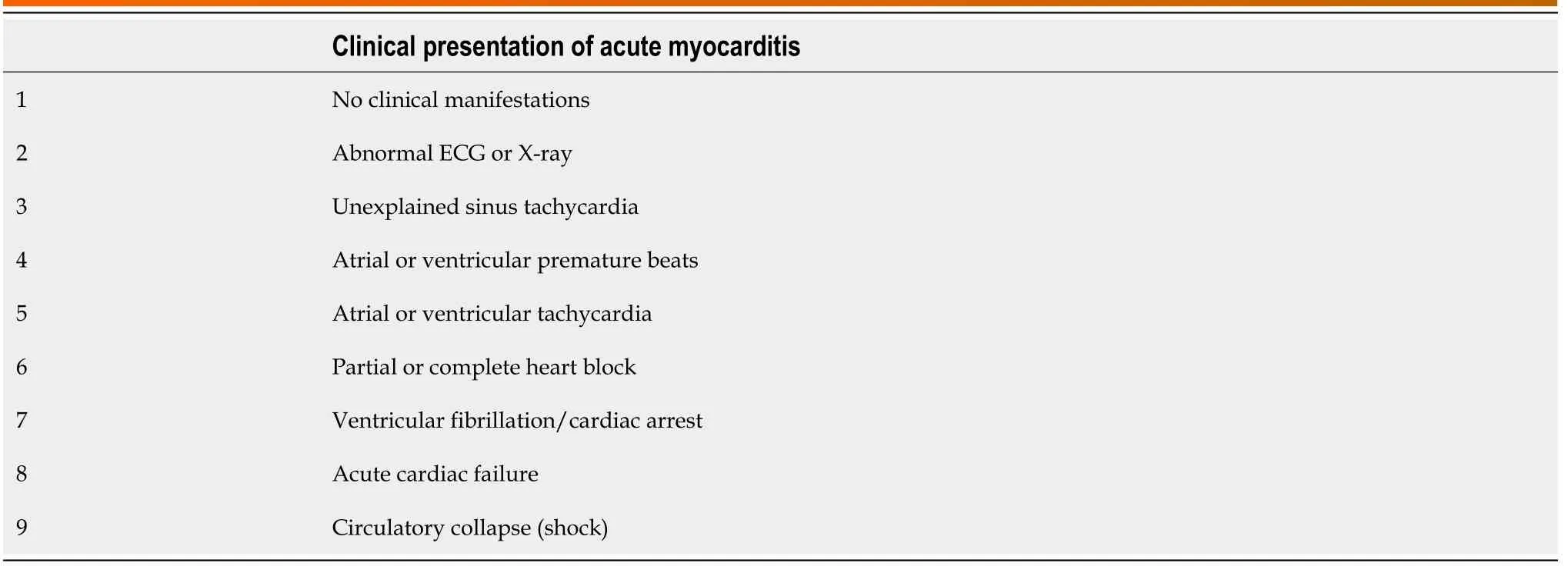
Table 1 Clinical presentation of the patients with acute myocarditis

Figure 1 Viruses causing myocarditis.
This review presents the incidence, clinical manifestation, electrocardiogram (ECG) abnormalities, cardiac biomarkers, echocardiography (ECHO), and cardiac imaging in DVI patients with cardiac involvement.
INCIDENCE AND CLINICAL MANIFESTATION
In 1998, Waliet al[24] conducted a study to evaluate cardiac involvement in 17 consecutive patients with DHF, by use of radionuclide ventriculography, ECHO, and ECG during the epidemic of DEN-2.They found that the mean left ventricular (LV) ejection fraction (LVEF) was 41.69%, and 7 patients (41.17%) had LVEF < 40%.Moreover, global hypokinesia was detected in 12 patients (70.59%) and was found to revert to normal within 3 wk.They concluded that acute reversible cardiac involvement was present in DHF or DSS and could be responsible for clinical shock.Hence, the role of ECG, ECHO, and radionuclide ventriculography in the clinical evaluation of DHF and DSS patients should be considered tools to improve the prognosis in these patients.
In the same year, Kabraet al[25] conducted a study to evaluate cardiac involvement in 54 pediatric patients admitted to the hospital with various grades of DVI (DF/DHF/DSS).They found that 9/54 (16.7%) children had LVEF < 50% and 2/54 (3.70%) had LVEF < 35%.They concluded that cardiac functions need to be assessed in patients with DVI, especially those with hypotension, with adequate hydration.In 2003, Khongphatthanayothinet al[26] reported an observational study of LV performance and hemodynamic abnormalities during different stages of DHF.They found that LVEF and velocity of circumferential fiber shortening adjusted for endsystolic meridional wall stress and end-diastolic volume were significantly lower during the toxic stage than after recovery.Moreover, the cardiac index was found to be low during the toxic stage and to remain subnormal during the convalescence stage due to sinus bradycardia.They concluded that the mechanism of decreased cardiac output during a toxic stage in DHF patients was complex, due to decreased preload, decreased LV performance, and subnormal heart rate response (Table 2).
In 2007, Kularatneet al[27] investigated cardiac complications in 120 DHF patients between 13-76 years of age.They found that 75/120 (62.5%) of the patients had ECG abnormalities (e.g., T inversion, ST depression, bundle branch blocks).Moreover, 5/75 (6.66%) of the patients had LVEF < 55%.They concluded that cardiac involvement in DHF was common and early recognition and differentiation from DSS is crucial.In the same year, Khongphatthanayothinet al[28] conducted a study to determine the prevalence of myocardial depression and its effect on the clinical severity in 91 patients with DF, DHF, and DSS.They found that during the critical stage, LVEF < 50% was present in 2 (6.7%), 5 (13.8%) and 9 (36.0%) patients with DF, DHF and DSS, respectively.Furthermore, all 14 patients in convalescent stage who had had LVEF < 50% at the critical stage presented LVEF ≥ 50%.They concluded that the proportion of patients with cardiac dysfunction was larger for those with higher clinical severity of the disease.
In 2009, Salgadoet al[29] conducted a prospective study to determine the incidence of myocarditis in 102 DHF children.They found that 11/102 (10.7%) of the children had signs of myocarditis, 10/102 (9.8%) needed inotropic drugs, 9/102 (8.8%) had bradyarrhythmias, and 2/102 (1.9%) had tachyarrhythmias.Investigations by ECHO demonstrated pericardial leakage in 2/102 (1.96%) and diminished LVEF in 1/102 (0.98%) of the patients.They concluded that the myocarditis in DHF patients was not rare and should be of concern during DHF management.In 2011, La-Orkhunet al[30] evaluated the cardiac arrhythmias and heart rate variability in 35 children with DVI using overnight 18-24-h Holter monitoring.They found that 10/35 (29%) of the patients had cardiac rhythm abnormalities [sinus pause, first-degree arterioventricular (AV) block, Mobitz type I second-degree AV block (Wenckebach), atrial ectopic beats, and ventricular ectopic beats] during the convalescent stage.There was no association between the clinical severity and the incidence of cardiac arrhythmia.They concluded that various benign bradyarrhythmias and ectopic beats are present in patients with DVI during the convalescent stage.
One year later, Yacoubet al[18] conducted an observational study to evaluate the cardiac involvement in 79 DVI patients (age range of 8-46 years).They found that the patients with severe dengue had worse cardiac function than those with dengue, in the form of LV systolic dysfunction and with increased left myocardial performance index.Furthermore, septal myocardial systolic velocities (S′) were also reduced.They concluded that the patients with severe dengue have evidence of systolic and diastolic cardiac impairments, with the septal and right ventricular wall being predominantly affected.Then, in 2013, Mirandaet al[31] conducted a study to evaluate the cardiac involvement in 81 DVI patients.They found that 12/81 (14.81%) of the patients had elevated troponin I and NTproBNP.Of the 10 patients, 1 showed reduced LVEF, 2 showed LV segmental abnormalities with preserved ejection fraction, and 1 showed a significant pericardial effusion with tamponade; cardiac magnetic resonance confirmed cardiac dysfunction in these 4 patients.They concluded that the heart could be affected by DENV in a considerable percentage of patients needing hospitalization.Overall, these date indicate that clinical presentations range from mild elevation of cardiac markers to myocarditis and pericarditis.These cardiac dysfunctions might be underdiagnosed in clinical practice and contribute to the high mortality rates of DHF and DSS.
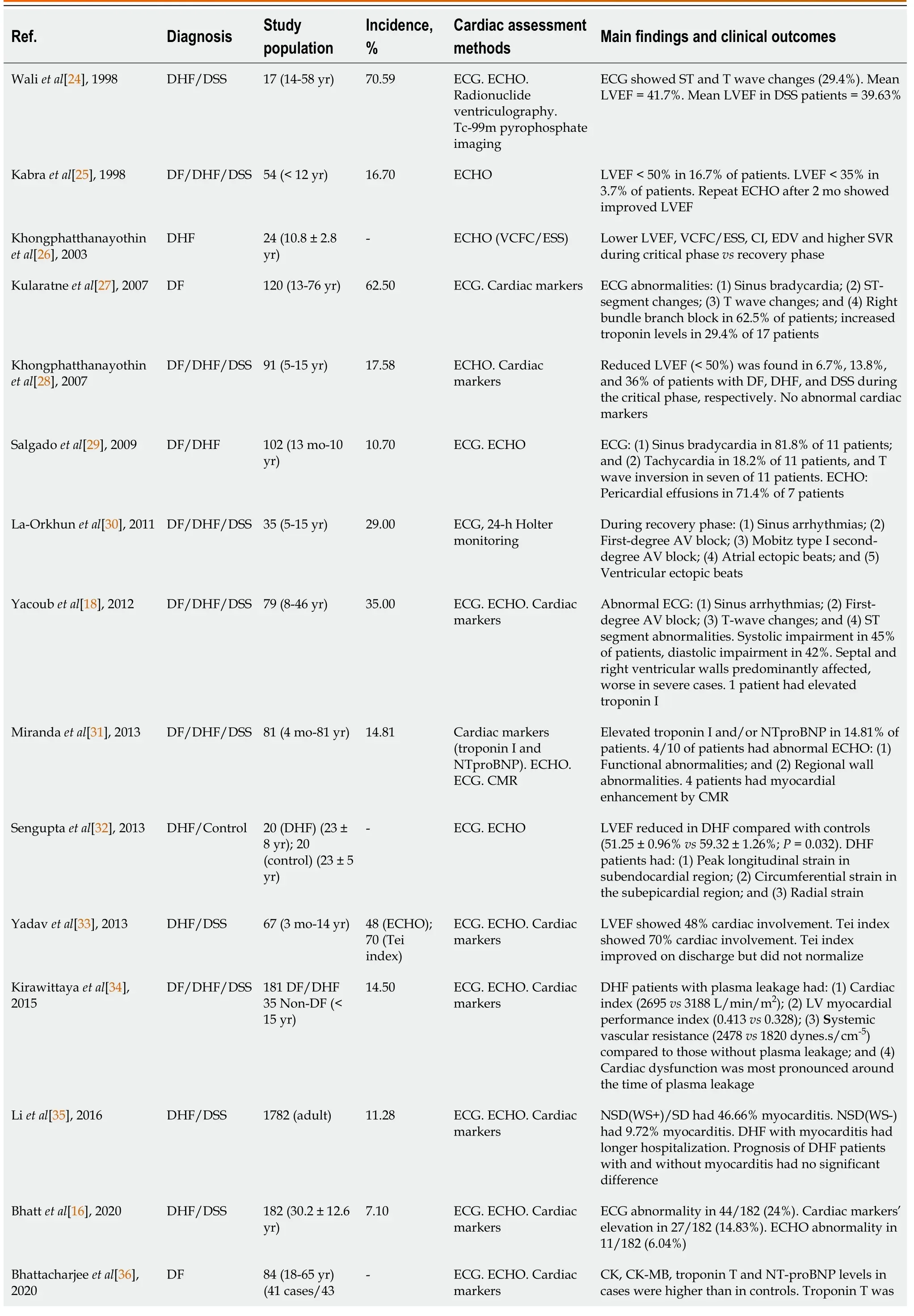
Table 2 Incidence and clinical manifestations of dengue viral infections with cardiac involvement
AV: Arterioventricular; CI: Cardiac index CK: Creatine kinase; CMR: Cardiac magnetic resonance; DF: Dengue fever; DHF: Dengue hemorrhagic fever; DSS: Dengue shock syndrome; ECHO: Echocardiography; EDV: End-diastolic volume; ECG: Electrocardiogram; LVEF: Left ventricular ejection fraction; NSD(WS)/SD: Non-severe dengue (warning sign)/severe dengue; SVR: Sustained virological response; VCFC/ESS: Velocity of circumferential fiber shortening adjusted for end-systolic meridional wall stress.
Later on, Senguptaet al[32] conducted a prospective study that used 2D ECHO images of the LV in the four-, three- and two-chamber views and parasternal shortaxis views at the basal, mid and apical levels to evaluate 40 participants (20 DHF, and 20 controls).They found that LV global ejection fraction was reduced in patients with DHF at presentation, as compared with controls.The peak longitudinal strain in patients with DHF was significantly attenuated in the subendocardial region, as compared with that in normal controls.DHF patients showed significantly higher radial strain than controls.They concluded that the identification of reduced LV longitudinal strain helps in understanding the mechanism of reduced LV myocardial performance seen in patients with DHF.
Yadavet al[33] conducted a study to evaluate myocardial involvement in infants and children with severe dengue using the Tei index, which combines systolic and diastolic time intervals to assess global ventricular function.They found fewer patients with severe dengue had myocardial involvement on admission by LVEF (48%) than by the Tei index (70%).They concluded that most of the patients with severe dengue had asymptomatic myocarditis, as evident by a deranged Tei index, which improved but did not normalize by the time of discharge.Hence, these groups of patients needed a longer follow-up period.
In 2015, Kirawittayaet al[34] conducted a study to characterize the incidence and changes in cardiac function by serial ECHO in 181 DF and DHF patients compared with 35 non-dengue patients.They found that DHF patients who developed plasma leakage had lower cardiac index (2695vs3188 L/min/m2, P= 0.003) and higher LV myocardial performance index (0.413vs0.328,P= 0.021) and systemic vascular resistance (2478vs1820 dynes.s/cm-5, P= 0.005) compared to those without plasma leakage.Moreover, cardiac dysfunction was most pronounced around the time of plasma leakage.They concluded that transient LV systolic and diastolic dysfunction was common in pediatric DHF patients and related to the severity of plasma leakage.
In the same year, Liet al[35] conducted a study to investigate the prevalence of myocarditis using ECG, ECHO, and cardiac enzymes tests in 1782 DHF patients.They found that 201/1782 (11.28%) of the patients had myocarditis.Moreover, the prevalence of myocarditis in non-severe dengue with warning signs and severe dengue was 46.66% compared with non-severe dengue without warning signs, which was 9.72%.DHF patients with myocarditis experienced longer hospitalization than those without myocarditis (7.17 ± 4.64vs5.98 ± 2.69,P= 0.008).They concluded that the incidence of myocarditis increased with the severity of DHF; moreover, the prognosis of DHF patients with and without myocarditis had no significant difference, even if myocarditis patients experienced longer hospital stay.
In 2020, Bhattet al[16] conducted a prospective observational study to evaluate the cardiac involvement among 182 admitted DHF patients of age ≥ 14 years.They found that 44/182 (24%) of the DHF patients demonstrated some ECG abnormalities, such as sinus tachycardia, sinus bradycardia, and non-specific ST changes.Moreover, cardiac markers were elevated in 27/182 (14.8%) of the DHF patients, and among these patients, 11/27 (40.7%) had ECHO evidence of myocarditis.The incidence of myocarditis in that study was 7.10%.The authors concluded that myocarditis among admitted dengue patients is not uncommon and may cause increased morbidity and mortality.Thus, there is a need to have a CPG to diagnose and treat myocarditis among DHF patients.
Bhattacharjeeet al[36] conducted a study to compare cardiac markers in DF patients with and without myocarditis.The patients were classified as cases (ECG and ECHO changes of LVEF < 50%;n= 41) and controls (age and sex-matched with normal ECG and ECHO;n= 43).They found that creatine kinase (CK), CK-MB, troponin T, and NT-proBNP concentrations were higher in the cases than in the controls.Serum troponin T and NT-proBNP were also found to be positively correlated to each other.They concluded that routine monitoring of cardiac markers, in conjunction with ECG and ECHO in early diagnosis and management of myocarditis, was needed for DVI patients.
In 2021, Cabrera-Regoet al[37] conducted a study to characterize the cardiovascular manifestations in 427 hospitalized patients with DVI.They found that 19.7% of these patients had cardiovascular manifestations.The manifestations were, specifically, sinus bradycardia (13.8%), atrial extrasystoles (4.9%), and ventricular extrasystoles (4.0%).Pericarditis and myocarditis were detected in 1.6% and 0.2%, respectively.The higher probability of presenting cardiac dysfunctions in DVI patients was decreased platelet count [odds ratio (OR = 1.13)], advanced age (OR = 1.70), male sex (OR = 1.94), and dengue with warning signs (OR = 3.29).They concluded that cardiovascular signs in patients with dengue are common and self-limited.It is essential to consider these findings and not underestimate cases with asymptomatic pericarditis or myocarditis.
Shahet al[38] conducted a study to evaluate the incidence and spectrum of cardiac abnormalities in 320 patients with DF.They found that 112/320 (35%) of DF patients had changes of cardiac manifestations, with 19.7% having sinus bradycardia, 13.1% having LVEF < 40%, 15% having elevated troponin T, 10.6% having elevated CK-MB, and 5.9% having elevated NTproBNP.The mortality rate was 4.38% among the entire patient population that presented cardiac involvement.They concluded that cardiac dysfunction in patients with DVI was common.Moreover, their findings showed that the abnormalities of ECG, ECHO, and cardiac markers were predictors of risk for adverse outcomes.
HISTOPATHOLOGICAL FINDINGS
An extensive series of histopathological findings regarding DHF patients were reported since 1967[15].In 1968, Burke[39] reported the autopsy findings of 12 DHF cases, all of which had cardiac involvement, including epicardial petechial hemorrhage, myocardium focal hemorrhage, and subendocardial hemorrhage in the posterior wall of the LV.Moreover, the author stated having not found evidence of myocarditis with normal blood vessels.In 2011, Weerakoonet al[20] reported that 5 autopsy DSS patients' histopathological findings showed evidence of florid myocarditis; the distinct histological findings of the myocardium were interstitial edema with inflammatory cell infiltration, necrosis of myocardial fibers, and evidence of pericarditis.They concluded that primary cardiac failure due to myocarditis in DHF is usually overlooked.
Management guidelines for DHF should address myocarditis as an important issue.The primary histopathological features are shown in Table 3[15,20,39-42].Moreover, DENV antigen has been detected with immunohistochemistry in fatal dengue with clinical myocarditis[13,20,31,43,44].Myocardial damage had also been demonstrated in patients dying of dengue[20,45].Weerakoonet al[20] showed severe myocardial damage with hemorrhage, interstitial edema with inflammatory cell infiltration, and necrosis of myocardial fibers.Moreover, Torreset al[46] demonstrated focal and diffuse myocarditis with cellular infiltration by lymphocytes, neutrophils, and eosinophils in the patients who died from dengue.Pericarditis, characterized by chest pain, has also been demonstrated in histopathology of DHF patients’ autopsies[20,47].This evidence may suggest that direct viral-induced cardiac tissue damage does take place.
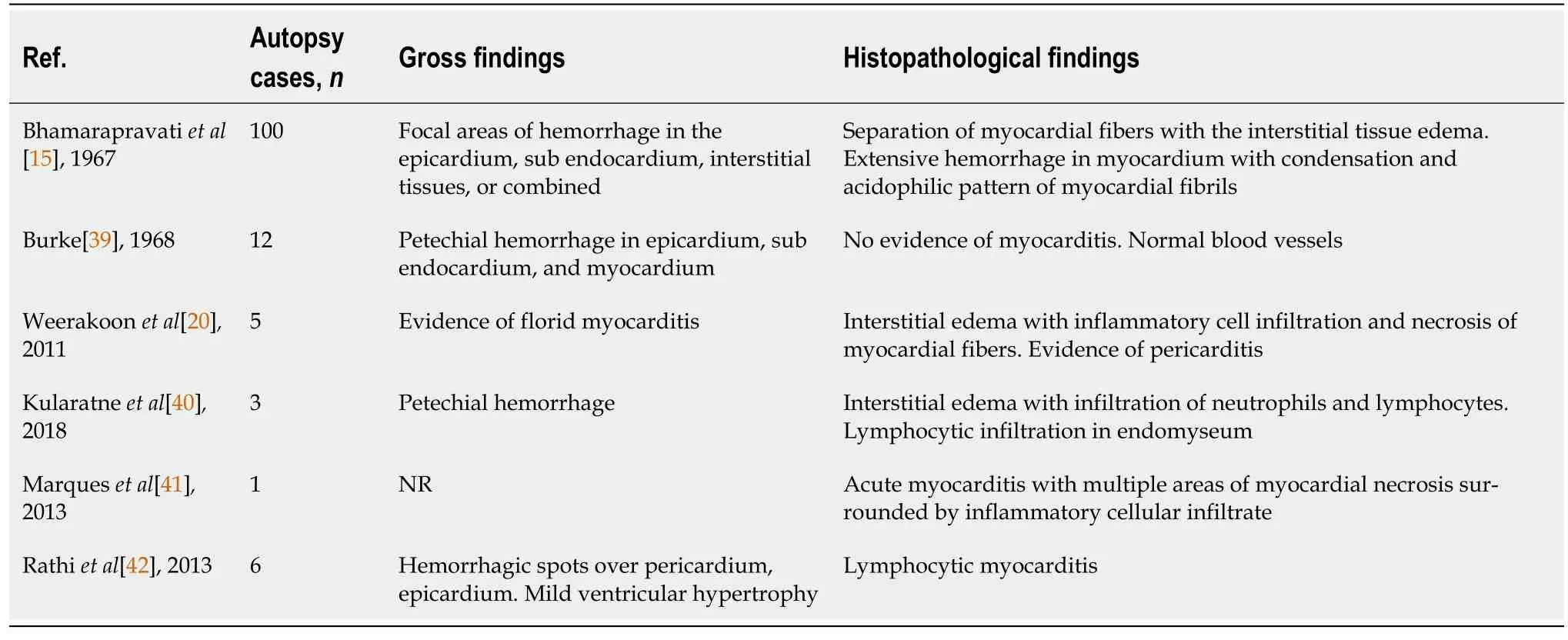
Table 3 Primary histopathological features of cardiac involvement in autopsy of dengue hemorrhagic fever patients
PATHOPHYSIOLOGY OF CARDIAC DYSFUNCTION IN DENGUE
Although the exact mechanisms leading to cardiac dysfunction in DVI are not clearly understood, two mechanisms of cardiac injury may cause it, either directly (viaDENV invasion and damage to the cardiac tissues) or indirectly (viacytokine-induced immune damage), or both may be at play[20,48].Numerous inflammatory mediators, such as cytokines, tumor necrosis factor-α, interleukins [(ILs),e.g., IL-1, IL-2, IL-6, IL-13, and IL-18] and cytotoxic factors, as well as increased capillary permeability, may play a vital role in the pathogenesis of cardiac dysfunction[49-51].
Cardiac dysfunction in DVI, although mild and self-limited, can be caused by SDD, which results in progressive and intractable acute heart failure with global hypokinesia and acute cardiac dilatation[24,28,29].After DENV enters the body, it will be attacked by macrophages, which in turn causes T cell activation.Activated T cells then release several inflammatory mediators and induce the complement system pathways’ activation (i.e.C3a and C5a).This process will cause inflammation and necrosis of the endothelial cell lining of blood vessels, leading to increased capillary permeability and vascular leakage.When the vascular leakage occurs in myocyte interstitial spaces, it will cause myocardial interstitial edema, leading to the impairment of myocardial contraction and reduced overall function.Moreover, intravascular blood volume decreases, leading to coronary circulation impairment.The release of numerous cytokine mediators also affects cardiac electrical conduction and suppresses cardiac contractility, leading to conduction block and arrhythmias.Another factor that may contribute to cardiac dysfunction in DHF patients is a derangement of calcium ion storage in the myocytes, directly leading to myocarditis[13,52,53].
MANAGEMENT
Cardiac dysfunction in DHF is primarily asymptomatic and transient, and there are no specific guidelines available for its management.Thus, routine ECG will provide clear evidence of myocarditis or abnormal cardiac rhythm in this group of patients.The patients who have abnormal ECG with clinical manifestations of cardiac involvement should be progressed to ECHO evaluation.The most critical problem has been determining whether myocarditis is playing a contributory role in a patient with shock.The goal of the management is to provide symptomatic and supportive care to maintain hemodynamic stability.These goals include maintenance of optimal intravascular volume, with use of diuretics and inotropic drugs as necessary.Moreover, special care should be taken not to cause iatrogenic fluid overload[54,55].
Corticosteroid treatment in SDD with myocarditis remains controversial.Only one study to date has shown rapid recovery of a child with myocarditis upon intravenous administration of methylprednisolone[56].Tachyarrhythmias, including atrial fibrillation (AF), have also been documented in patients with dengue but are much less common than bradyarrhythmias.A few studies have supported the use of antiarrhythmic drugs in non-self-limiting AF[57-59]; although, the CPG has still not been clearly defined.However, the efficacy of channel blockers and β-blockers for AF treatment caused by DVI is not conclusive because of co-existing hypotension.Notably, close and regular monitoring of the clinical presentation of DHF patients is crucial.Furthermore, a vigorous treatment regimen to maintain normal hemodynamic stability is the critical point of DHF patient management.
CONCLUSION
Cardiac dysfunction has been reported to be more severe in patients with DSS than in those with DF or non-shock DHF.The pathophysiology of myocardial damage in DHF patients is not yet fully elucidated.It may result either from direct DENV damage of the cardiomyocytes or an indirect cytokine-mediated immunological response, or even a combined effect.Cardiac involvement in DHF is not uncommon.Although acute, transient and self-limited, acute cardiac dysfunction in DHF may lead to clinically severe state and a fatal outcome.Early detection of cardiac involvement in DVI patients, prompt resuscitation of hemodynamic instability, avoidance of circulatory overload, and sparing the essential tools for critical management are vital in managing DHF patients with severe cardiac dysfunction.
 World Journal of Meta-Analysis2021年3期
World Journal of Meta-Analysis2021年3期
- World Journal of Meta-Analysis的其它文章
- Post COVID-19 infection: Long-term effects on liver and kidneys
- Immune response to Helicobacter pylori infection and gastric cancer development
- Glycated haemoglobin reduction and fixed ratio combinations of analogue basal insulin and glucagon-like peptide-1 receptor agonists: A systematic review
- Impact of Streptococcus pyogenes infection in susceptibility to psoriasis: A systematic review and meta-analysis
- Extraintestinal infection of Listeria monocytogenes and susceptibility to spontaneous abortion during pregnancy: A systematic review and meta-analysis
