Post COVID-19 infection: Long-term effects on liver and kidneys
Swati Srivastava, Iti Garg
Swati Srivastava, Iti Garg, Defence Institute of Physiology and Allied Sciences, Defence Research and Development Organization, New Delhi 110054, Indiana, India
Abstract Coronavirus disease 2019 is a pandemic, which has affected millions of people across the globe in the year 2020.This disease is caused by a virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), that belongs to the family of coronaviruses and primarily affects the respiratory system.This infection has a wide spectrum of clinical manifestations ranging from asymptomatic form to mild, moderate and severe forms depending upon the age, comorbidity and immunity of an affected individual.Hyper-inflammatory response due to SARSCoV-2 adversely affect several internal organs.Besides lung injury, which is the main outcome of SARS-CoV-2 infection, it has been reported to adversely impact other organs including the liver and kidneys.SARS-CoV-2 virus can also have a direct adverse impact on liver as well as kidneys due to systemic inflammatory response or drug toxicity, leading to elevated levels of liver injury markers and acute kidney injury.Clinical outcomes of SARS-CoV-2 infection could be worse in patients suffering from pre-existing liver and kidney disease.So far, there have been several reports on the mechanism of liver and kidney injury during SARSCoV-2 viral attack.However, the long-term impact of this infection on these organs is yet to be understood.This review summarizes the possible causes and effects of SARS-CoV-2 on the liver and kidneys during the infection and post recovery based on available literature.
Key Words: Alanine aminotransferase; Aspartate aminotransferase; Acute Kidney injury; COVID-19, Kidneys; Liver; SARS-CoV-2
INTRODUCTION
Coronaviruses are a large family of viruses causing a variety of diseases.In recent times, severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome-CoV) have caused respiratory illness (pneumonia) to thousands of people worldwide, leading to death of many patients.Novel coronavirus-induced pneumonia caused due to severe acute respiratory syndrome coronavirus 2 (SARSCoV-2) was declared a Public Health Emergency of International Concern on January 30, 2020[1] and later named as coronavirus disease 2019 (COVID-19) by the World Health Organization (WHO) on the February 11, 2020.SARS-CoV-2 (COVID-19) viral infection was first reported in December 2019 in Wuhan, Hubei Province, China (CDC 2019), and it spread to almost all the parts of the world in a short span of time.
COVID-19 infection causes severe pulmonary disease and has devastating effects.Itinduces a systemic disease attacking multiple organs, potentially causing major damage, including mortality and long-term effects.The mortality rate due to COVID-19 infection is 2%-5% in the general population.However, patients with comorbidities, such as hypertension, diabetes and chronic obstructive pulmonary disease, are considered at higherrisk, with a mortality rate above 50%[2].
There is no definite treatment for infection caused by SARS-CoV-2.Therapeutics currently under consideration by the WHO includeinterleukin6-blockers, colchicines, monoclonal antibodies, anticoagulants and vitamin D along with systemic corticosteroids in patients having severe and critical condition[3].However, treatment protocols across different countries vary wherein several antiviral candidates are in use for the treatment of infected patients like, the Food and Drug Administration approved remdesivir[4], corticosteroids, oseltamivir (Tamiflu), arbidol hydrochloride, hydroxychloroquine and convalescent plasma therapy[5], although the WHO does not recommend these treatment strategies.Several broad-spectrum antibiotics along with anti-inflammatory drugs are given to prevent acute respiratory distress syndrome.There has been a worldwide endeavour to design a safe and effective vaccine for protection against it.In this regard, several potential vaccines are under various stages of clinical trials.
The infection caused by SARS-CoV-2 virus is predominantly a respiratory disease.However, its adverse effects on other organ system remains unclear.The most common clinical presentation includes respiratory tract involvement with mild to high fever, shortness of breath and cough.However, SARS-CoV-2 infection has a wide spectrum of symptoms ranging from asymptomatic cases and very mild cases with minor symptoms of sore throat and loss of smell or taste[6] to acute respiratory failure and damage to other organs including acute kidney injury (AKI), liver damage, cerebrovascular stroke and gastroenteritis[7,8].There are several reports on adverse effects of SARS-CoV-2 infection on liver and kidney functions causing liver injury and acute renal injury.The purpose of this review is to ascertain the damaging effects of SARS-CoV-2 infection on the liver and kidneys.
ORIGIN, TRANSMISSION AND PATHOGENETIC MECHANISM OF SARSCOV-2
Coronaviruses are enveloped viruses with a positive sense single-stranded RNA genome with sizes between 26-32kb[9].Four genera of coronavirus viz., α, β, γ, δ have been identified so far, with human coronaviruses detected in α coronavirus and β coronavirus genera[10].SARS-CoV-2 is a positive-stranded RNA virusbelonging to the genus Beta-coronavirus.It has a crown like structure due to the presence of spike glycoproteins on the envelope[11].Scientists are trying to find the animal host of this novel coronavirus.However, most groups agree that the intermediate hosts are bats, pangolins or sea animals[12].Sequencing of the SARS-CoV-2 genome and its phylogenetic analysis revealed that it has 88%-89% similarity with two bat-derived SARS-like coronaviruses, bat-SL-CoVZC45 and bat-SL-CoVZXC21[12].
The virus SARS-CoV-2 mostly enters the body through inhalation, binds to epithelial cells in the nasal cavity and starts replicating[13].Like SARS-CoV, angiotensin converting enzyme 2 (ACE2) acts as the main receptor for SARS-CoV-2, which mediates internalization of the virus[14].The ACE2 protein is expressed in a variety of human cells, including type II alveolar cells, oral, oesophageal, ileal epithelial cells, myocardial cells, proximal tubule cells of the kidneys as well as urothelial cells of the bladder.The cleavage of the spike (S) protein of SARS-CoV-2 by a cellular enzyme named furin at the S1/S2 site is essential for viral entry to the lung cells[15].The activated S protein is primed byTMPRSS2and gets attached to ACE2 receptors.The virus replicates and migrates down the respiratory tract along the conducting airways, thus triggering the innate immune response.In the next stage of infection, the virus further reaches gas exchange units of the lung and infects alveolar type II cells.
CLINICAL MANIFESTATIONS OF SARS-COV-2
Although individuals of any age group can succumb to SARS-CoV-2 infection, susceptibility largely varies according to age and comorbidities.Its common clinical features in adults include fever, dry cough, sore throat, headache, fatigue, myalgia and breathlessness[16,17].The disease manifestations may range from mild pneumonia to moderate pneumonia, which may result in hypoxia requiring hospitalization and critical illness requiring mechanical ventilation, multiorgan dysfunction and possibly death[18].Individual age, underlying comorbidities and severity of the disease may increase the risk of death due to SARS-CoV-2 up to 49% in critically ill patients[19].However, the actual mortality rate due to this infection varies in different parts of the world.
IMPACT OF COVID-19 ON LIVER
Liver is a vital organ in the human body.COVID-19 infection can either have serious impact on individuals with pre-existing liver disease or can have direct adverse effects on the liver (Figure 1).Although several recently published reports establish that patients with liver diseases are at increased risk and severity of COVID-19 infection, interaction of pre-existing liver disease with SARS-CoV-2 has not been investigated in detail.There have been several studies to demonstrate adverse effects of SARS-CoV-2virus on the liver, and its impairment post SARS-CoV-2 infection is also an emerging concern.Previous studies of SARS coronavirus have shown that up to 60% of patients had a liver impairment showing viral nucleic acid and damage in a liver biopsy[20-22].Authors in these studies noted that this could be due to treatment of patients with high doses of antibiotics, hepatotoxic antiviral drugs and steroids.Because the process of RNA shedding is well described in the gastrointestinal tract and the production of ACE2 is higher in the colon, biliary system and liver, it is very likely that SARS-CoV-2 may have active replication in these sites, which might in turn result in direct or indirect tissue injury.Because liver is one of the potential entry targets for SARS-CoV-2, the liver damage caused due to infection by SARS-CoV-2 can be attributed to several factors including direct damage by penetrating virus, inflammatory or immune response, increased risk of thrombosis and liver lesions caused by anti-COVID-19 drug therapy[15,23].
Several independent reports are available to demonstrate liver function abnormalities in COVID-19 patients.Elevated levels of liver injury markers such as alanine aminotrans-ferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferaseand total bilirubin levelshave been reported in patients of SARS-CoV-2[24-30].A few selected cohort studies have been summarized in Table 1 showing levels of AST and ALT in COVID-19 patients having liver abnormalities either at the time of viral infection or post viral infection.

Table 1 Studies showing liver function abnormalities in coronavirus disease 2019 patients
Apart from cohort studies, numerous case reports and meta-analyses are also available, which depict severe clinical outcome of viral infection to those patients who were having pre- and post-liver abnormalities[31,32].Liver injury in SARS-CoV-2 infection is one side of the coin, but little is known about SARS-CoV-2 clinical presentation in the context of liver transplant.It is a well-known fact that these patients are immunosuppressed and are more susceptible to various opportunistic infections.Few case reports are available, which mainly focused on patients who underwent liver transplant a few months or year before SARS-CoV-2 infection but after getting SARS-CoV-2 infection, disease progressed rapidly from mild to critical stage and ultimately led to death despite giving various therapies and treatment[33-35].Few case reports showed promising recovery of these kinds of patients after dynamic surveillance and treatment[35,36].There is a prerequisite to plan strategies to manage SARS-CoV-2 infection in the post-transplant situation to reduce mortality.
Youssefet al[37] reported a meta-analysis of 3428 patients having SARS-CoV-2 infection from 20 retrospective studies and found that these patients exhibited elevated levels of ALT and AST.They concluded that dysfunctionality of the liver is associated with a critical outcome of SARS-CoV-2 infection, and precise monitoring is very much required in these patients to avoid serious clinical outcomes[37].Recent meta-analysis done by Del Zompoet al[38] included 20724 COVID-19 patients out of 36 studies and showed that there is an elevation of AST 26.5% and ALT 22.8% at the time of admission.In another meta-analysis, it has been also demonstrated that liver enzymes are firmly linked with 12882 confirmed COVID-19 patients as they showed that COVID 19 infected patients has elevated levels of AST (41.1%) and ALT (29.1%)[39].It has been reported that COVID-19 associated liver injury is more common in severe COVID-19 than non-severe COVID-19.Wonget al[40] reported the pooled odds ratio for elevated ALT (odds ratio = 2.5, 95% confidence interval: 1.6-3.7,I2= 57%) and AST (odds ratio = 3.4, 95% confidence interval: 2.3-5.0,I2= 56) were higher subjects in critical 5961 COVID-19 patients.
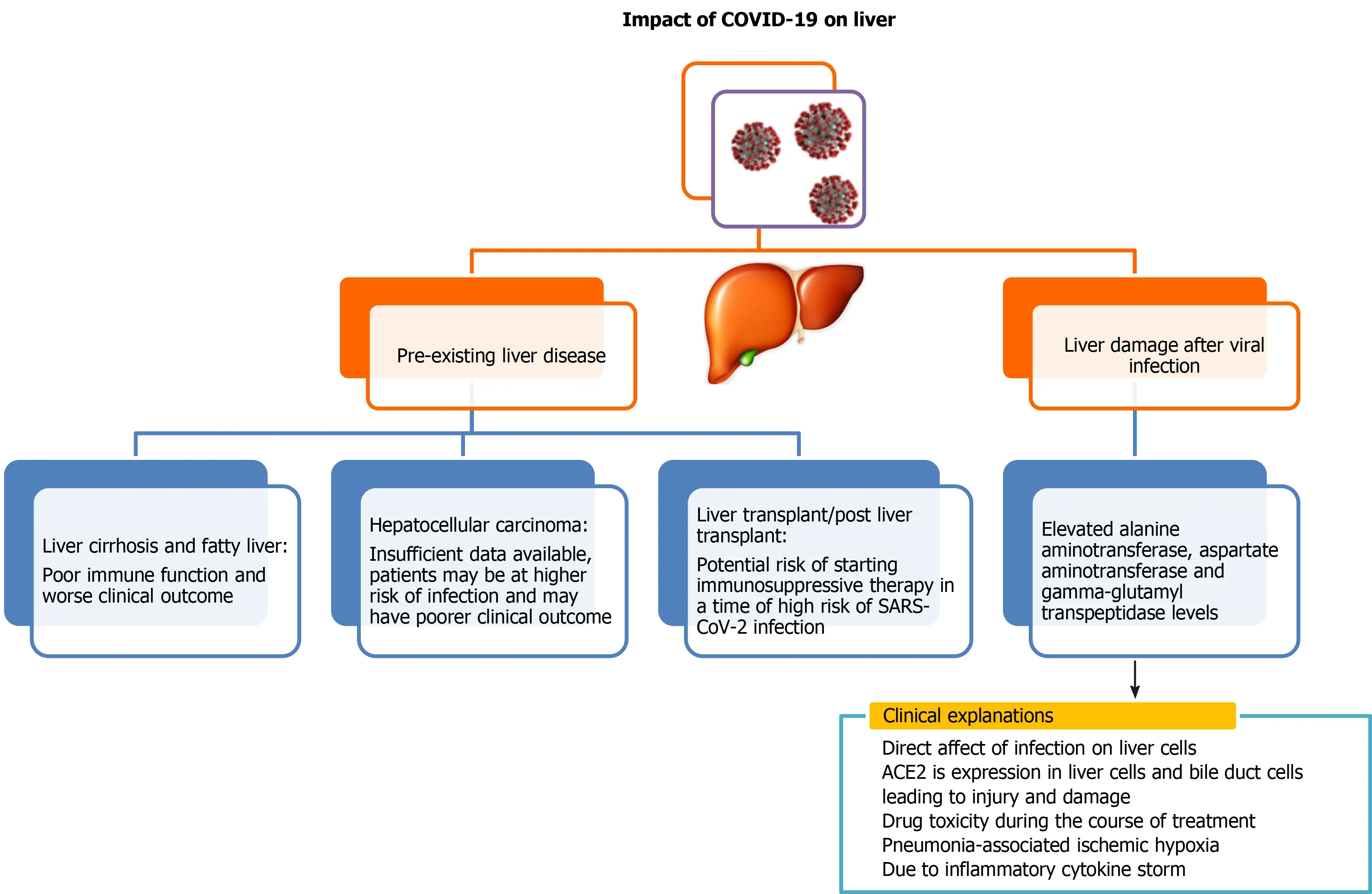
Figure 1 Liver injury due to coronavirus disease 2019 attack.
Díazet al[41] performed a meta-analysis to characterize hepatic pathological findings in COVID-19 patients.They included 18 studies, which were all case reports and case series from autopsies and reported that 55.1% of patients had hepatic steatosis, 34.7% had congestion of hepatic sinuses, 29.4% had venous thrombosis, 20.5% patients were with fibrosis, 13.5% patients represented Kupffer cell hyperplasia, 13.2% had portal inflammation, and 11.6% had lobular inflammation.Thus, there was a high prevalence of hepatic steatosis and vascular thrombosis as major histological liver features[41].
Finally, there is an utmost need to pay more attention towards the occurrence of liver damage in the diagnosis and treatment of SARS-CoV-2 infection.It is also advisable to clinicians to closely monitor the progression of liver dysfunction in mild to moderate as well as severe COVID-19 patients so that appropriate medical treatment could be given based on severity.It has become of utmost importance to get deep insight into the COVID-19 pathogenesis of liver in patients pre- and postinfection to develop an effective treatment regime for patients.
Liver impairment in alcoholic and nonalcoholic COVID-19 patients
Scanty information is available regarding liver impairment in alcoholic COVID-19 patients in comparison to nonalcoholic COVID-19 patients.Although, it is well known that alcohol is a major hepatotoxin, and its consumption is responsible for more than 40% of all deaths from liver disease as it interferes with the immune system and compromises its function[42].A wide spectrum of liver disease ranging from mild to severe comes under the umbrella of alcoholic hepatitis, which increases the risk of severe COVID-19 infection.It has been observed that the patients with alcoholic liver disease infected with SARS-CoV-2 have a worse prognosis.As we are evolving everyday for current pandemics, there are not straight and clear recommendations for the management of alcoholic liver disease in the COVID-19 pandemic[43].There is little information on the interaction of alcoholic hepatitis and COVID-19[44]; however, it is well-known that patients with advanced alcoholic liver disease are more prone to develop numerous kinds of respiratory distress[45].
COVID-19 AND AKI
Coronavirus infections have been associated with multiple organ dysfunction, including AKI (Figure 2).Several reports have shown higher frequencies of renal abnormalities in COVID-19 patients.Some studies have classified kidney impairment as an independent risk factor for mortality in COVID-19 patients admitted to hospitals[46,47].It is important for clinicians to understand whether accompanying AKI phenomenon occurs due to infection or is an important marker of disease severity[48].AKI occurrence at an early stage is considered a negative prognostic factor for survival and is more common in severely infected patients, especially those admitted to an intensive care unit (ICU)[49,50].Individuals with no underlying kidney problems also show signs of kidney damage after getting infected with SARS-CoV-2.Renal injury by SARS-CoV-2 can also be attributed to multiple factors such as direct injury due to virus infection or due to systemic effects including host immune clearance and immune response, endothelium-mediated vasculitis, thrombus formation, glucose and lipid metabolism disorder and hypoxia[51].In the early stages of pandemic spread, incidence of AKI was reported to be in 3% to 9% of patients.However, later studies reported an incidence rate of 15%[52,53].The COVID-19 associated AKI is managed by avoiding nephrotoxic drugs and by including supportive treatment like renal replacement therapy[47].Continuous renal replacement therapy by hemofiltration and hemodiafiltration has been used in the past to treat SARS, Middle East respiratory syndrome and sepsis.Thus, it is speculated that continuous renal replacement therapy may be beneficial in patients with COVID-19[54]; however, it needs to be critically evaluated.
Studies report that normal kidneys and intestinal tract have higher ACE2 expression compared to the lungs[28].ACE2 receptors on the cell walls of the kidney allow the entry of virus into the cells.Thus, kidneys can be directly impacted by the SARS-CoV-2 virus.Diaoet al[55] examined post-mortem kidney tissue from 6 infected patients and observed severe acute tubular necrosis and lymphocyte infiltration[56].Additionally, SARS-CoV-2 nucleocapsid protein and clusters has been detected in kidney tubules by immunohistochemistry[55] and in the tubular epithelium and podocytes using electron microscopy[56].Thus, this pathogenetic nature of a virus causing injury in tubular, glomerular and vascular parts of the kidney leads to AKI.In a recent observational study of 85 deceased patients, it was found that 54% of the patients had severe AKI, and non-recovery from severe AKI was associated with the presence of pigmented casts, as found on kidney biopsies.Researchers further reported that inflammatory markers and medications were associated with specific histopathologic findings in patients dying due to SARS-CoV-2 virus infection[57].Some selected studies showing kidney abnormalities in COVID-19 patients have been listed in Table 2.
COVID-19 in chronic kidney diseasepatients
Patients with chronic kidney disease have functional defects in innate and adaptive immunity, thus having a persistent proinflammatory state.These patients are at higher risk of infections in the upper respiratory tract; hence they could be prone to severe SARS-CoV-2 infection[47].Although, no systematic study has been done on this so far, Henry and Lippi[58] found a significant association of chronic kidney disease with severe COVID-19while analysing different available studies.
COVID-19 effect on chronic dialysis patients
Comorbidity such as nephropathy could lead to poor prognosis of COVID-19.Wang[59] studied 230 haemodialysis (HD) COVID-19 patients at Renmin Hospital, Wuhan University.They found 37 individuals amongst them were infected with SARS-CoV-2.Most of these patients presented mild symptoms and did not required admission to an intensive care unit.During this observational study, 7HD patients died, out of which 6 died due to SARS-CoV-2 infection.Another study on HD patients revealed that the most common symptoms of COVID-19, such as fever, cough, and dyspnoea, were not present in these patients.Infact, diarrhoea was the most common symptom, which made the diagnosis even more difficult[60].In a large-scale retrospective study of 7154 HD patients, SARS-CoV-2 infection was found in 2% of patients, and only about 50% of them had fever while about 20% were asymptomatic.Also, the mortality rate was as high as 31%, much higher compared to the general population[61].Studies report that HD patients with SARS-CoV-2 infection have reduced inflammatory response, such as levels of circulating CD4 and CD8 T cells, natural killer cells and proinflammatory cytokines and mild symptoms with a lower risk of acute respiratory distress syndrome compared to other general SARS-CoV-2 infected patients[62].However, this reduced or impaired inflammatory response may lead to a severe outcome of the disease.Thus, HD patients might need to be extra cautious and take all preventive measures to avoid contact with an infected person.
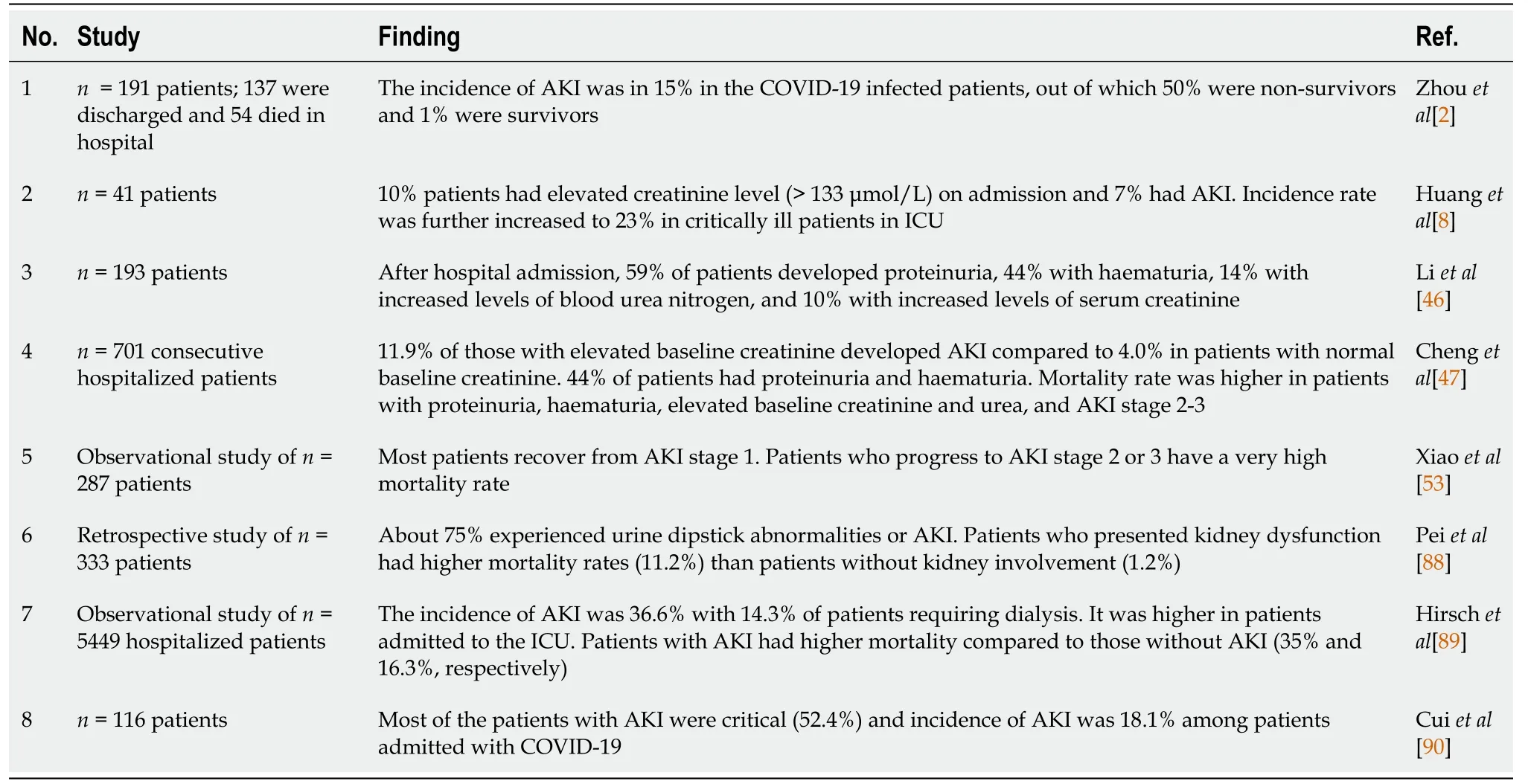
Table 2 Studies showing kidney abnormalities in coronavirus disease 2019 patients

Figure 2 Kidney injury due to coronavirus disease 2019 attack.
COVID-19 in kidney transplant recipients
To prevent graft rejection and to prevent inflammatory response, kidney transplant recipients are given immunosuppressants.Many countries with community transfer of COVID-19 have withheld transplantation procedures unless required in highly selected cases when required as a life-saving procedure.Common symptoms of COVID-19 in transplant recipients include fever, cough, asthenia, myalgias and diarrhoea[63].In different studies on COVID-19 infection in transplant patients showed numerous radiopacity and patchy shadows on chest radiographs[64-66], while in another study on 15 kidney recipients, 33% had no acute radiographic findings (Columbia University Kidney Transplant Program)[67].
Complete withdrawal of immunosuppressants such as calcineurin inhibitors is not recommended in COVID-19 patients in the absence of pneumonia, according to the European Renal Association-European Dialysis and Transplant Association guidelines[68].However, reduction in its dose and withdrawal of mycophenolate, azathioprine or mTOR-inhibitors can be considered based on the severity of infection.Also, in such cases the prescription of antiviral and anti-inflammatory drugs should be done with caution, considering their drug-drug interactions with immunosuppressants[68].Sudden withdrawal of immunosuppressants to clear viral load may lead to immune reconstitution and kidney rejection.
POST COVID-19 RECOVERY
Most people who get infected by SARS-CoV-2have mild or moderate symptoms and can recover completely after supportive medical care.Whereas some people, especially the elderly population with weaker immune systems and comorbidities, might suffer from severe symptoms and do not survive[69].Currently there are no drugs licensed for the treatment or prevention of COVID-19.As treatment with several different drugs is under trial, the WHO is keeping a close watch on the efforts to develop medicines to treat COVID-19.In such cases, misuse of several drugs can lead to serious side effects and complications[70].Meanwhile, non-pharmaceutical measures such as personal hygiene like hand washing and face masks, environmental sanitizing, social distancing and community-based decisions such as closure of schools, pubs, workplace restrictionsetc.are followed at various levels to contain the infection spread.
The majority of people infected with SARS-CoV-2 make a complete recovery in 2-3 wk.Inconclusive studies are available on how long a person remains infectious after the symptoms of infection vanish.Doctors and medical experts across the globe are putting their best efforts to fight this novel coronavirus pandemic.With every passing day new information about its early symptoms, spread, variants, possible treatment strategies and post recovery complications is being added.Still there is no definite answer to the long-term implications of COVID-19.However, some emerging data point out that people suffer from shortness of breath, fatigue, joint pain and headache post recovery from infection[71].Although, a patient’s recovery may happen in about 2 wk of medical care, people may suffer from other ailments of the kidney, lungs and heart post-recovery.This condition is referred to as ‘post COVID-19 syndrome’ or ‘long COVID19’, which occurs due to persistent symptoms or delayed long-term complications beyond 4 wk.Older aged people, especially those with comorbidities, are more likely to experience lingering COVID-19 symptoms.Nalbandianet al[72] recently summarized the epidemiology and organ-specific sequelae of post-acute COVID-19 and the management considerations needed for interdisciplinary comprehensive care of such patients.
Most of the human viral diseases such as influenza virus do not produce a stable immune response.Understanding the immunity development and its long-lasting effects post SARS-CoV-2 infection needs systematic large-scale studies.Conclusive data on post infection immunity against COVID-19 is lacking.However, antibodies present post recovery would confer to immunity against re-infection, atleast temporarily.During recovery from COVID-19, immunoglobulin (Ig) M and IgG antibodies develop within days to weeks from the onset of symptoms[73,74].However, there is not a clear and direct relationship between detectable antibody response and clinical improvement in a patient’s condition[73,74].Viral load peaks during the early stage of infection and then gradually declines as antibodies develop during the subsequent 2-3 wk[74,75].The stability of neutralizing antibodies, primarily IgG, against COVID-19 is yet to be confirmed.During infection with SARS-CoV, IgG remained high for over 4-5 mo and subsequently declined during the next 2 years to 3 years[76].It persisted upto 34 mo in patients recovered from Middle East respiratory syndrome-CoV[77].
There have been reports from different parts of the world about re-infection, possible by a distinct molecular form of the same virus[78].A few studies have shown that patients who had mild symptoms of COVID-19 developed a weaker and shorter immune response and show a decrease in antibody levels after 2-3 mo of infection[79,80].After almost a year of the COVID-19 pandemic, there is no consensus regarding persistence of neutralizing antibodies and re-infection by SARS-CoV-2.
CONCLUSION
COVID-19 or SARS-CoV-2 infected patients with pre-existing liver and kidney comorbidities are likely to have a poorer clinical prognosis.Such patients are at high risk of severe infection, and the mortality rate is higher compared to that of the general population.They should take utmost precautions to avoid contact with infected persons.SARS-CoV-2 infection can adversely affect liver and kidney functions in healthy individuals.Data available so far indicates that SARS-CoV-2 causes acute kidney and liver damage.However, the extent of damage and long-term consequences are yet to be elucidated.Currently in the absence of specific therapy for this viral infection and newly developed vaccines being unavailable to the majority of populations worldwide, further clinical studies are needed to understand COVID-19 pathology in relation to liver and kidney damage.This could further help clinicians to modify therapeutic approaches for better management of SARS-CoV-2 infection.
ACKNOWLEDGEMENTS
We acknowledge and thank all clinicians, medical staff and researchers who collected the clinical data of COVID-19 patients, which is very helpful in further understanding and formulating strategies to deal with this pandemic.
 World Journal of Meta-Analysis2021年3期
World Journal of Meta-Analysis2021年3期
- World Journal of Meta-Analysis的其它文章
- Immune response to Helicobacter pylori infection and gastric cancer development
- Dengue hemorrhagic fever and cardiac involvement
- Glycated haemoglobin reduction and fixed ratio combinations of analogue basal insulin and glucagon-like peptide-1 receptor agonists: A systematic review
- Impact of Streptococcus pyogenes infection in susceptibility to psoriasis: A systematic review and meta-analysis
- Extraintestinal infection of Listeria monocytogenes and susceptibility to spontaneous abortion during pregnancy: A systematic review and meta-analysis
