Determination of bisphosphonates anti-resorptive properties based on three forms of ceramic materials:Sorption and release process evaluation
Monik Zielińsk,Ew Chmielewsk,Tomsz Buhwld,Adm Voelkel,Pweł Kfrski
aPoznań University of Technology,Institute of Chemical Technology and Engineering,Ul.Berdychowo 4,60-965,Poznań,Poland
bWrocław University of Science and Technology,Faculty of Chemistry,Department of Bioorganic Chemistry,Wybrze˙ze Wyspiańskiego 27,50-370,Wrocław,Poland
cPoznań University of Technology,Institute of Materials Research and Quantum Engineering,Piotrowo 3,60-965,Poznan,Poland
Keywords:
Monolithic column
Polymer composites
Bisphosphonates
Hydroxyapatite coatings
Hydroxyapatite composite
A B S T R A C T
There is a strong need to search for more effective compounds with bone anti-resorptive properties,which will cause fewer complications than commonly used bisphosphonates.To achieve this goal,it is necessary to search for new techniques to characterize the interactions between bone and drug.By studying their interaction with hydroxyapatite(HA),this study used three forms of ceramic materials,two of which are bone-stimulating materials,to assess the suitability of new active substances with antiresorptive properties.In this study,three methods based on HA in loose form,polycaprolactone/HA(a polymer-ceramic materials containing HA),and polymer-ceramic monolithic in-needle extraction(MINE)device(a polymer inert skeleton),respectively,were used.The Affinity of risedronate(a standard compound)and sixteen aminomethylenebisphosphonates(new compounds with potential antiresorptive properties)to HA was defined according to the above-mentioned methods.Ten monolithic materials based on 2-hydroxyethyl methacrylate/ethylene dimethacrylate are prepared and studied,of which one was selected for more-detailed further research.Simulated body fluids containing bisphosphonates were passed through the MINE device.In this way,sorption-desorption of bisphosphonates was evaluated using this MINE device.The paper presents the advantages and disadvantages of each technique and its suitability for assessing new active substances.All three methods allow for the selection of several compounds with potentially higher anti-resorptive properties than risedronate,in hope that it reflects their higher bone Affinity and release ability.
1.Introduction
Geminal bisphosphonates(BPs)are characterized by a strong Affinity for the calcium component of both natural and synthetic hydroxyapatite(HA)bone minerals.Therefore,they have been widely applied as therapeutic agents for several bone-related diseases,especially osteoporosis.The chemical structure of BPs diametrically influences drugs properties,including the uptake and retention by the skeleton.The most potent BPs are those containing amino groups in their alkyl side chain.Aminomethylenebisphosphonates are the fourth generation of these drugs,with zoledronate being the most successful example[1].
The binding of BPs to bone surfaces is a reversible physicochemical process,and the release of the BPs can occur through at least two mechanisms:chemical desorption and osteoclastic resorption.Desorption or reattachment of BPs from a skeleton is an important property because it causes higher solution concentration in the vicinity of the bone mineral and around bone cells,such as osteoclasts[2].BPs with lower Affinity to HA will lead to higher desorption and thus a greater diffusion in the bone.For example,a low-Affinity BP,clodronate,compared with etidronate,demonstrates greater diffusion through bone,including within the osteocyte network[3].
The examination of the Affinity of BPs for bone and its distribution in the bone has already been extensively studied.Most research involves the determination of the Langmuir isotherms adsorption of BPs or the adsorption of BPs on powder HA.An example may be the adsorption of risedronate studied by Errassifi et al.[4]at the temperature of the human body(37°C).Synthetic nanocrystalline apatite was used as a model of bone mineral.The adsorption plateau was reached at relatively low risedronate concentrations,indicating a high Affinity of risedronate molecules to the mineral surface.However,there are very few studies describing the behavior of individual BPs during microanatomical distribution.Simultaneously,there is a lack of comparison of the individual BPs,which would enable an analysis of the differences[5].
One of the proposals to fill this gap was the use of liquid chromatography to assess the interactions of active substances with potential anti-resorptive properties with a column filled with HA[6].Comparison of the retention times of various bisphosphonates allowed for constructive conclusions regarding drug-mineral affinities.However,this method does not allow to separate sorption and desorption processes since both of them occur repeatedly one after the other at chromatographic column.Therefore,in this work,new methods were sought to precisely specify the adsorption process and desorption process of chosen BPs on HA.
Polymer-ceramic monolithic in-needle extraction(MINE)device based on poly(2-hydroxyethyl methacrylate-co-ethylene dimethacrylate)was previously used to examine the interactions between potentially anti-resorptive drugs and bones[7,8].Krenkova et al.[9]initially proposed this material as a technique for protein separation as well as for selective enrichment of phosphopeptides.The polymer-ceramic MINE device is a system by which the sorption and desorption processes of potential anti-resorptive drugs can be examined with good reproducibility.The conclusion from previous work was that the MINE device could be useful in determining the binding Affinity of potential anti-resorptive drugs to HA in the future.
By three methods(Fig.1):with hydroxyapatite in loose form,as well as using two newly prepared forms of bone-simulating materials;polymer-ceramic films based on polycaprolactone(PCL)and HA;and polymer-ceramic MINE device,this study aimed to determine Affinities of series of structurally diverse BPs to the HA treated as a model of bone.The goal was to determine not only the degree of sorption but also that of desorption of the BPs because BPs should have high Affinity and should be relatively easily released.

Fig.1.Varied methods of sorption and release process evaluation.
2.Experimental
2.1.Reagents and chemicals
Methanol p.a.sodium chloride(99%),and hydrochloric acid(36%-38%)were obtained from POCh(Gliwice,Poland).HA(≥90%)with an average particle size of 13μm,ethylene dimethacrylate(EDMA),2-hydroxyethyl methacrylate(HEMA),isobutanol(≥99%),dodecanol(98%),tetrahydrofuran(THF)(≥99%),cyclohexanol(≥99%),(3-mercaptopropyl)trimethoxysilane(95%)(SCA),2,2′-azobisisobutyronitrile(AIBN)0.2 M solution in toluene,potassium phosphate dibasic trihydrate(99%),potassium phosphate monobasic(99%),and tris(hydroxymethyl)aminomethane(99.8%)(TRIS)were obtained from Sigma-Aldrich(Steinheim,Germany).High purity potassium chloride was obtained from Chempur(Poland).Risedronate sodium(R)was purchased from Sigma-Aldrich(St.Louis,MO,USA).
Stainless steel needles(internal diameter 2.7 mm)and gas-tight syringes(10 mL)were obtained from Danlab(Białystok,Poland).
Sixteen BPs were used in this study,and their chemical names and structures are listed in Table 1.Compounds were prepared according to the procedure described previously[10-19].For compounds BP15 and BP16,amine(0.03 mol),triethyl orthoformate(0.033 mol,4.40 mL or 0.066 mol,8.8 mL),and diethyl phosphite(0.063 mol,8.16 mL or 0.126 mol,16.32 mL)were heated and simultaneously stirred for 15 h at ~130°C on the heating plate(125°C in a reaction medium)of a Radley's Carousel apparatus.The mixture was cooled,and the volatile components were removed using a rotary evaporator.The resulting mixture was dissolved in ethyl acetate(100 mL)and purified by washing with water(100 mL),saturated sodium chloride solution(100 mL),and again with water(100 mL).The solution was then dried over anhydrous MgSO4,and the solvent was evaporated under a vacuum.The crude reaction product was obtained after boiling in 20 cm3of 6 M hydrochloric acid for 12 h.After cooling,the volatile components were removed using a rotary evaporator,and the resulting oil was dissolved in hot water,discolored with active charcoal,and purified by crystallization with water/ethanol mixture.
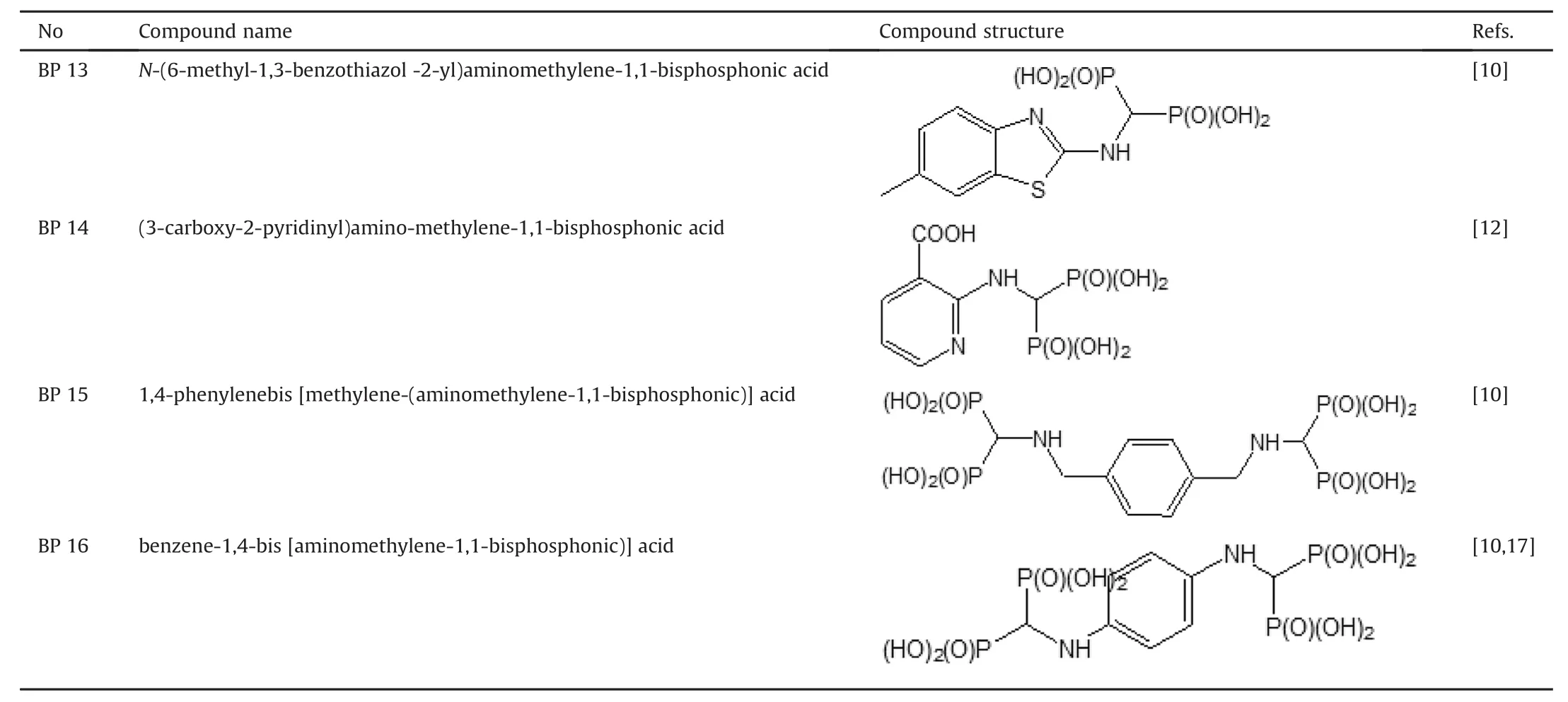
Table 1(continued)
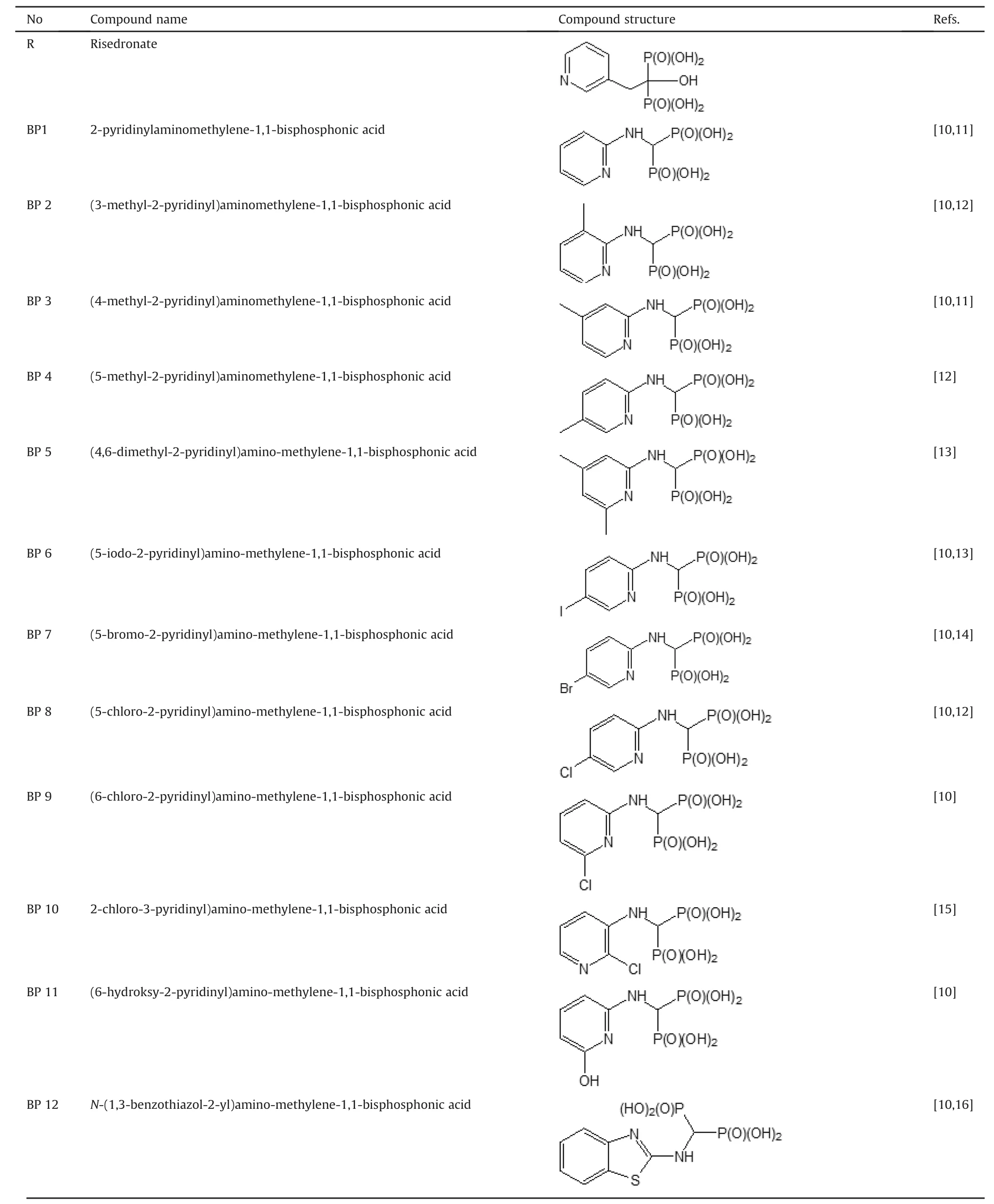
Table 1 Structures of bisphosphonates used in this study.
2.2.Simulated body fluid(SBF)
SBF was prepared by dissolving sodium chloride(8.0 g/L)in TRIS buffer solution(12.1 g/L).The pH of the obtained solution was adjusted to 7.4 using HCl as previously described[7].
2.3.Polymer-ceramic materials
2.3.1.PCL/HA films
The preparation of PCL/HA composites was done by merging two solutions[20].First,PCL was dissolved in THF at 60°C.Second,HA was dispersed in THF.The second solution was added to the first one and mixed for 30 min.Afterward,the Eppendorf repeater M4 pipette was used to prepare a series of similar films.All obtained materials were weighed and measured with an electronic caliper,and outliers were rejected.Subsequently,the HA layer was increased using the previously described method(with minor modifications)by mineralizing four layers of HA[21].Each film was immersed into CaCl2/Tris-HCl solution(pH 7.4)for 30 min.After washing with water and drying,the resulting material was added to 120 mM Na2HPO4solution for 30 min.This process consisted of two steps and was repeated four times.
Pure PCL film(without HA in its structure)was also prepared to determine the Affinity of tested compounds to the polymer part,which should be inert to BPs.
2.3.2.HEMA/EDMA/HA monolithic materials
The surface of each stainless steel needle was precisely covered with a silanization agent using the previously described procedure[7].Based on the previous procedure[8],ten monolithic materials were prepared,including nine new ones.Proportions of the components in the polymerization mixture are listed in Table 2.Each polymerization mixture was treated in the same manner:purged with nitrogen for 30 min under mixing conditions and then transferred into needles.The polymerization process was carried out for 20 h at 70°C.Subsequently,to get rid of the solvent constituting the porogenic agent,rubber plugs were removed from the needles,and the material was heated at 110°C and finally washed with methanol and water.

Table 2 Ratio of components used for HEMA/EDMA/HA monolithic materials preparation.
2.4.Bisphosphonates Affinity
2.4.1.Method based on HA in loose form
Sorption of BPs was initiated by weighing 30 mg of HA in an analytical balance with an accuracy of+/- 0.1 mg.Subsequently,3 mL of SBF containing BP(0.164 mM)was poured into the polypropylene tubes and placed in a shaker for 30 min,2 h,and 24 h.Sorption of potential anti-resorptive compounds on HA was measured using a UV-Vis spectrophotometer after centrifugation(speed 4000 rpm).
2.4.2.Method based on PCL/HA
The study of sorption and desorption using PCL/HA consisted of placing one material in one polypropylene cuvette.The cuvettes were filled with 1 mL of SBF and 0.164 mM of each analyte(BPs)and sealed with a lid.The BP concentration in the solution was measured without the removal of the absorbent.The desorption step was initiated by the transfer of desiccated materials to new cuvettes filled with 0.15 M phosphate buffer(1 mL).
2.4.3.Method based on polymer-ceramic MINE device
Using risedronate as model BP,ten materials were characterized by determining their permeability and sorption and desorption properties.The most advantageous monolithic material was selected for performing all tests for the sixteen BPs.
Piston pump CL-20 A(Shimadzu,Japan)was used for the uniform flow rate during the sorption and desorption processes(0.5 mL/min).
For sorption,3 mL of SBF containing each analyte(BPs)with a concentration of 0.164 mM was passed through a needle and analyzed using a UV-Vis spectrophotometer.For desorption,0.15 M phosphate buffer with pH 7.8 was used.
2.5.Raman microspectroscopy analysis
InVia confocal Raman microspectroscope(Renishaw,Gloucestershire,UK),using Raman spectral maps,was applied for the determination of pure PCL film,PCL containing HA and PCL/HA,as well as HA distribution in these materials.Raman spectra were collected using 785 nm laser in the range of 200 cm-1-1800 cm-1.Raman spectral maps were recorded in areas of 2000μm × 2000μm with steps of 50μm.
2.6.UV-Vis analysis
UV-Vis spectrophotometer JASCO V-630(JASCO,Japan)was applied for the determination of BPs concentration.The measurements were carried out in the range of 220-400 nm.
2.7.Permeability
The permeability parameter of the porous monolith,which depends on the porosity and pore size,was determined experimentally by measuring the pressure drop across the monolithic material ΔP(Pa)while maintaining the appropriate flow rate F(μL/min)and including the viscosity of the mobile phase ŋ (Pa ·s),length of the monolithic material L(dm),and radius of the monolithic material r(dm)according to the formula:

2.8.Data evaluation
Each result presented in the work was based on five repetitions.A Dixon's Q test was applied to determine and reject statistical outliers with a critical value of 0.71 at 95% Confidence[22].
3.Results and discussion
In this study,sixteen BPs with potential anti-resorptive properties and risedronate sodium,as a reference compound(R),were examined using three different HA-based materials treated as models of bone.
3.1.Method based on HA in loose form
The study of BPs Affinity to bones began with their sorption on HA powder.Almost all analyzed compounds exhibited high Affinity to HA(Fig.2),which is the main bone component.Sorption at the lowest level was determined for BP16.Interestingly,this compound exhibited a relatively substantial activity in vitro test carried out on murine macrophage cell line,RAW 264.7[23].However,it is unstable in aqueous solutions and thus was eliminated from further studies.In addition,three other compounds(BP13-BP15)were not as strongly sorbed by HA as the other BPs.Compounds BP14 and BP15,which possess two bisphosphonic moieties in their structures,were expected to have a strong Affinity to HA.However,they were bound relatively weakly.
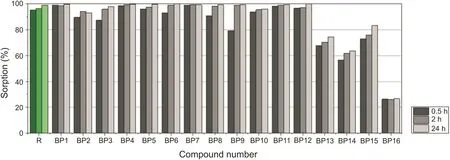
Fig.2.Aminobisphosphonates sorption on HA.
3.2.Method based on PCL/HA
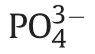

Fig.3.(A)Raman spectra,(B)Raman spectral maps,and(C)SEM images of pure PCL film,PCL with HA( film based on HA in dissolved PCL),and PCL/HA film after four cycles of the build-up layers of HA.
The sorption(Fig.4A)and desorption processes(Fig.4B)were carried out for 15 BPs,and risedronate was taken as a reference.Compound BP1,structurally related to risedronate,was sorbed at a higher level than the reference(R).Once more,compounds BP13-BP15 exhibited the lowest Affinity to HA.A similar pattern of activity was observed in studies using pure HA and PCL/HA films.The higher Affinity to HA than risedronate was found in compounds BP1-BP4,BP6-BP9,and BP12.Therefore,aminomethylenebisphosphonates with a methyl substituent in the pyridine ring as well as those substituted with bromine,chlorine,or iodine could lead to a stronger binding to bones.The results of the desorption process showed that only five of them desorbed faster than risedronate(R).Those which exhibited strong sorption and were desorbed faster than the reference were marked with green boxes in fig.4B.This information is crucial because,when BPs are released more quickly,their concentrations increase in the vicinity of the bone mineral and around bone cells such as osteoclasts[25].
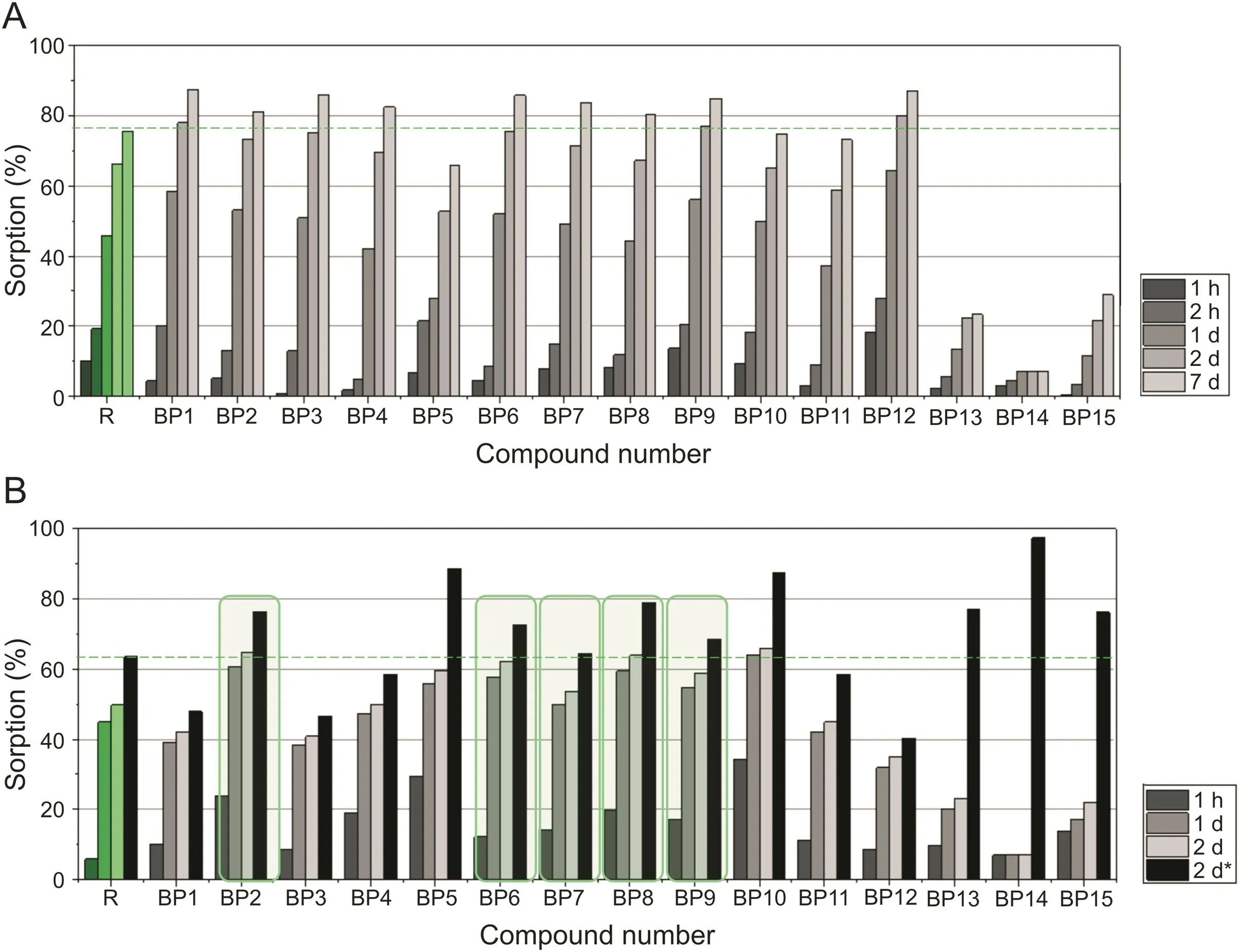
Fig.4.Aminobisphosphonates(A)sorption and(B)desorption on PCL/HA films.*Two days of desorption-results in relation to the sorbed mass.
Compounds BP1,BP3,BP4,and,especially,BP12 were sorbed more strongly than risedronate and less efficiently desorbed.This means that they have a relatively strong bone Affinity.
3.3.Method based on polymer-ceramic MINE device
Polymer-ceramic monolithic materials(N1-N10)were obtained by polymerization of mixtures containing HEMA,EDMA,and HA according to the procedures described in Section Experimental.All investigations were carried out using three identical needles,which gave flow resistance at the same level and equal flow rate.The flow rate of the sample through the monolithic material was chosen as 0.5 mL/min,as described previously[26,27].The repeatability of the method(extraction using three identical needles)was acceptable and relative standard deviation(RSD)values varied between 0.1% and 1.5%.
During the selection of porogenic agents,several factors were guided,and the most important of them were high viscosity and low boiling point.High viscosity will prevent rapid sedimentation of HA from the polymerization mixture,whereas the low boiling point will affect the rate and effectiveness of the removal of the porogenic agent.
First,the permeability of the monolithic material during liquid flow was checked(Fig.5A).Due to the use of a material based on brittle HA,it was assumed that the pressure in the needle should not exceed 100 bar since it was noticed that in certain cases some materials became unstable after crossing this limit.Therefore,it was necessary to reject needles N9 and N10 from further studies.
The second step relied on checking the sorption capacity of the prepared monoliths.The largest amount of risedronate was sorbed using needle N8(Fig.5B)with the lowest permeability value found for this needle(after exclusion of needles N9 and N10).More importantly,it allowed us to achieve desorption at the highest level among all tested materials(Fig.5C).Based on these results,needle N8 was selected as the most favorable.

Fig.5.Selection and optimization of In-needle HEMA/EDMA/HA monolithic materials.(A)Permeability of monoliths in needles,(B)risedronate sorption using N1-8 needles,(C)risedronate desorption using N1-N8 needles,and(D)selection of eluent volume for needle N8.
The third step concerned the right choice of the amount of eluting agent during the examination of the release of the tested BPs.Based on Fig.5D,it was noticed that the volume should be 10 mL,which is suitable for carrying out a controlled desorption process,followed by the use of another 10 mL for conditioning.Two red lines marked in this Fig.5D separate the range used for desorption studies.The results of the studies carried out using 5 mL( first line)are shown in fig.5C,while those of desorption studies carried out using 10 mL(second line)are shown in fig.6B.
Aminomethylenebisphosphonate Affinity to HA(ceramic part of the monolithic material)was determined using a MINE device.For this purpose,the sorption and desorption processes were carried out.
The sorption capacity,indicating the mass of analyte retained on the monolithic material,was determined(Fig.6A)by passing each of the tested BPs through the MINE device.The highest sorption capacity,almost 95%,was achieved for compounds BP7 and BP9.Five compounds,namely,BP1,BP4,BP7,BP9,and BP12,exhibited a higher sorption percentage than control risedronate(R)while comparing only the first milliliter.Additionally,over 80% of sorption was noted for nine compounds:BP1,BP3,BP4,BP7-BP12.It is an important finding since compound BP12 appeared to be of choice for in vivo test when curing induced osteoporosis in sheep[10].Although compound BP9 has not been studied on sheep,in vitro test indicated that it was equipotent with compound BP12.
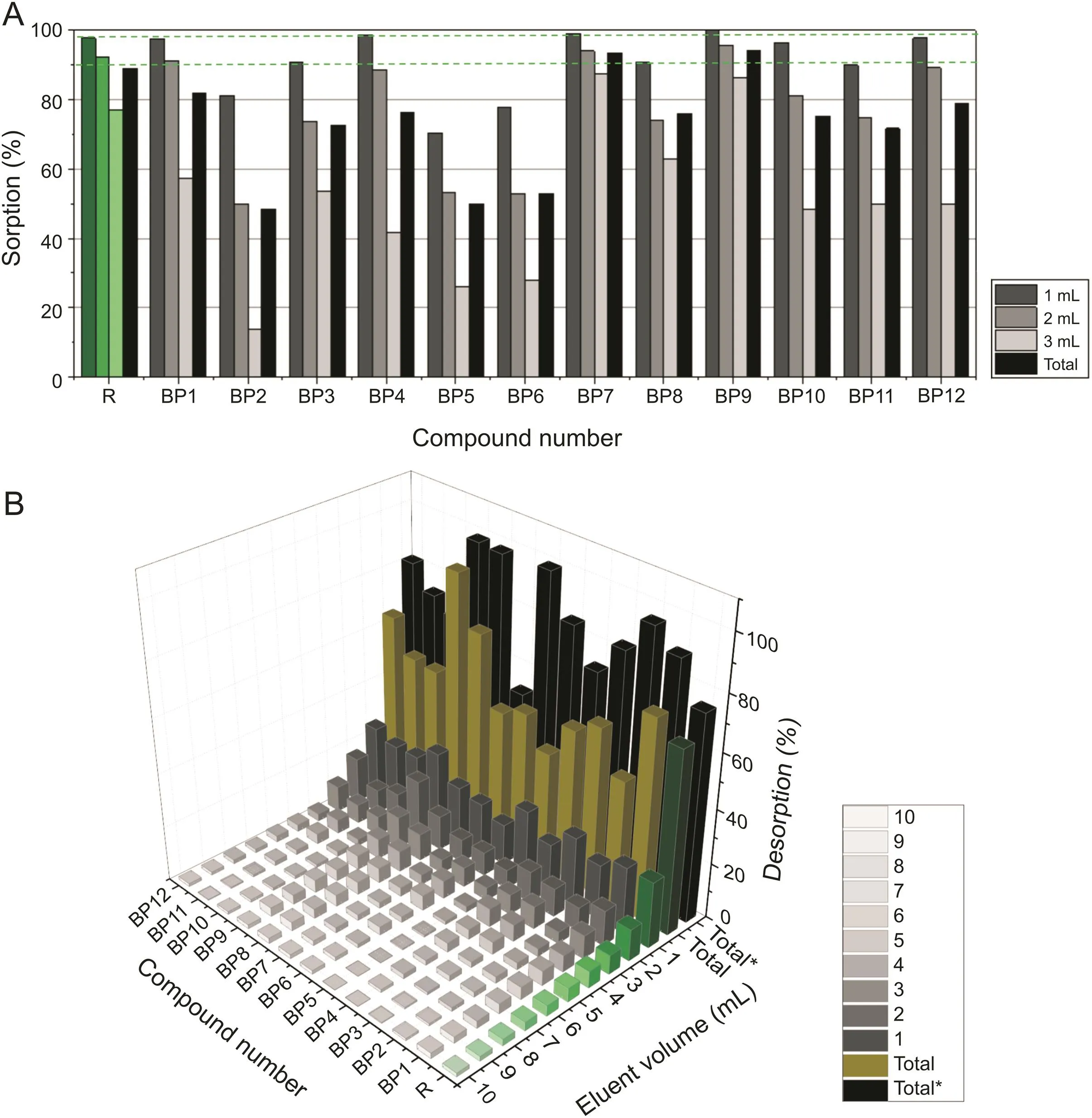
Fig.6.(A)Sorption and(B)desorption of bisphosphonates with using needle N8.*Total deposition-results in relation to the sorbed mass.
The desorption process,as mentioned earlier,also demonstrates the strength of the interaction between the aminomethylenebisphosphonate and HA.A higher Affinity for the HA can be proved by the low elution of the drug.On the other hand,the BPs should be desorbed relatively quickly because drugs released from bone have a greater recycling potential,and the desorbed drug returns onto bone surfaces faster[1].Moreover,the strong adsorption of drugs to the bone tissue is suspected to cause jaw osteonecrosis,a dangerous side effect of medication with BPs[28,29].The BPs with a high Affinity for bone mineral would be expected to exhibit a greater degree of return to bone surfaces during reattachment(resulting from higher sorption and lower desorption).
From the analytical point of view,the desorption step leads to elution of the compounds,which in turn enables the complete extraction process as well as enables the conditioning of the monolithic material for further use.
Four compounds(BP2,BP6,BP8,and BP9)were almost completely desorbed(at a level above 95%)considering the mass of each BP which was previously sorbed(Total*value in fig.6B).Furthermore,three other compounds(BP1,BP5,and BP12)were desorbed at a higher level than control risedronate(R).
The kinetics of desorption is also an interesting factor.Comparing the concentration of the compounds eluted in the first milliliter,it can be concluded that compounds BP1,BP3,BP9,and BP12 were removed faster from used monolith than from risedronate.These compounds were also extensively desorbed with the first 3 mL of the eluent.In addition, five compounds were desorbed above the 75% level in the first 5 mL(BP2,BP3,BP6,BP9,and BP12).
A closer look at those compounds that were sorbed at a higher level than risedronate(based on Fig.6A)indicates that compounds BP1,BP9,and BP12 demonstrated interesting properties;they sorbed on a ceramic monolith at a high level and,subsequently,extensively desorbed.This is a desired feature of potential antiosteoporotic agents.For the introduced mass,BP9 recovery achieved 92%.Considering the adsorbed amount,this result was even higher and reached 98%.Compound BP7 had the highest Affinity to HA,which proved a high level of sorption and resistance to desorption.The speculation on the sorption/desorption significance finds support in the high antiosteoporotic activity of BP12 in vivo studies[10].It is worth to note that for BP9 the level of sorption is equal to 70% for PCA/HA,but the ability to desorb is low for BP12(40%).
Among the tested BPs,only compound BP16 exhibited high activity in vitro test and was rejected from further studies because of its instability in buffer solutions after a prolonged time.All the other compounds were moderately active,being nearly equipotent to incadronate and other popular antiosteoporetic drugs.Furthermore,compound BP12 tested on sheep with induced osteoporosis revealed very promising drug-like properties.It also behaves specifically in the present study:being relatively strongly adsorbed and relatively fast-desorbed from HA.The remaining compounds showed a high recovery wherein their sorption was at a low level.This justifies the speculation that BPs lacking hydroxylic moiety would be more weakly bound to bone tissue and,thus,might be of choice from the point of view of side effects.
4.Conclusions
This study compares the results of three different model methods of testing BPs Affinity to the bone.A simple sorption study on HA in loose form gives only general information,while two new methods provided more specific data.The PCL/HA films and the MINE device are both useful tools for the determination of binding Affinity of potential anti-resorptive drugs to bones.However,there are differences in the results achieved by these methods,which are caused by two factors.First,the contact time of the BP with the ceramic material during sorption significantly varies.For PCL/HA films,it ranges from 30 min to several hundred hours,and for monoliths,the contact takes a few minutes.The same difference appears during the desorption process,but there is an additional factor that generates variation in desorption efficiency.The elution fluid was intentionally not exchanged during the desorption process.Fortunately,it was possible to distinguish which compounds had undergone intense desorption and which were again sorbed until the equilibrium state was achieved.This can be seen only by comparing the results of desorption with PCL/HA and the MINE device.For example,BP9 and BP12 were strongly sorbed on both types of ceramic materials.However,their desorption results vary.Both compounds were desorbed at a very high degree from monoliths in needles,while for PCL/HA,the difference is as follows:70% for BP9 and only 40% desorption for BP12.Therefore,it can be concluded that BP12 has a stronger Affinity for HA than BP9;however,as the recycling of BPs and the transfer back onto bone surfaces is an important concept,BP9 will be more easily released.
Currently used anti-resorptive drugs are characterized by longlasting residence in bone tissue.This might be caused by side effects,such as jaw osteonecrosis.Moreover,these drugs must be present in the circulating blood and available for reuptake into the bone.Therefore,the search for new candidates characterized by strong sorption and better desorption properties is one of the strategies for designing new medicines.The desorption process for risedronate reached the 70% level.Several compounds studied in this work showed sorption and desorption at higher levels than those found for control compounds.This means that they have some potential as new anti-resorptive drug candidates.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by the Ministry of Science and Higher Education grants and subsidy of the Ministry of Science and Higher Education.
 Journal of Pharmaceutical Analysis2021年3期
Journal of Pharmaceutical Analysis2021年3期
- Journal of Pharmaceutical Analysis的其它文章
- Molecular detection of SARS-CoV-2 being challenged by virus variation and asymptomatic infection
- The potential of miRNA-based therapeutics in severe acute respiratory syndrome coronavirus 2(SARS-CoV-2)infection:A review
- Potential treatment with Chinese and Western medicine targeting NSP14 of SARS-CoV-2
- Accurate and sensitive determination of hydroxychloroquine sulfate used on COVID-19 patients in human urine,serum and saliva samples by GC-MS
- Fast saccharide mapping method for quality consistency evaluation of commercial xylooligosaccharides collected in China
- Dispersive liquid-liquid microextraction,an effective tool for the determination of synthetic cannabinoids in oral fluid by liquid chromatography-tandem mass spectrometry
