Accurate and sensitive determination of hydroxychloroquine sulfate used on COVID-19 patients in human urine,serum and saliva samples by GC-MS
Süleymn Bodur,Sezin Errpt,ömer Thir Günkr,Sezgin Bkırdere,b,*
aYıldız Technical University,Faculty of Art and Science,Department of Chemistry,34210,Davutpasa,Esenler,Istanbul,Turkey
bTurkish Academy of Sciences(TÜBA),Piyade Sokak No:27,Çankaya,06690,Ankara,Turkey
Keywords:
Hydroxychloroquine sulfate
GC-MS
Biological samples
Dispersive solid phase extraction
Liquid phase microextraction
A B S T R A C T
A rapid,accurate,and sensitive analytical method,ultrasonication-assisted spraying based fine droplet formation-liquid phase microextraction-gas chromatography-mass spectrometry(UA-SFDF-LPME-GCMS),was proposed for the determination of trace amounts of hydroxychloroquine sulfate in human serum,urine,and saliva samples.To determine the best extraction strategy,several liquid and solid phase extraction methods were investigated for their efficiencies in isolation and preconcentration of hydroxychloroquine sulfate from biological matrices.The UA-SFDF-LPME method was determined to be the best extraction method as it was operationally simple and provided accurate results.Variables such as the extraction solvent,spraying number,sodium hydroxide concentration and volume,sample volume,mixing method,and mixing period were optimized for the proposed method using the onevariable-at-a-time approach.In addition,Tukey's method based on a post hoc comparison test was employed to evaluate the significant difference between the parameters inspected.After the optimization studies,the limit of detection(LOD)and limit of quantification(LOQ)were determined to be 0.7 and 2.4μg/kg,respectively.The sensitivity of the GC-MS system based on the LOD was enhanced approximately 440-fold when the UA-SFDF-LPME method was employed.Spiking experiments were also conducted for the human serum,urine,and saliva samples to determine the applicability and accuracy of the proposed method.Recoveries for the human serum,urine,and saliva samples were found to be in the ranges of 93.9%-101.7%,95.2%-105.0%,and 93.1%-102.3%,respectively.These results were satisfactory and indicated that the hydroxychloroquine sulfate level in the above biological samples could be analyzed using the proposed method.
1.Introduction
In December 2019,a new coronavirus was reported by authorities.This pathogen was previously unknown and was named as severe acute respiratory syndrome coronavirus-2(SARS-CoV-2)[1].Several drugs have been used to date to alleviate the severity of coronavirus disease-2019 (COVID-19)[1,2].Chloroquine and hydroxychloroquine are widely used as drug active compounds for the treatment of malaria[3].Hydroxychloroquine is the hydroxyl derivative of chloroquine that is synthesized to impart higher water solubility,lower toxicity,and fewer side effects than chloroquine[4].Literature review on the effect of hydroxychloroquine on coronavirus suggests that it can inhibit the virus,replication process and its fusion to the cell membrane[5,6].Unfortunately,hydroxychloroquine has side effects such as vomiting,diarrhea,and gastrointestinal diseases.The more serious side effects include retinopathy and QTc interval prolongation,which can cause ventricular arrhythmias and sudden cardiac death[5].It has been also reported that hydroxychloroquine usage causes itching,intravascular hemolysis,rashes,and bone marrow suppression[7].Therefore,an accurate,sensitive,and rapid analytical method is required for the determination of hydroxychloroquine in biological samples to scientifically evaluate its possible effects on humans.
Generally,hydroxychloroquine is qualified and quantified by hyphenated and electroanalytical techniques such as high performance liquid chromatography-ultraviolet detection(HPLCUV)[8],high performance liquid chromatography- fluorescence detection(HPLC-FLD)[9],liquid chromatography-tandem mass spectrometry(LC-MS/MS)[10],liquid chromatography-high resolution mass spectrometry(LC-HRMS)[11],potentiometry[12],differential pulse voltammetry[13],and square-wave voltammetry[14].Gas chromatographs with an appropriate detector are also an important analytical instrument for drug analyses[15,16].There are only a few reports on the determination of hydroxychloroquine using GC systems.Furthermore,GC systems have been combined with several detectors such as flame ionization detector,electron capture detector,thermionic ionization detector,photoionization detector,helium ionization detector,thermal conductivity detector,flame photometric detector,and optical detectors for the qualitative and quantitative determination,depending on the properties of the analyte(s)[17].In addition to these systems,gas chromatography-mass spectrometry(GC-MS)is a powerful hyphenated technique for the separation and determination of various compounds.GC-MS systems generate mass spectrum based on the fragmentation of the analyte(s);therefore,structure(s)of the analyte(s)can be identified from their mass spectra[18].
GC-MS systems are not sensitive for trace and ultra-trace quantification of most of the analyte(s). Thus, sample preparationmethods are frequently developed prior to instrumental measurements to isolate and preconcentrate the analyte(s) from complexmatrices. There are several methods for the extraction and preconcentration of compounds such as dispersive liquid-liquidmicroextraction (DLLME) [19], switchable-hydrophilicity solventliquid-liquid microextraction (SHS-LLME) [20], hollow fiber liquidphase microextraction (HF-LPME) [21], solidified floating organicdrop microextraction (SFODME) [22], dispersive solid phase microextraction (DSPME) [23], solid phase microextraction (SPME) [24],single drop microextraction (SDME) [25], and spraying-based finedroplet formation-liquid phase microextraction (SFDF-LPME) [26]to increase the detection power of the instrument. In the SFDF-LPMEmethod, the extraction solvent is dispersed into the standard/samplesolution with the help of air pressure, without the dispersive solvent;therefore, organic solvent consumption is lowered[26].
In this study,several liquid phase microextraction and solid phase extraction methods were assessed to determine the most efficient method for the preconcentration of hydroxychloroquine sulfate.The SFDF-LPME method was selected to extract and preconcentrate the analyte prior to GC-MS measurement.Parameters of the SFDF-LPME method were optimized by the one-variable-ata-time approach.Tukey's method based on a post hoc comparison test was utilized to evaluate the significant difference in pair-wise comparisons and determine the optimum extraction conditions.After determining the limit of detection(LOD),limit of quantification(LOQ),and linear range for the developed method,spiking experiments were conducted for three different biological samples to evaluate the accuracy and applicability of the proposed method.
2.Materials and methods
2.1.Chemicals and standards
Hydroxychloroquine sulfate(98.6% purity)was kindly supplied by Neutec Pharmaceutical Company(İstanbul,Turkey).A main stock solution of hydroxychloroquine sulfate(3.0 g/kg,mass based)was prepared by dissolving the required amount of the solid standard in ultrapure water.Dichloromethane(99.9% purity),ammonia(25.0%-30.0% assay),acetonitrile(≥99.8% purity),chloroform(≥99.0% purity),and 1,2-dichloroethane(≥99.5% purity)were purchased from Merck(Darmstadt,Germany).Ethanol(99.9% purity)was obtained from IsoLab Laborgeräte GmbH Chemicals(Eschau,Germany).Sodium hydroxide(98% purity)was purchased from Ak Kimya(Yalova,Turkey).Ultrapure water obtained from an Elga Flex 3 water purification system was used to prepare the standards and samples.Centrifuge(BKC-TL5II)and ultrasonication bath(UC-20SDII)were purchased from BIOBASE(Zhangqiu,China),while the vortex apparatus(M1010002 model)was purchased from IsoLab Laborgeräte GmbH Chemicals(Eschau,Germany).
2.2.Instrumentation
Hydroxychloroquine sulfate was analyzed on an Agilent 6890 gas chromatograph equipped with an Agilent 5973 mass spectrometry detector(CA,USA).The chromatographic separation was carried out on an HP5MS column with the following dimensions:30 m length,250 μm inner diameter,and 0.25 μm film thickness.Helium was used as the mobile phase at a flow rate of 2.8 mL/min.Inlet temperature and injection volume were set at 290°C and 1.0μL,respectively,and the splitless injection mode was employed.Only a single ramp from 100 to 300°C(held for 3.5 min)at 40°C/min was set in the oven temperature program to perform the chromatographic separation.The MS unit was operated in the single ion monitoring mode for a certain period around the retention time of the analyte.The MS source and quad temperature were set to 230 and 150°C,respectively.An electron impact ionization source at 70 eV was used as the ionization source.The quantifier and qualifier ions for the analyte were 245 and 247,respectively.
2.3.UA-SFDF-LPME method
Twelve milliliters of the standard/sample solution was transferred into a 15 mL centrifuge tube.Sodium hydroxide solution(80 g/kg,0.50 mL)was added into the tube to eliminate the sulfate ions in the analyte.A spraying system consisting of a solvent container,spray pump,and screw cap was used to spray dichloromethane into the aqueous solution.For this,the screw cap was placed into the centrifuge tube,and then the tube was inverted and dichloromethane was sprayed into the solution.After this,the tube was covered with a new screw cap and placed into the ultrasonication bath(80 kHz)at room temperature for 45 s.Centrifugation was performed at 3461 g for 2.0 min.The aqueous phase was removed,and the dichloromethane phase was transferred to a clean insert vial for the GC-MS measurements.
2.4.Sample preparation
Human serum,urine,and saliva samples used for the recovery studies were provided by the volunteers in our research laboratory.The saliva samples were collected from the volunteers by spitting.All the volunteers refrained from smoking,eating,and drinking alcoholic beverages and soft drinks for 1 h prior to analyses and washed their mouth with distilled water before providing the sample.All the samples were subjected to a protein precipitation process to alleviate the matrix effects and precipitation in the application of the UA-SFDF-LPME method.Human serum and saliva samples(1.33 g)were first spiked with the desired concentration and then treated with 2.40 g of acetonitrile.After this,masses of all the spiked solutions were made up to 4.0 g by adding ultrapure water.Supernatant and precipitate were separated by centrifuging at 4420 g for 5.0 min.The supernatant(3.0 g)was transferred into a clean centrifuge tube and then diluted to 40 g with ultrapure water.In addition,1.33 g of urine was weighed and spiked at different concentrations.Concentrated ammonia solution(0.53 g)and 1.87 g of acetonitrile were consecutively added into the spiked sample.Finally,the spiked urine sample was diluted to 4.0 g with ultrapure water.After centrifuging at 4420 g for 5.0 min,3.0 g of the supernatant was made up to 40 g by adding ultrapure water.
2.5.Data analysis
A post hoc comparison with the analysis of variance(ANOVA)is a statistical method to determine the significantly different numerical results[27].Tukey's honestly significant difference(HSD)test has been also utilized to assess the differences in pair comparisons[28].In the optimization studies,the post hoc comparison was conducted by processing the mean values with the help of the JASP 0.9.1.0 software.Different letters(a,b,c)present significant differences in terms of Ptukeyvalues of pairwise comparisons at a 95% confidence interval.When two results were identical according to the post hoc test,the parameters were indicated with the same letter.
3.Results and discussion
The first step for the preconcentration of hydroxychloroquine sulfate was to determine an efficient microextraction method.Various parameters having a significant influence on the extraction yield were individually optimized.The one-variable-at-a-time approach was employed to determine the optimum conditions for the selected microextraction method.All the results were evaluated according to their peak area values and statistical comparisons.All the optimization experiments were performed in triplicate to calculate the means and standard deviations of the results.Concentration of the standard solutions used in the optimization experiments varied between 0.25 and 2.5 mg/kg.
3.1.Selection of the microextraction method
Different microextraction methods were compared to determine a rapid and efficient method for the preconcentration of the analyte.First,DLLME was applied to an 8.0 mL of aqueous solution by injecting 2.0 mL of ethanol and 200μL of chloroform mix after the addition of 0.50 mL of sodium hydroxide(40.0 g/kg).Second,SHS-LLME was implemented as follows:1.0 mL of protonated N,N-dimethyl benzylamine was added into an 8.0 mL of aqueous solution,followed by the addition of 1.0 mL of 1.0 M sodium hydroxide.The SFDF-LPME method was applied to an 8.0 mL of aqueous sample by spraying chloroform(twice)after the addition of 0.50 mL of sodium hydroxide(40.0 g/kg).
DSPE was also employed in an attempt to achieve high extraction yields.For this,20 mg of fe3O4,stearic acid-coated Fe3O4,nickel,cobalt,reduced graphene oxide-Fe3O4,zirconium,and citric acid-coated Fe3O4nanoparticles were individually studied.Chloroform(200μL)was used as the eluent to collect the analyte from the nanoparticle surface.Additional experiments were performed for stearic acid-coated Fe3O4nanoparticles by adding sodium hydroxide(0.50 mL,40.0 g/kg).
However,there were no detectable signals for any of the nanoparticles.Under the tested conditions,target analyte could not be adsorbed onto the selected nanoparticle or eluted from the surface of the nanoparticles.Therefore,Fig.1 does not contain the results concerning the tested DSPE.

Fig.1.Comparison of different microextraction methods for the preconcentration of hydroxychloroquine sulfate.aCompared with SFDF-LPME,P<0.05;bcompared with DLLME and SHS-LLME,P<0.05.DLLME:dispersive liquid-liquid microextraction;SFDF-LPME:spraying-based fine droplet formation liquid phase microextraction;SHS-LLME:switchable-hydrophilicity solvent liquid-liquid microextraction.
Results corresponding to the DLLME,SHS-LLME,and SFDF-LPME methods are given in fig.1.
Based on the peak values,the SFDF-LPME method was concluded to be the best microextraction method among the three methods.All these methods yielded different results based on ANOVA tests.SFDF-LPME was also superior to the other methods in terms of its simplicity,rapidness,cost-effectiveness,and extraction efficiency.The spraying system used in the SFDF-LPME method was developed in our previous study and detailed in the corresponding report[26].
3.2.Type of extraction solvent
The choice of the extraction solvent is a critical step for achieving efficient extraction.Analyte solubility in the selected extraction solvent directly affects analyte isolation and preconcentration from the sample matrix based on the like to like principle,which states that polar analytes dissolve in polar solvents while non-polar analytes dissolve in non-polar solvents.It is also important to achieve a clear and distinct phase when an extraction solvent is added into an aqueous solution[29].Chloroform,1,2-dichloroethane,and dichloromethane were selected as the candidate extraction solvents to preconcentrate the target analyte.Fig.2 shows that the highest signals were obtained when dichloromethane was sprayed into the aqueous solution.Dichloromethane is moderately soluble in water(2 g/100 mL at 20°C)[30],resulting in a decrease in the organic layer at the end of the extraction process.Therefore,dichloromethane can have a higher preconcentration factor than the other solvents,and the solubility of hydroxychloroquine in this solvent may also be higher than that in other solvents.The results obtained with dichloromethane were statistically different from those obtained with the other extraction solvents at 95% confidence interval.
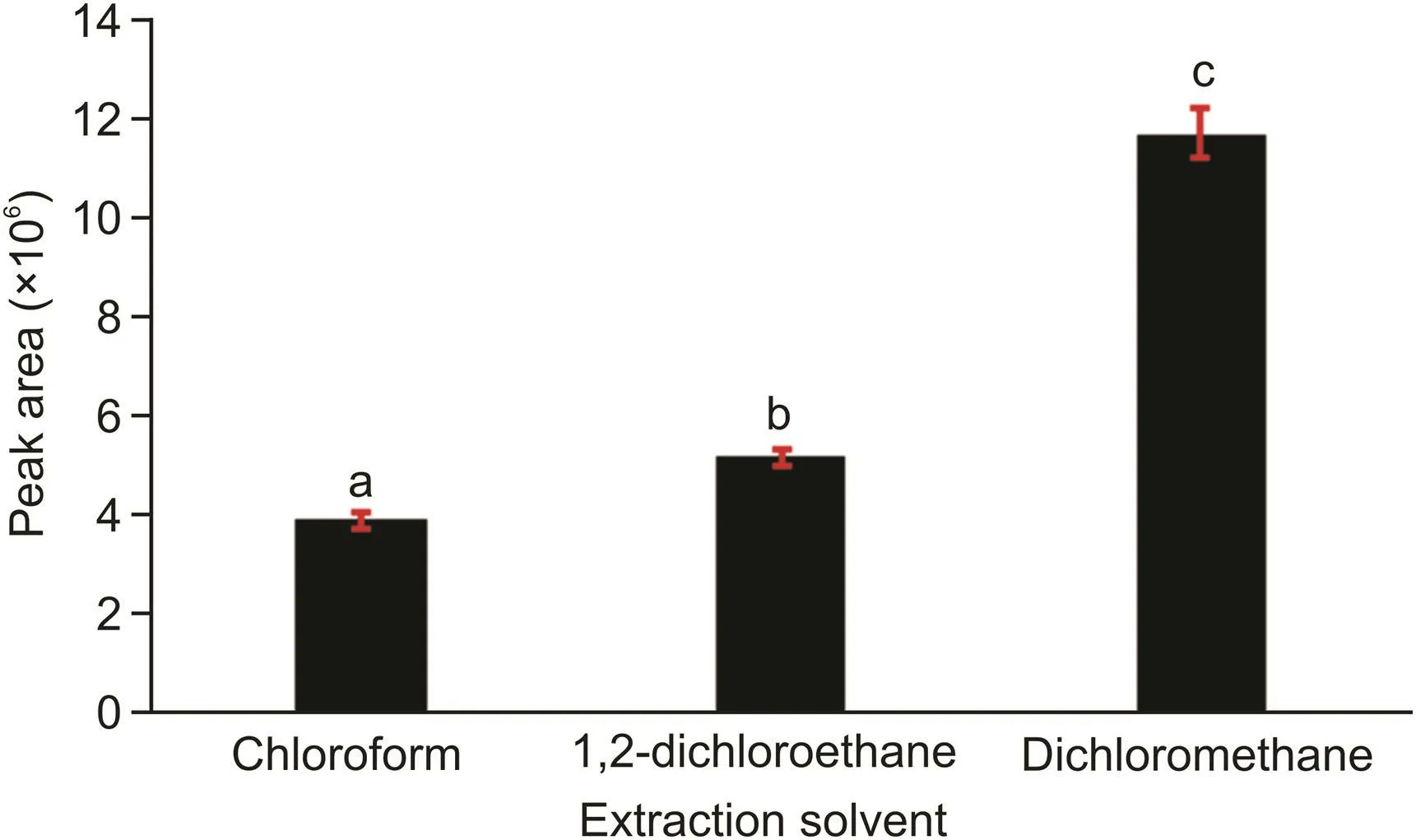
Fig.2.Optimization of the extraction solvent.aCompared with 1,2-dichloroethane and dichloromethane,P<0.05;bcompared with chloroform and dichloromethane,P<0.05;ccompared with chloroform and 1,2-dichloroethane,P<0.05.
3.3.Sample volume
In microextraction strategies,sample volume optimization is also important to attain trace levels of the analyte and improve the preconcentration factor.Meanwhile,the sample volume should be applicable to the selected analytical method[31].To obtain high preconcentration factors and low detection limits,different sample volumes in the range of 8.0 mL and 12 mL were individually examined.Volumes higher than 12 mL were not included in this optimization step due to leaking of the aqueous solution through the screw cap.Peak area values gradually increased with increased sample volumes,which can be associated with the increased number of analyte molecules in the aqueous solution extracted using the selected extraction solvent.Further,all the sample volumes gave statistically different results according to ANOVA tests.Hence,12 mL was chosen as the optimum sample volume.
3.4.Sodium hydroxide concentration and volume
Sodium hydroxide was also used to remove the sulfate ions from the analyte sample.When no sodium hydroxide was added,no analytical signal corresponding to the analyte was detected.After this,20,40,and 80 g/kg of sodium hydroxide solutions were tested to determine the optimum concentration.The optimized concentration was limited to 80 g/kg because higher concentrations led to the formation of white precipitates between the aqueous and organic layers.Fig.S1 shows that the highest peak areas were obtained for 80 g/kg of sodium hydroxide.
To evaluate the effect of sodium hydroxide on analyte mass transfer from the aqueous solution to organic phase,the volume of sodium hydroxide was varied from 0.25 to 1.0 mL.A gaussian curve was obtained for three volumes.The highest signals corresponded to 0.50 mL of sodium hydroxide(Fig.S2).Therefore,further experiments were performed with the addition of 0.50 mL of 80 g/kg sodium hydroxide solution.
3.5.Spraying number
There are several methods to obtain a better distribution of the extraction solvent throughout the aqueous solution.The use of dispersive solvent is one of the strategies to achieve fine droplets of the extraction solvent[32].However,dispersive solvents such as ethanol,methanol,and isopropyl alcohol lead to additional cost and chemical usage[26].Spraying with the help of air pressure is an easy and rapid way to disperse the extraction solvent throughout the aqueous solution.Here,the spraying number is closely related to the volume of the extraction solvent in aqueous solution,which also influences the preconcentration factor and extraction yield.In this optimization step,the spraying number of the extraction solvent was adjusted to its optimum value by testing with 1,2,3,and 4 sprays.There was no phase separation for one spray of dichloromethane.Hence,two sprays were selected to be the optimum number of sprays because of the highest yield and preconcentration factor.
3.6.Mixing method and mixing period
Mixing can promote the extraction efficiency of the analyte.Therefore,vortexing,ultrasonication,and mechanical shaking were employed,and the analytical results and mixing performances were compared.One experiment was also performed without mixing.Among the three methods,ultrasonication was concluded to be the optimum mixing method owing to the highest peak values obtained using this method(Fig.3).In addition,the results obtained with ultrasonication were statistically different than those obtained with the other mixing methods,while similar results were obtained with mechanical shaking and vortexing based on the post hoc comparison tests.
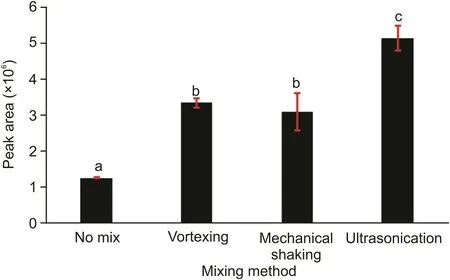
Fig.3.Optimization of mixing method.aCompared with vortex,mechanical shaking and ultrasonication bath,P<0.05;bcompared with no mix and ultrasonication bath,P<0.05;ccompared with no mix,vortex and mechanical shaking,P<0.05.
The mixing period was also optimized between 15 and 60 s.The peak values were lowest for 15 and 30 s,and 45 s was chosen as optimum ultrasonication period(Fig.S3)since the peak area was marginally higher than that for 60 s.
3.7.Linearity,LOD,and LOQ
The developed microextraction method was evaluated in terms of linearity,LOD,LOQ,and enhancement in detection power.First,a calibration curve for the GC-MS system was constructed by plotting the peak areas versus analyte concentration in the range of 1.1-103.8 mg/kg.Under the optimum microextraction and instrumental conditions,good linearity was observed for the UA-SFDFLPME-GC-MS system in the range of 3.6-1236.6 μg/kg.The LOD and LOQ values were calculated on the basis of 3 s/m and 10 s/m(s:standard deviation of the lowest concentration in the calibration plot,m:slope of the calibration plot),respectively[33].The LOD values for the GC-MS and UA-SFDF-LPME-GC-MS systems were found to be 319 and 0.7μg/kg,respectively,indicating that there was a 440-fold enhancement in the detection power of the GC-MS system.The analytical performances of both the systems are given in Table 1[8,10,34].
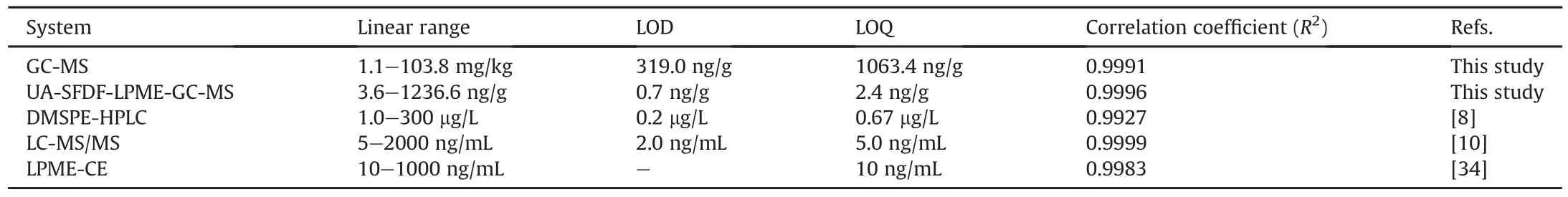
Table 1 Analytical performance of all the systems and comparison with other studies.
Previous studies have mainly focused on the determination of hydroxychloroquine by LC.Compared to DMSPE-HPLC method using Ni@MIL-100(Fe)@MIP[8],LC-MS/MS[10],and liquid-phase microextraction based on polypropylene hollow fibers capillary electrophoresis(LPME-CE)[34],the proposed UA-SFDF-LPME-GCMS system achieved ng/g levels of the analyte using organic solvents in the microliter scale.In this regard,other methods reported in literature have some disadvantages such as high solvent consumption,high amounts of waste generation,requirement of tedious steps,and high cost.
3.8.Recovery studies
The developed UA-SFDF-LPME method was validated by recovery tests to assess the method's applicability and accuracy.Human serum,urine,and saliva samples were first prepared.Upon blank analyses,there was no detectable concentration of the analyte in any of the samples.As a result,all the samples were spiked with four different concentrations of the analyte.Under the optimum conditions,the percent recovery was calculated to be in the range of 29.7%-42.8% after applying the external calibration method(Table 2).It is clear that all the samples had negative interference effects on the analytical signals.Thus,the matrix matching calibration strategy was individually applied to the human serum,urine,and saliva samples by preparing calibration standards(six different concentrations between 10.0 and 500.0μg/kg)with their sample matrices.After performing protein precipitation and microextraction under the optimized conditions,the percent recovery was found to be close to 100% for the human serum,urine,and saliva samples.Table 2 shows the percent recoveries with their standard deviations for all the spiked samples.When matrix dilutions were taken into account in the calculation,the LOD/LOQ values for the human serum,urine,and saliva samples were 45.1/150.2 ng/g,32.8/109.4 ng/g,and 48.6/162.1 ng/g,respectively.Furthermore,68.6%,56.1%,and 69.6% matrix effects arising from the human serum,urine,and saliva matrices,respectively,were observed on the analyte when the slopes of the external and matrix matching calibrations were compared.Matrix effects were calculated by multiplying(CE-CM)/CEwith 100,where CEis the slope of the external calibration,and CMis the slope of the matrix matching calibration.Chromatograms of the standard solution and spiked human serum,urine,and saliva samples are given in fig.S4.

Table 2 Percent recoveries with their standard deviations for the human serum,urine,and saliva samples.
4.Conclusion
In this work,hydroxychloroquine sulfate was preconcentrated by a new microextraction method,i.e.,UA-SFDF-LPME.Additionally,several microextraction methods were evaluated to attain low detection limits for hydroxychloroquine sulfate.After selecting the UA-SFDF-LPME method,some parameters that could affect the analyte extraction were optimized by the one-variable-at-a-time approach.With respect to the LOD value obtained from the direct GC-MS system,a 440-fold enhancement in the detection power was observed for the UA-SFDF-LPME-GC-MS system.The proposed method was successfully applied to spiked human serum,urine,and saliva samples,with high percent recoveries in the range of 93.1%-105.0%.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by the Health Institutes of Turkey(TÜSEB)(Project No.2020CV01-8946).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2021.01.006.
 Journal of Pharmaceutical Analysis2021年3期
Journal of Pharmaceutical Analysis2021年3期
- Journal of Pharmaceutical Analysis的其它文章
- Molecular detection of SARS-CoV-2 being challenged by virus variation and asymptomatic infection
- The potential of miRNA-based therapeutics in severe acute respiratory syndrome coronavirus 2(SARS-CoV-2)infection:A review
- Potential treatment with Chinese and Western medicine targeting NSP14 of SARS-CoV-2
- Fast saccharide mapping method for quality consistency evaluation of commercial xylooligosaccharides collected in China
- Dispersive liquid-liquid microextraction,an effective tool for the determination of synthetic cannabinoids in oral fluid by liquid chromatography-tandem mass spectrometry
- Identification and quantification of the bioactive components in Osmanthus fragrans roots by HPLC-MS/MS
