Identification and quantification of the bioactive components in Osmanthus fragrans roots by HPLC-MS/MS
Xiaoyan Liao,Yuan Hong,Zilin Chen,*
aKey Laboratory of Combinatorial Biosynthesis and Drug Discovery,Ministry of Education;Hubei Province Engineering and Technology Research Center for Fluorinated Pharmaceuticals;School of Pharmaceutical Sciences,Wuhan University,Wuhan,430071,China
bState Key Laboratory of Transducer Technology,Chinese Academy of Sciences,Beijing,100080,China
Keywords:
Osmanthus fragrans roots HPLC-MS/MS
Identification
Quantitation
Bioactive constituents
Network pharmacology
A B S T R A C T
The roots of O.fragrans are also a valuable resource in addition to its flowers and fruits.In this study,the HPLC-MS/MS method used for analyzing the chemical constituents in O.fragrans roots extract was developed,which showed high sensitivity for both qualitative and quantitative analyses.Thirty-two compounds were first discovered in O.fragrans roots,one compound of which was reported for the first time.The simultaneous determination method for acteoside,isoacteoside,oleuropein and phillyrin was validated to be sensitive and accurate.Then it was applied to determine the content of bioactive components in O.fragrans roots from different cultivars.The content of oleuropein and phillyrin in the twelve batches was relatively stable,while the content of acteoside and isoacteoside varied greatly.Moreover,the therapeutic material basis and mechanism of O.fragrans roots exerting its traditional pharmacodynamics were analyzed by network pharmacology.The results showed that O.fragrans roots might be effective for the treatment of Inflammation,cardiovascular diseases,cancer,and rheumatoid arthritis,which is consistent with the traditional pharmacodynamics of O.fragrans roots.This work can provide an analytical method for the comprehensive development of O.fragrans roots.
1.Introduction
Osmanthus fragrans Lour.(O.fragrans)is famous for its pleasant smell,and it is widely planted in Chinese landscaping.The O.fragrans flowers are commonly used in the production of aromatic essential oil,as well as in health products and food additives,which can bring economic benefits to the local.So far,most of the researches related to O.fragrans focused on the exploration of biological activities and practical application of the flowers.In recent years,there have been some researches on O.fragrans fruit too[1,2].
in fact,the resource utilization of O.fragrans is not only limited to flowers and fruits,the roots can also be used as compositions of functional beverages and tea.In the usage of traditional Chinese medicine,O.fragrans roots are commonly used to relieve stomachache,toothache,rheumatism numbness,muscle and bone pain[3].In order to understand the material basis of the application of O.fragrans roots as a traditional Chinese medicine,the chemical constituents of O.fragrans roots have been studied[3-6].It has been found that there are many flavonoids,lignans,iridoids and phenylethanols,but the studies on chemical constituents of O.fragrans roots are still limited.Column chromatography is a usual technique for isolation and purification,but it is difficult for the separation of those only occurring in minor or trace quantities or being co-eluted.The characteristics such as the high separation efficiency of LC and the high sensitivity and molecular structure elucidation ability of MS were integrated by HPLC-MS/MS.The different fragmentation patterns of the isomers were propitious for illuminating the fine distinction,and it can provide direction for the accurate separation and purification of monomer components in order to avoid plenty of duplication of labor.In this study,the phenylethanoid glycosides and lignans in the O.fragrans roots were identified by HPLC-MS/MS.And the high sensitive HPLC-MS/MS method was established to determine the content of acteoside,isocacteoside,oleuropein and phillyrin in different varieties of O.fragrans roots.
At present,the effective ingredients of O.fragrans roots are yet not comprehensively studied.Only the unknown lignans isolated from O.fragrans roots are proved to have anti-inflammatory and analgesic effects[7],which could not clearly clarify the basis of traditional medicinal effects of O.fragrans roots.Network pharmacology is based on the theory of system biology and multidirectional pharmacology.The network analysis of life and disease system has become a new and comprehensive method to reveal the material basis of traditional Chinese medicine and clarify the mechanism of their traditional therapeutic effects.Therefore,the therapeutic material basis and mechanism of O.fragrans roots exerting its traditional pharmacodynamics were analyzed by network pharmacology.It will facilitate the comprehensive development of O.fragrans roots resources.
2.Materials and methods
2.1.Materials and chemicals
Acteoside was obtained from Shanghai Yuanye Biotechnology Co.,Ltd.(Shanghai,China).Isoacteoside,oleuropein and phillyrin were purchased from Ruifensi Biotechnology Co.,Ltd.(Chengdu,China).Methanol and acetonitrile obtained from Fisher Scientific(Fairlawn,NJ,USA),and formic acid obtained from Aladdin Reagent Co.,Ltd.(Shanghai,China)were all of HPLC-grade.Ultrapure water was bought from Wahaha Group Co.,Ltd.(Hangzhou,China).All the other reagents and chemicals were of analytical grade otherwise mentioned.
O.fragrans roots were gathered in Wuhan,China,in May 2018.Twelve batches of O.fragrans roots(Whu-X-2,Whu-XS-1,Whu-Y-4,Whu-X-1,Whu-Y-1,Whu-Y-2,Whu-X-10,Whu-Y-7,Whu-Y-9,Whu-XD-9,Whu-YD-5,and Whu-YD-6)were labeled No.1-12 successively.They were stored in herbaria in School of Pharmaceutical Sciences of Wuhan University.And they were identified by Dr.Jun Tang of School of Pharmaceutical Sciences of Wuhan University.Batches Whu-X-1,Whu-Y-1 and Whu-Y-2 are O.fragrans Albus group bearing fruits.Batches Whu-X-2,Whu-XS-1 and Whu-Y-4 are O.fragrans Luteus group bearing fruits.Batches Whu-XD-9,Whu-YD-5 and Whu-YD-6 are O.fragrans Aurantiacus group that could not bear fruits.Batches Whu-X-10,Whu-Y-7 and Whu-Y-9 belong to O.fragrans Albus group that could not bear fruits.Samples were dried in an oven at 60°C,then smashed and screened,and at last kept at-20°C before use.
2.2.Pretreatment of samples and reference standards
The preparation method of the samples for identification was the same as that in the previous study[1].And for quantitative analysis,the samples preparing method was as follows:0.1 g of the O.fragrans roots was taken and mixed with 12 mL of 60% methanol in ultrasonic extraction for 45 min.Extracted samples were filtered through a 0.22 μm membrane filter and diluted by adding 950 μL methanol to 50μL aliquot of the sample.The standards of acteoside,isoacteoside,oleuropein and phillyrin were weighed and dissolved in methanol and used as stock solutions.The final concentrations ranged from 454 to 509μg/mL.The stock solutions were stored at 4°C and then diluted to a certain concentration to prepare standard working mixture solutions.For limit of quantitation(LOQ;S/N≥10)determination,dilutions and injections of the standard were made until S/N reached approximately 10.
2.3.HPLC-MS/MS analysis
Low resolution MS and MS/MS analysis was obtained on an API4000 Qtrap mass spectrometer equipped with an electrospray interface(ESI)(AB Sciex,Framingham,MA,USA).Equipment control and data analysis were performed using Analyst software ver.1.6.2(AB Sciex).The main parameters included the ion spray voltage,-4500 V(4500 V in positive ion mode);temperature,350°C;curtain gas,35 psi;nebulizer gas,50 psi;heater gas,50 psi;and collision gas,medium(high in EPI scan).Mass spectra were recorded in the m/z 100-1200 range with accurate mass measurements of all mass peaks.The MS/MS spectra were acquired by EPI scans in negative ion mode at CE of-40 V with CE spread of-15 V.The declustering potential for both scan types was set at-80 V.High resolution MS data were recorded by an UltiMate 3000 Rapid Separation Liquid Chromatography System(Thermo Scientific,Germering,Germany)coupled with a Q Exactive Focus mass spectrometer(Thermo Scientific,Waltham,MA,USA)controlled by the Xcalibur 3.0 software(Thermo Scientific,Waltham,MA,USA).Chromatographic separations were carried out on a Sepax BR-C18column(100 mm × 2.1 mm i.d.,3μm;Sepax Technologies,Newark,USA)with a flow rate of 0.2 mL/min.The optimized mobile phase was a mixture of water with 0.1% aqueous formic acid(A)and acetonitrile(B).For identification samples,the gradient was operated as follows:0-20min15%-45% B;20-25 min,held at 95% B;and 25-35 min,held at 15% B.For the determination of bioactive constituents,the initial ratio was changed from 15% to 12% B for better sensitivity.The injection volume was 2μL.
2.4.Network pharmacology analysis
For the prediction and retrieval of target chemical constituents,the identified compounds were taken as target compounds,and then the ADME character was analyzed in ETCM database(http://www.nrc.ac.cn:9090/ETCM)[8].The compounds whose druglikeness weight was greater than 0.1 were selected for target prediction.Then based on chemical similarity,targets prediction and retrieval were carried out in the database of STITCH(http://stitch.embl.de),SwissTarget Prediction(http://www.swisstargetprediction.ch)[9,10],and SymMap(http://www.symmap.org).Compound targets retrieved from various databases were merged.Cytoscape 3.6.0 was used to construct a network with HPRD database(http://www.hprd.org/)as the background and prediction target as the interest gene.At the same time,neighbor nodes were selected to analyze the network topology,and then the core nodes were selected.It is generally believed that the median of degree greater than or equal to twice the connectivity of all nodes has an important contribution to the entire network[11],and thus is regarded as the core node.
The database applied for annotation,visualization,and integrated discovery was DAVID database(https://david.ncifcrf.gov/home.jsp,ver.6.7),both for disease and gene ontology(GO)analyses.We chose the diseases when P value and FDR value were less than 0.001 and 0.5,respectively,and counted number was greater than 4 for further analysis.So,the diseases that O.fragrans roots participated in the treatment could be figured out.Compounds,targets and diseases were visualized by Cytoscape 3.6.0 with cluster maker.
3.Results and discussion
3.1.Characterization of the chemical constituents in n-butanol extract
The chemical material basis of O.fragrans roots was analyzed by HPLC-MS/MS.Both negative ion mode and enhanced product ion mode were adopted to the identification of corresponding signals.As shown in Table 1 and Figs.1,S1 and S2,thirty-six compoundswere identified from the O.fragrans roots extract tentatively.Thirty-two components,except R4,R23,R34 and R35,were first discovered in O.fragrans roots.Many new isomers were found and distinguished by the sensitive analysis through EPI scan.Moreover,the structure of compound R36 was first reported in this work.The characterization was validated by the data of high resolution mass spectrometry to further Affirm the exact molecular weight of those constituents.Lignans and phenolic compounds including secoiridoid and phenylethanoid glucosides were demonstrated to be the major constituents.Most of the phenylethanoid glucosides were hydroxyphenethyl alcohol and aglycone of methyloleoside derivants,linked to glucose,rhamnose or apiose with different combinations.

Fig.1.Chemical structures of main components identified in the extract of O.fragrans roots(Glc:β-D-glucopyranosyl;Caff:Caffeyl;Rha:Rhamnosyl;Api:Apiosyl).

Table 1 Characterization of the chemical constituents of O.fragrans roots by HPLC-ESI-MS/MS.
3.1.1.Phenylethanoid glucoside derivatives
Both acteoside,R15,and isoacteoside,R21,which appeared at a retention time(tR)of 10.15 min and 11.25 min,respectively,exhibited a parent ion at m/z 623.1,generated main fragments at m/z 461.2 by loss of a glucose reside(162 Da)and showed characteristic fragments(m/z 179.1,161.1 and 135.0)of a caffeoyl group.The identification of R15 and R21 was Affirmed by comparison to commercial standards.β-hydroxyacteoside isomers,R9 and R11,were presented at m/z 639.1 and demonstrated the characteristic fragments of a caffeoyl group.The major fragment at m/z 621.0 could be caused by the loss of H2O,which further generated the fragment at m/z 459.1 by the loss of C9H6O3(162 Da,characteristic fragments of a caffeoyl group).The quasi-molecular ions of R14,R16 and R19(m/z 653.1),were identified as β-methoxyacteoside isomers.They exhibited a loss of 32 Da to raise main ions at m/z 621.1,indicating the presence of a methoxy moiety[12].The other fragment ions were the same as that of compound R11.Compound R6 was detected at m/z 487.0,showing that a C8H8O2group was departed from acteoside,and it was equal to the dehydrated group of hydroxytyrosol(136 Da).Since the characteristic fragments of a caffeoyl group were exhibited in its MS/MS spectra,compound R6 was provisionally assigned as caffeoyl rutinose ester.Compound R2 was observed at m/z 461.1 and showed the fragments at m/z 315.1 and 297.0,attributed to the loss of a rhamnose(164 Da)or rhamnose residue(146 Da).The fragment ion at m/z 315.1 gave rise to the ion at m/z 135.1 by loss of a glucose(180 Da),indicating the presence of an hydroxytyrosol moiety.So,compound R2 was tentatively identified as forsythoside e[13].Compound R1,also observed at m/z 461.1,was only assigned as a vanillin acid derivative by the product ions at m/z 167.0 and 152.0.Osmanthuside H[14,15],R3,showed a quasi-molecular ion at m/z 431.1.The main fragment 299.0 was due to the loss of apiose residue(132 Da),and the characteristic fragments(m/z 137.0 and 119.0)of tyrosol group were produced by the successive loss of a glucose residue(180 Da).
3.1.2.Methyloleoside derivatives
Both compounds R8 and R10 were obtained at m/z 403.1(tR5.36 min and 7.19 min),which were assigned as oleoside-11-methyl ester and secoxyloganin,respectively.They showed the major product ions at m/z 371.1 by the neutral losses of CH3OH and the ion at m/z 223.1 attributed to the loss of glucose.The ions at m/z 223.1,191.1,181.1,and 149.1 appeared in the MS/MS spectra of them,which were the characteristic fragment ions of methyloleoside aglycone.Compound R17(tR10.57 min,m/z 951.2)was assigned as glucopyranosyl di-methyloleoside.The main fragment ions at m/z 403.2,which subsequently generated the ions at m/z 371.2,223.1 and 179.2,were indicative of a methyloleoside subunit.The main product ion at m/z 789.2 was generated by the loss of a glucose residue.It suggested that another methyloleoside moiety was combined with the methyloleoside moiety to generate a dimethyloleoside residue by dehydration.
3.1.3.Derivative based on both phenylethanoid and methyloleoside
Compound R33 showed a parent ion at m/z 1009.2 and produced the main fragment at m/z 623.2 by the loss of methyloleoside residue(386 Da)which subsequently gave rise to the ions at m/z 461.2 and 161.0,indicating the presence of an acteoside/isoacteoside group.The fragment ion at m/z 847.2 resulted from the loss of a glucose residue.It immediately generated the main fragments at m/z 777.2 by further neutral loss of C4H6O(70 Da)and at m/z 745.2 by double loss of C4H6O and CH3OH(102 Da).So,compound R33 was assigned as oleoacteoside/isooleoacteoside[16,17].Another derivative was found with m/z 1025.2(compound R27)and assigned as oleohydroxyacteoside,which shared the same fragmentation pattern with compound R33.The ions at m/z 639.2,863.1,793.2 and 761.2 were all 16 Da greater than the characteristic fragment ions of compound R33.The main fragment at m/z 1007.2 was due to the loss of H2O,which afterwards generated the fragment at m/z 621.1 and 459.0 by the loss of methyloleoside moiety and C9H6O3in sequence.
Ligstroside(R35,tR16.22 min)was shown to be one of the major components of O.fragrans roots and had a quasi-molecular ion at m/z 523.1.The product ion at m/z 361.1 was obtained by the loss of a glucose residue,which produced the main fragments at m/z 291.1 and 259.1 by loss of C4H6O and further loss of CH3OH.Then the ions at m/z 171.1 and 139.1 were attributed to the loss of dehydrated residue of hydroxyphenethyl alcohol(120 Da),and the ion at m/z 111.1 arose from the latter ion by the loss of CO(28 Da).The typical ion at m/z 223.1 was generated by the successive loss of a glucose residue and hydroxyphenethyl alcohol[14,18].A ligstroside derivative R31 was found at m/z 539.1,which shared the similar fragmentation pattern with ligstroside.In the case,it was identified as oleuropein(hydroxyligstroside)[18-21].The fragments of the ion at m/z 539.1(m/z 377.2,307.1 and 275.0)were 16 Da greater than the characteristic fragments(m/z 361.1,291.2 and 259.1).The ions at m/z 223.1,191.1,and 149.1 were the characteristic fragments of methyloleoside aglycone.Then the fragment at m/z 377.2 gave rise to the product ion at m/z 153.1 by the loss of methyloleoside aglycone,which indicated that the hydroxy group was combined with the hydroxyphenethyl alcohol moiety.It was Affirmed by the comparison with a commercial reference standard.Compound R28 was an isomer of oleuropein,and the extra main fragment at m/z 359.1 arose by the successive loss of a glucose residue and H2O.The appearance of the fragments at m/z 291.1 arose from m/z 377.1 by loss of C4H6O2(86 Da)instead of m/z 307.1 by loss of C4H6O(70 Da),indicating that the hydroxy group was combined with the methyloleoside aglycone.In addition,the fragments at m/z 419.0,401.0,and 239.0 arose by the loss of dehydrated residue of hydroxyphenethyl alcohol,successive loss of H2O,and then loss of a glucose residue.So,all the hints indicated that the hydroxy group was located on methyloleoside aglycone and it was assigned as 10-Hydroxyligstroside[22].Another ligstroside derivatives were found with m/z 555.1(R13)and m/z 569.1(R30),which were identified as hydroxyoleuropein[14,23]and methoxyoleuropein[23,24].The fragments of[M-Glu-H]-,[M-Glu-C4H6O-H]-,and[M-Glu-C4H6OCH3OH-H]-were found at m/z 393.1,323.1,and 291.1 of R13,and m/z 407.0,337.1,and 305.1 of R30,respectively.The typical fragments of the ion of m/z 403.1(m/z 371.1 and 223.0 by the loss of CH3OH and glucose,respectively)appeared.The main product ions at m/z 403.1 were obtained by the loss of C8H8O3(152 Da)in hydroxyoleuropein and by the loss of C9H10O3(166 Da)in methoxyoleuropein,showing that the hydroxy or the methoxy moiety was located on the phenylethanol group.And the main product ions at m/z 537.1 were generated by the loss of H2O and CH3OH.The ion at m/z 151.0 arose by the successive loss of a methyloleoside residue(386 Da)and CH3OH,and further generated the ion at m/z 123.0 by loss of CO.Compound R29(tR13.47 min,m/z 685.1)was assigned as excelside B[25],which was discovered to have a formula 162 Da greater than ligstroside.The characteristic fragments of ligstroside were observed at m/z 361.1,291.1,259.1,223.1 and 139.1.The fragment ion at m/z 361.1 was likely due to loss of 324 Da,indicating that the glucose group was combined with another glucose group to transform to a diglucoside moiety.
3.1.4.Lignans
Lignans are abundant in the O.fragrans roots,and a few lignans have been separated and identified.Pinoresinol 8-O-β-D-glucopyranoside(R23,tR11.46 min)was the major component of O.fragrans roots and showed a quasi-molecular ion at m/z 535.1,which were the most intense chromatographic peaks.Its isomer,compound R18,was detected at tR10.73 min.They showed similar fragmentation patterns [19,26]. Both of the fragment at m/z 355.1([M-C6H12O6-H]-),attributed to loss of a glucose(180 Da),and the fragment at m/z 373.1([M-C6H10O5-H]-),due to loss of a glucose residue(162 Da),were observed in their MS/MS spectra.The two ions could generate the product ions by neutral loss of CH2O(30 Da)or double loss of CH2O respectively,and then gave rise to the ions by loss of a CH3·or were followed by another loss of a CH3·.The ions at m/z 151.0 and 136.0 were the typical fragments of a 4-hydroxy-3-methoxy-benzophenone.The fragment at m/z 188.0 arose from the ion at m/z 355.1 by successive loss of a 4-hydroxy-3-methoxybenzophenone and a CH3·.The ion at m/z 181.1 was produced by neutral loss of C10H10O2(162 Da) from the ion of[M-C6H10O5-CH2O-H]-at m/z 343.1.It was found that the intensity of the fragment at m/z 355.1 was much higher than that of the fragment at m/z 373.1 in the MS/MS spectra of compound R23,but lower in the MS/MS spectra of its isomer.It was found in the literature[26]that there are a typical neutral loss of 162 Da in phenolic glycosides and a neutral loss of 180 Da in benzyl hydroxy glycoside.Hence,the glucose was located at 8-OH in pinoresinol 8-O-β-D-glucopyranoside,and the glucose was located at 4-OH or 4′-OH in the isomer.Compound R24 detected at m/z 565.1 was identified as fraxiresinol 1-O-β-D-glucoside[27,28]which contained a methoxy substitution of pinoresinol 8-O-β-D-glucopyranoside.By the similar fragment pattern,the fragments at m/z 403.1,385.1,373.1,355.2,325.2,310.2,295.2,280.2,181.1,and 166.1 were found in the derivative with 30 Da greater than the characteristic product ions(m/z 373.1,355.1,343.1,325.2,295.2,280.2,265.2,250.2,151.1 and 136.1)in pinoresinol 8-O-β-D-glucopyranoside.The intensity of the ion at m/z 385.1([M-C6H12O6-H]-)was much higher than that of the ion at m/z 403.1.So,the methoxy group was located at C-3'.Other similar lignans were found at m/z 519.1(R22 and R26)and m/z 549.1(R20 and R32),which were deduced as pinoresinol 4-β-D-glucoside isomers[19,26,29]and medioresinol 4-O-β-D-glucopyranoside isomers[30,31].The typical ions of[M-C6H10O5-H]-,[M-C6H10O5-CH3·-H]-,and[M-C6H10O5-CH2O-H]-of pinoresinol 4-β-D-glucoside isomers were observed.The characteristic ions of 4-hydroxy-3-methoxybenzophenone at m/z 151.0 and 136.0 were observed in compounds R22 and R26.The fragments at m/z 549.1(m/z 387.1,372.2 and 357.2)were 30 Da greater than the characteristic product ions of pinoresinol 4-β-D-glucoside(m/z 357.1,342.2 and 327.2),indicating the presence of a methoxy group,so they were assigned as medioresinol 4-O-β-D-glucopyranoside isomers.A different fragment pattern of the third pinoresinol 4-β-D-glucoside isomer was observed in compound R25.The dominant product ions were at m/z 339.1,324.2 and 309.2,which were the typical ions of[M-C6H12O6-H]-,[M-C6H12O6-CH3·-H]-,and[M-C6H12O6-CH2O-H]-.It indicated that there might be a hydroxy group located in C-8.Another lignan,compound R34,was found at m/z 533.1 and identified as phillyrin,which was Affirmed by comparison to commercial reference standard.The main fragment ion at m/z 371.1 was generated by loss of a glucose residue.Then it could give rise to the ions at m/z 356.4 and 326.1 by loss of a CH3·and successive loss of CH2O[32].Compound R36 exhibited a quasi-molecular ion at m/z 919.2 with characteristic product ions of methyloleoside(m/z 403.1,385.1,and 223.0)and phillyrin (m/z371.2 and 356.2).So,R36 was assigned as methyloleoside-phillyrin.The main fragment ion at m/z 757.2 was attributed to the loss of a glucose residue,which further gave rise to the product ions at m/z 687.2 and 655.3 by neutral loss of C4H6O(70 Da)and by successive loss of CH3OH(32 Da).The fragment at m/z 547.1 was produced by loss of phillyrin aglycone(372 Da).Moreover,methyloleoside-phillyrin,R36,was reported for the first time,and its MS/MS spectra and the fragmentation pathways are shown in fig.2.
Another type of lignan,compound R4,was detected at m/z 537.1,and was assigned as olivil 4-O-β-D-glucopyranoside[23,33-36],which had been separated and identified[5].It showed the typical fragment of [M-C6H10O5-H]-, [M-C6H10O5-CH3·-H]-and[M-C6H10O5-CH2O-H]-,and then the latter fragment lost double H2O continuously to give rise to the ions at m/z 327.1 and 309.1.The dehydration product ion at m/z 519.1 of its isomer,compound R5,was observed,indicating it may be a β-hydroxyl of benzyl.The dominant fragment at m/z 339.1 was produced by successive loss of H2O and a glucose,so glycoside bond might be located at 9-OH.Compounds R7 and R12 were found at m/z 521.1.In the MS/MS spectra of compound R7,the dominant fragment at m/z 359.1 was due to loss of a glucose residue and successive loss of CH3·to generate the ion at m/z 344.1.While in the MS/MS spectra of compound R12,the prominent fragment was at m/z 329.1,which was attributed to successive loss of a glucose residue and CH2O.Hence,compounds R7 and R12 were proposed as lariciresinol glucopyranoside isomers that the glucose was located at 4-OH or 4′-OH in the isomer because of loss of a glucose residue[34].
Overall,the fragments of phenylethanoid could be produced by neutral losses of glycosyl and glycosyl residues(180 Da and 162 Da for glucose,164 Da and 146 Da for rhamnose,150 Da and 132 Da for apiose).Caffeoyl group could generate characteristic fragments at m/z 179,161(by loss of H2O)and 135(by loss of CO2).Tyrosol group could generate characteristic fragments at m/z 137 and 119.Methyloleoside subunit could result in the neutral loss of 386 Da or 224 Da or give rise to fragments at m/z 403 and 223.For different types of lignans,we found the typical neutral loss of 162 Da in phenolic glycosides and a neutral loss of 180 Da in benzyl hydroxy glycoside.These different fragments of the isomers could be conducive to distinguishing these isomers.Hence,the proposed HPLC-MS/MS in this work is simple and efficient to discover different sets of similar constituents compared with traditional methods.
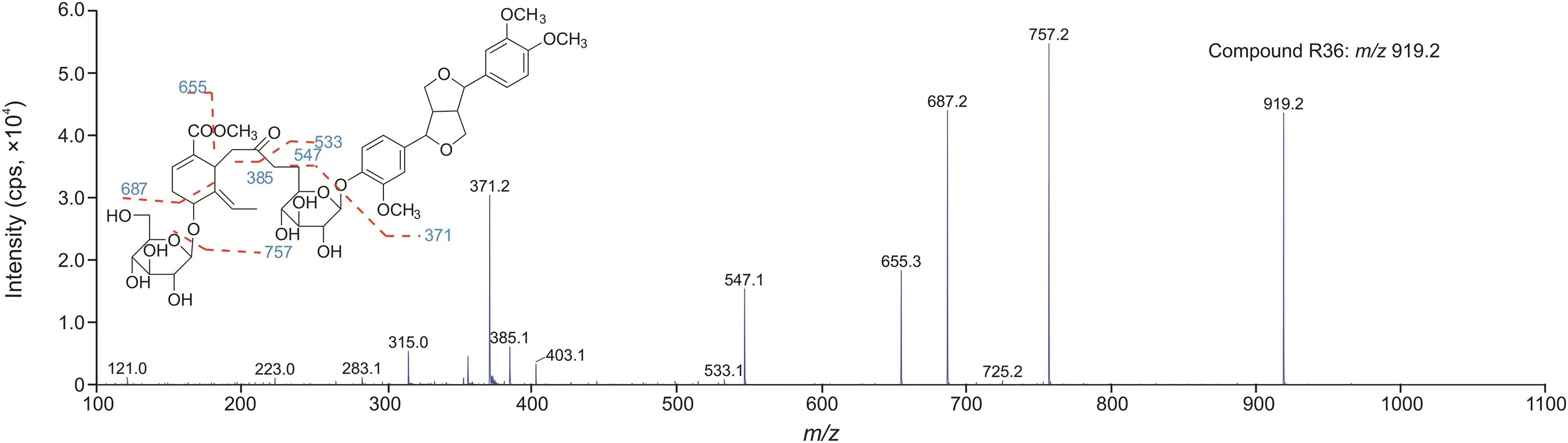
Fig.2.MS/MS spectra and fragmentation pathways of compound R36.
3.2.Optimization of extract factors and HPLC-MS/MS conditions
In order to extract acteoside,isoacteoside,oleuropein and phillyrin,considering the extraction efficiency both of phenylethanoid glycoside and lignans,the variables factors in the extraction process were optimized,including the extraction solvent ranging from 60% to 100% methanol,the volume of extraction solvent ranging from 8 to 20 mL,and extraction time ranging from 15 to 60 min.The ultimate optimized method for quantitation is described as follows:0.1 g of O.fragrans roots was taken and mixed with 12 mL of 60% methanol in ultrasonic extraction for 45 min.
Multiple reaction monitoring(MRM)scan mode was employed for the quantitative analysis.In order to obtain high sensitivity,three factors,namely,collision energy,declustering potential,and collision cell exit potential,were specified to find the parent/daughter ion pairs with high responses(shown in Table S1).
3.3.Method validation
Linearity equations were obtained by plotting corresponding peak areas versus different concentrations.All the linearity equations exhibited excellent linearity with correlation coefficients(r)higher than 0.9996.The LOQs were 4.51 ng/mL for acteoside,4.09 ng/mL for isoacteoside,4.58 ng/mL for oleuropein and 4.45 ng/mL for phillyrin.Hence,the HPLC-MS/MS method for simultaneous determination of the four analytes has higher sensitivity than other methods in literature[37-40].In order to test the intra-day and inter-day precision of the method,mixtures of standards were freshly prepared and injected five times on the same day and on 3 consecutive days,respectively.RSDs of the repeatability test of six samples ranged from1.59% to3.64%,and RSDs of the stability test of the sample stored at 15°C and analyzed after 0,4,8,12,16 h,19 h and 23 h ranged from 1.79% to 3.24%,as shown in Table 2.The accuracy of the proposed method was assessed,in which 0.05 g of the O.fragrans roots powder was mixed with a known amount of four reference substances,and then extracted by the sample preparation method.The results are shown in Table 2,and it indicated that the HPLC-MS/MS method possessed good accuracy with recoveries ranging from 95.68% to 104.56% while all the RSDs were smaller than 5%.
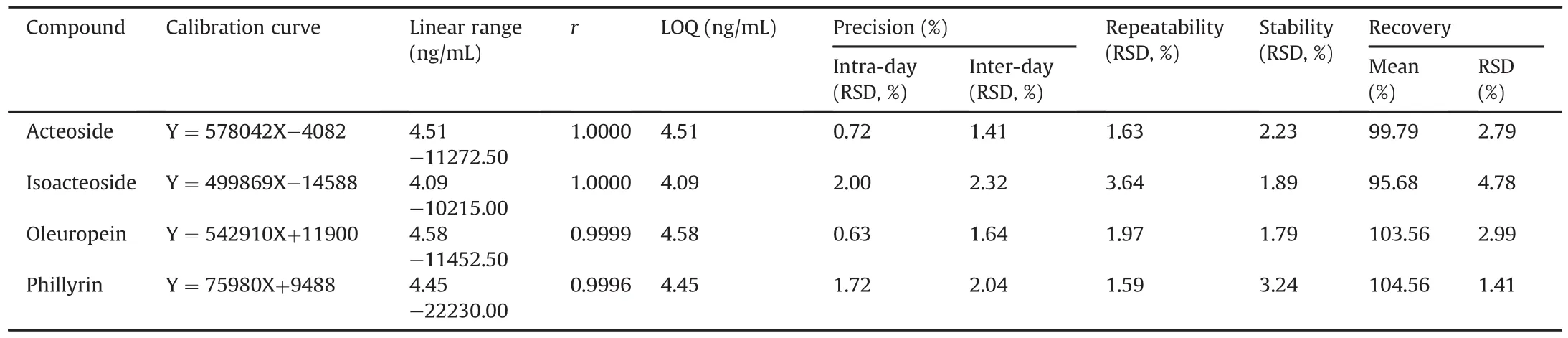
Table 2 Method validation using four target analytes.
3.4.Application in O.fragrans roots
Four bioactive components in O.fragrans roots were determined by the proposed HPLC-MS/MS method.Phillyrin,oleuropein,acteoside and isoacteoside belong to lignans,iridoids and phenylethanols,respectively,and they were proved to have many physiological activities.They were supposed to be the bioactive components in O.fragrans roots,and used to assess the quality of different cultivars.The typical MRM chromatograms of four bioactive compounds are shown in fig.3,and the content comparisons of them in twelve batches of O.fragrans roots are listed in Table 3.The contents of oleuropein and phillyrin in the twelve batches are relatively stable.The content of acteoside and isoacteoside varied up to 10 times.
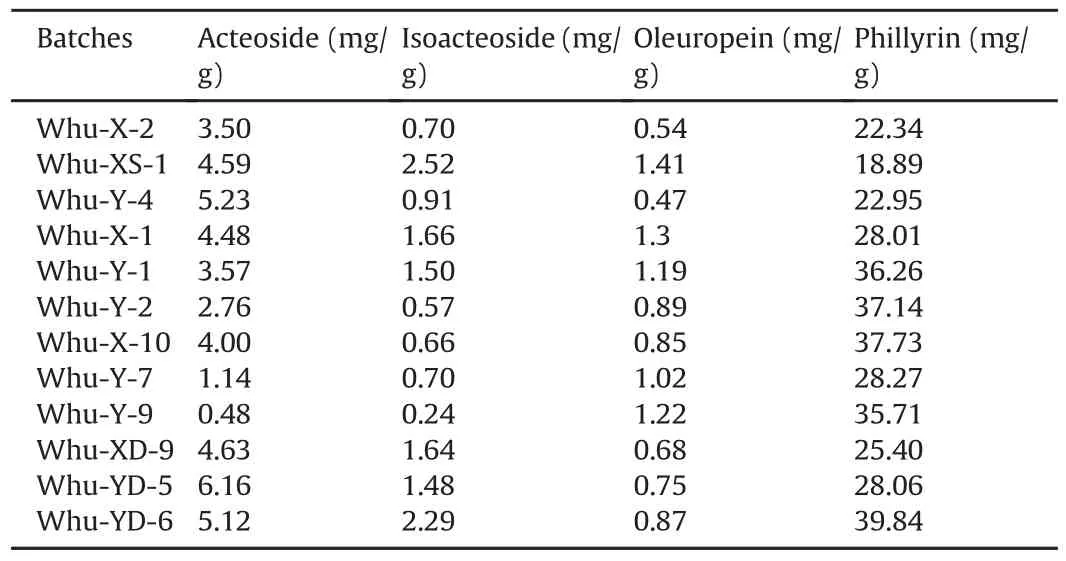
Table 3 Content comparisons of four compounds in twelve batches of O.fragrans roots.
The twelve bathes of samples could be split into 4 groups.It is noteworthy that the content of phillyrin in the batches from O.fragrans Luteus group bearing fruits is commonly lower than that in other batches.And the content of acteoside and isoacteoside in the batches from O.fragrans Aurantiacus group that could not bear fruits is the highest.The differences of the bioactive component contents might be due to different cultivars,but more sample batches are required for statistical analysis.
3.5.Network pharmacological analysis
The targets of O.fragrans roots are shown in Table S2.And the important targets of core nodes(Table S3)are membrane proteins,serine/threonine protein kinases,aerobic and hypoxic transcription regulators,MAP kinases,tubulin,matrix metalloproteinases(MMPs),tyrosine protein phosphatase,calcium osmotic cation channels and so on.
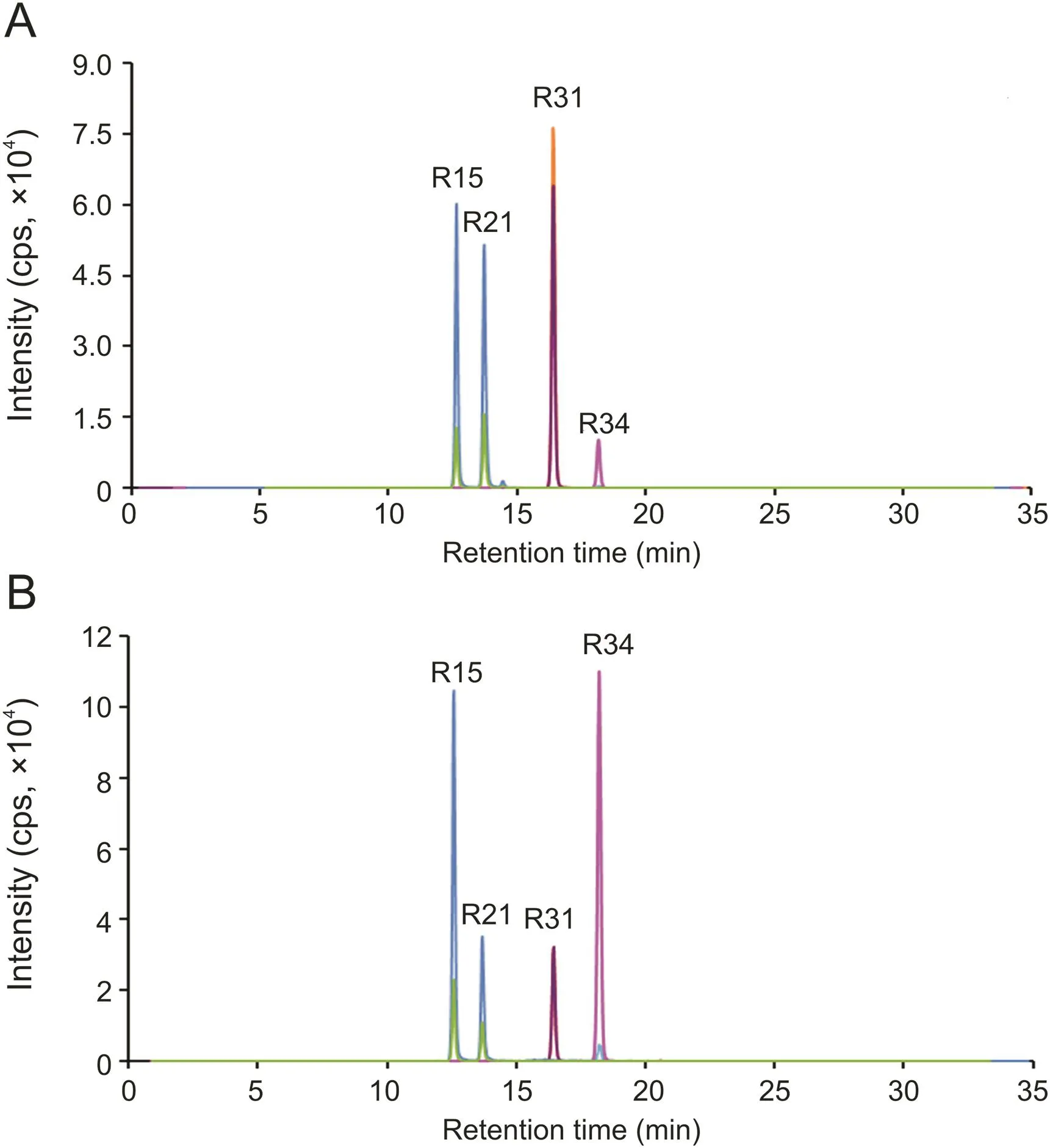
Fig.3.Typical MRM chromatograms of four bioactive compounds:(A)standard solutions;and(B)extracts.Peak identification:R15,Acteoside;R21,isoacteoside;R31,oleuropein;R34,phillyrin.
in fig.4 and Table S4,it is shown that the diseases involved are mainly Inflammation,cardiovascular diseases and cancer.It can be seen that rheumatoid arthritis is one of the related diseases,which is consistent with the traditional pharmacodynamics of O.fragrans roots,such as ‘treating muscle pain’and ‘treating rheumatism’.Its related targets are protein kinase C Theta(PRKCQ),MMP9,MMP3,MMP2 and MMP1.Gene ontology annotations related to the gene PRKCQ include transferase activity, transferring phosphoruscontaining groups and protein tyrosine kinase activity.MMPs are zinc-dependent endopeptidases and the major proteases in extracellular matrix(ECM)degradation.MMPs are capable of degrading several extracellular molecules and a number of bioactive molecules.On the other hand,the result in Table S5 shows that MMPs play a role mainly by influencing the biological processes related to collagen decomposition and extracellular matrix decomposition.The compounds related to these targets are 10-hydroxylstroigside,ligstroside,oleuropein,osmanthuside H,and secoxyloganin.The result above provides evidence for us to study the mechanism of O.fragrans roots exerting traditional pharmacodynamics.
4.Conclusion
Effective and sensitive HPLC-MS methods for characterization and quantitation of the bioactive constituents in O.fragrans roots were developed.Thirty-two constituents were first discovered in O.fragrans roots,one compound of which was reported for the first time.The simultaneous determination method for acteoside,isoacteoside,oleuropein and phillyrin was validated to be sensitive and accurate.Then it was applied to determine the content of bioactive components in O.fragrans roots from different cultivars.Moreover,network pharmacological analysis shows that O.fragrans roots might be effective for the treatment of Inflammation,cardiovascular diseases,cancer,and rheumatoid arthritis,which is consistent with the traditional pharmacodynamics of O.fragrans roots.This work will facilitate the comprehensive development of O.fragrans roots.

Fig.4.Interaction network of the effect substances(in red),diseases(in royal blue),the important targets(in orange)and other targets(in blue).
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China(Grant Nos.81872828 and 81573384)and the Large-scale Instrument and Equipment Sharing Foundation of Wuhan University(LF20191065).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2020.06.010.
 Journal of Pharmaceutical Analysis2021年3期
Journal of Pharmaceutical Analysis2021年3期
- Journal of Pharmaceutical Analysis的其它文章
- Molecular detection of SARS-CoV-2 being challenged by virus variation and asymptomatic infection
- The potential of miRNA-based therapeutics in severe acute respiratory syndrome coronavirus 2(SARS-CoV-2)infection:A review
- Potential treatment with Chinese and Western medicine targeting NSP14 of SARS-CoV-2
- Accurate and sensitive determination of hydroxychloroquine sulfate used on COVID-19 patients in human urine,serum and saliva samples by GC-MS
- Fast saccharide mapping method for quality consistency evaluation of commercial xylooligosaccharides collected in China
- Dispersive liquid-liquid microextraction,an effective tool for the determination of synthetic cannabinoids in oral fluid by liquid chromatography-tandem mass spectrometry
