LINE-1在肿瘤早期诊断和治疗中的研究与应用
寇艳妮,岑山,李晓宇
LINE-1在肿瘤早期诊断和治疗中的研究与应用
寇艳妮,岑山,李晓宇
中国医学科学院&北京协和医学院,医药生物技术研究所免疫生物学室,北京 100050
长散布元件-1 (long interspersed elements-1, LINE-1)约占人类基因组的17%,是人类基因组中唯一具有自主转座能力的转座子。LINE-1可通过逆转录转座过程插入到新的基因位点上,从而会导致基因组的不稳定。因而机体对LINE-1的复制和转座有着严格的限制,在正常体细胞中几乎检测不到LINE-1的表达。然而,在绝大多数的肿瘤组织或癌组织中LINE-1的表达却普遍存在,提示LINE-1的表达和转座与肿瘤的发生发展密切相关。LINE-1在肿瘤细胞中的差异表达可以作为肿瘤早期诊断的标志物,同时也可作为肿瘤治疗预后评价的重要指标。与此同时,LINE-1作为肿瘤治疗潜在靶点的可行性也在评估和验证中。本文介绍了在临床方面LINE-1作为肿瘤诊断、预后方面的应用,以及作为肿瘤治疗潜在靶点的研究进展,以期为临床上肿瘤的诊断和治疗提供一些参考。
长散布元件-1;肿瘤;诊断;治疗;预后
转座子是一类可以在同一染色体内或在不同染色体间自由移动的基因序列,其中“转座”即是指其位置的转移。根据转座方式的不同,转座子可分为DNA转座子和RNA转座子两类:DNA转座子的转座是利用转座酶将自身的DNA片段从原有的基因位点上切下后,再整合到基因组的其他位点上;而RNA转座子又被称为逆转录转座子(retrotransposons),它首先通过转录生成RNA中间体,然后以该RNA为模板逆转录生成cDNA并插入到新的基因位点上。逆转录转座子通过这样“复制–粘贴”的转座机制在人类基因组中得以扩增和蔓延,从而在真核基因组中积累了大量重复序列。长散布元件-1 (long interspersed elements-1, LINE-1)是逆转录转座子的一种,也是人类基因组中现今唯一具有自主转座活性的转座子,约占人类基因组的17%[1]。LINE-1曾在物种形成和生物进化中发挥了至关重要的作用,但其逆转录转座活性对于基因组维持稳定性来说也是巨大的威胁。LINE-1的逆转录转座能够引起基因组DNA的异常转录、选择性剪接、插入突变、DNA损伤从而导致基因组不稳定[2,3]。大量的证据表明,LINE-1通过逆转录转座直接或间接地参与了肿瘤的发生和发展,在正常组织和癌旁组织中LINE-1的表达与癌组织中的也有不同,评估LINE-1作为肿瘤早期诊断标志物、预后评价指标和抗癌药物靶点的潜力,能够为更新肿瘤诊断和预后手段以及肿瘤的治疗提供理论依据,也能为肿瘤的早期发现和个性化治疗提供借鉴和参考[1,4~6]。本文就近年来LINE-1在肿瘤中的表达以及作为生物标志物在肿瘤的早期诊断、治疗和预后判定中的应用进展作一综述。
1 LINE-1的结构与功能
LINE-1全长6 kb,包括含有内部启动子的5′端非翻译区(untranslated region, UTR)、两个不重叠的开放式读码框架(open reading frame, ORF)ORF1和ORF2以及以poly(A)尾结尾的3′端UTR,灵长类动物的LINE-1 5ʹUTR还含有一个物种特异性的ORF0编码区。ORF1和ORF2编码的蛋白对LINE-1的逆转录转座是不可缺少的。ORF1p由338个氨基酸组成,大小约40 kDa,其C端相当保守,参与RNA结合;N端具有亮氨酸拉链的结构特征,能够介导蛋白质之间的相互作用;ORF2p约150 kDa,包括三个保守的结构域,分别是N端的核酸内切酶结构域,具有识别AA|TTTT序列并行使切割双链DNA的功能;中间的逆转录酶结构域,具有逆转录酶活性;C端的锌指状结构域,参与DNA、蛋白质和RNA的识别和结合[7]。
LINE-1转录生成LINE-1 mRNA,LINE-1 mRNA既作为基因组DNA模板,又行使mRNA的功能。LINE-1 mRNA在细胞质中翻译表达出ORF1p和ORF2p两种蛋白。ORF1p和ORF2p与LINE-1 mRNA顺式结合,组装成核糖核蛋白复合物(ribonucleoprotein complexes, RNPs)转运入核,随后通过靶位点引导逆转录过程(target-site primed reverse transcription, TPRT)插入到新的基因位点上(图1)[8]。除自身可以自主转座以外,LINE-1还能协助不具有自主转座能力的转座子或重复序列进行转座[9]。与其他类型的逆转录转座子一样,LINE-1通过逆转录转座向基因组提供可进行非等位基因重组的序列,它的扩增极大地塑造了多细胞生物的基因组多样性。
之前的证据显示,LINE-1的逆转录转座频繁地发生在生殖细胞、神经细胞以及成年人的大脑中[10,11],除此之外,由于LINE-1会通过逆转录转座机制向基因组插入LINE-1或其他转座子,从而改变或破坏基因的结构和功能,为了维持基因组的稳定性,宿主通过表观遗传修饰和非编码小RNA调控网络等管控机制严格控制着LINE-1的转座活性,所以,LINE-1在分化程度高的正常体细胞组织中表达水平很低,甚至不表达[12]。LINE-1仍有可能被遗传毒性刺激、氧化刺激、运动刺激和生理刺激所激活[11]。研究表明,LINE-1在大多数肿瘤组织中都出现了启动子低甲基化,LINE-1 mRNA、ORF1p和ORF2p过表达的情况,是多种类型肿瘤的共同的特征(表1)[13~32]。
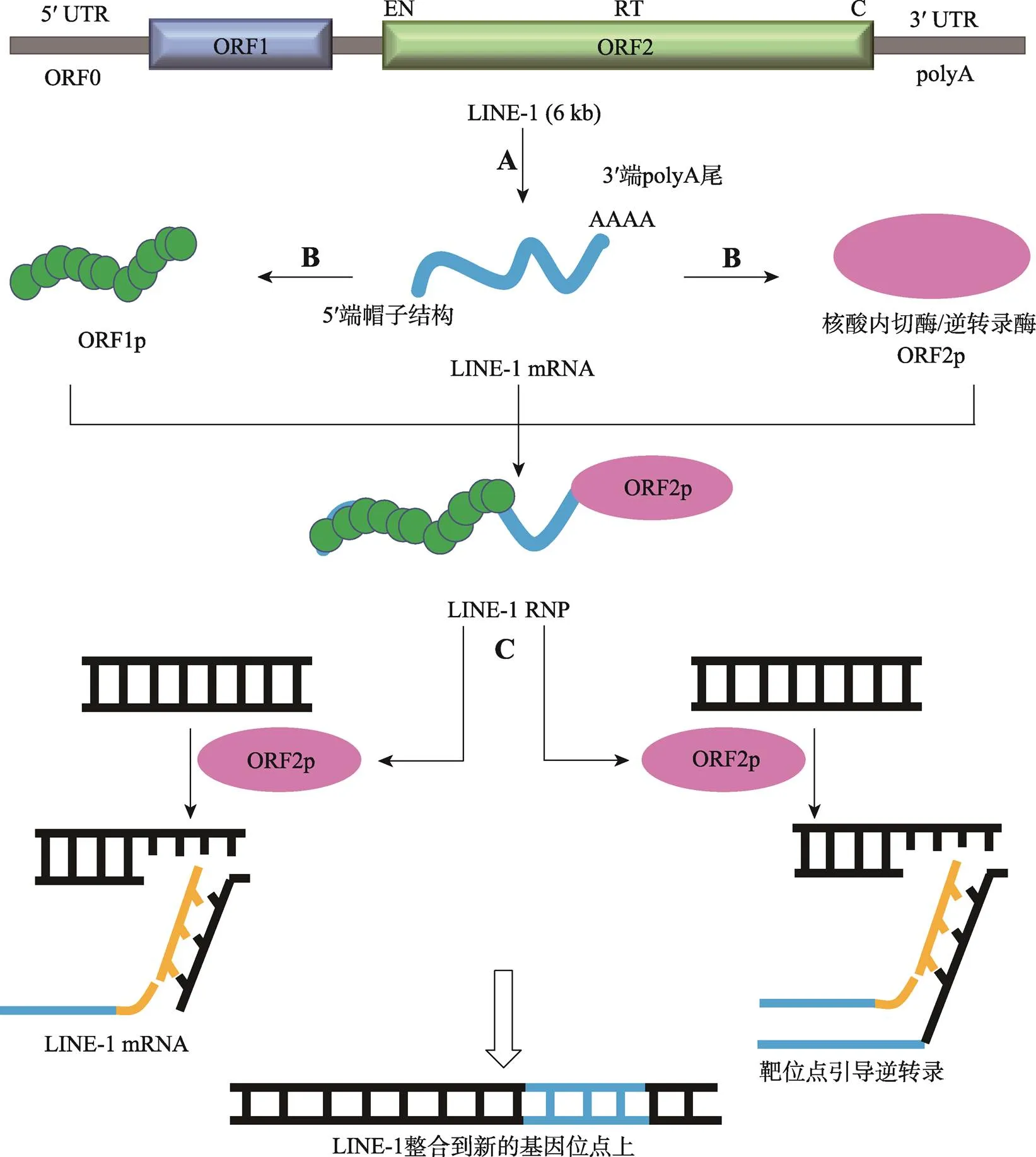
图1 LINE-1的转座过程
A:LINE-1转录生成LINE-1 mRNA。B:LINE-1 mRNA翻译表达出ORF1p和ORF2p两种蛋白质,ORF1p和ORF2p与LINE-1 mRNA相结合生成LINE-1 RNP。C: ORF2p具有核酸内切酶活性和逆转录酶活性,通过靶位点引导逆转录过程将LINE-1整合到新的基因位点上。

表1 LINE-1的转座与相关肿瘤/癌症
2 LINE-1作为肿瘤诊断的生物标志物
2.1 LINE-1在肿瘤中的低甲基化现象
DNA甲基化是真核生物表观遗传调控机制之一,其中,脊椎动物基因组的DNA甲基化修饰主要发生在DNA的胞嘧啶–鸟嘌呤二核苷酸(cytosine phosphate-guanosine, CpG)岛[33]。一般认为,CpG岛的甲基化可以通过影响转录因子结合或改变染色质结构抑制相应RNA转录。全基因组DNA低甲基化是恶性肿瘤中常见的表观遗传现象,并且这种现象可能在肿瘤的发生发展过程中发挥关键作用。LINE-1作为基因组中广泛散在的高度重复序列,其启动子区富含CpG岛,CpG岛的甲基化大多发生在与LINE-1相关的启动子序列中,因此,LINE-1启动子甲基化状态已成为全基因组甲基化状态的替代指标[34]。
LINE-1启动子的高度甲基化是抑制LINE-1转录的重要手段[35],而启动子的去甲基化意味着机体为LINE-1 mRNA的转录松绑。Chalitchagorn等[36]的研究显示,包括乳腺癌、结直肠癌、肺癌、头颈部癌、膀胱癌、食道癌、肝癌、前列腺和胃癌在内的大多数肿瘤组织样本中LINE-1启动子的甲基化程度都显著低于正常组织。与此同时,只有在某些恶性肿瘤细胞中才能检测到完整的LINE-1 mRNA[21,22],正常体细胞基因组中几乎不含完整的LINE-1 mRNA[37]。Rodić等[38]对LINE-1启动子的甲基化程度与卵巢癌癌变阶段之间的关系进行了深入的研究,他们利用联合亚硫酸氢钠限制性内切酶分析法(combined bisulfite restriction analysis PCR, COBRA PCR)对不同癌变阶段的上皮性卵巢癌组织进行检测,结果表明,随着肿瘤的恶化,LINE-1甲基化水平显著降低,且LINE-1的甲基化水平与上皮卵巢癌的FIGO分期和肿瘤分级密切相关。
癌前病变是具备癌变可能性的良性病变,及早发现癌前病变并及时干预对于恶性肿瘤的防治具有重要意义。有研究表明,结直肠癌和乳腺癌的癌前病变与恶化的癌组织中的LINE-1甲基化水平都显著低于正常组织,这表明LINE-1的低甲基化现象预示着肿瘤的发生,提示LINE-1低甲基化有成为肿瘤诊断生物标志物的潜力[36,39]。
2.2 ORF1p的过表达是恶性肿瘤的标志之一
ORF1p是RNA结合蛋白,尽管其发挥的确切作用尚不清楚,但ORF1p是LINE-1逆转录转座所必须的[2]。Rodić等[40]首次检测了1027个肿瘤组织样本中ORF1p的表达,结果显示,约47%的肿瘤组织样本表达ORF1p,而在非肿瘤性体细胞组织中则检测不到ORF1p的表达。不同种类的肿瘤中ORF1p表达水平不同,有研究表明,ORF1p在90%以上的乳腺癌和卵巢癌、近90%的胰腺癌、50%~60%食管癌和结肠癌、50%的肺癌和40%前列腺癌中均可以检测到过表达,而肾癌、肝癌、宫颈癌、原发性胶质母细胞瘤和低级别B细胞淋巴瘤则几乎不表达ORF1p[1,41]。ORF1p的表达水平与肿瘤的恶化程度相关,Rodić等[25,26]还在89%的胰腺导管腺癌组织中都检测到了ORF1p的表达,仅在27%的胰腺上皮内瘤组织可以检测到ORF1p,且胰腺导管腺癌原发部位组织和转移癌组织中ORF1p表达无显著性差异,这表明ORF1p的表达可能是一种获得性特征,常见于肿瘤晚期的高级病变。目前,有研究团队为了推进了ORF1p作为生物标志物在肿瘤诊断上的临床应用,正在开发一种检测和测量血液中的ORF1p含量的方法,用于更好地诊断前列腺癌[42]。
2.3 ORF2p在肿瘤组织中的表达
ORF2p具有逆转录酶和核酸内切酶活性,用逆转录酶抑制剂处理癌细胞能引起细胞增殖下降,其核酸内切酶结构域能够识别并切断特定基因组DNA序列,造成DNA损伤[43]。ORF2p通过非常规机制进行少量的翻译表达[44],这使研究ORF2p变得十分困难,所以相对于ORF1p,ORF2p之于癌症的数据是有限的。多项研究表明[45,46],正常乳腺组织中检测不到ORF2p的表达,但在肿瘤发生早期,在乳腺组织还没有出现明显的组织学改变且检测不到癌症标志物和表皮生长因子受体的时候,ORF2p的表达就出现了增加;与此同时,随着肿瘤发展进阶,ORF2p的表达还会进一步的上调。有研究小组在探究ORF2p在前列腺癌和结直肠癌中的表达时发现,内源性ORF2p的过表达最早发生在前列腺上皮内瘤和肠腺瘤这两种癌前病变中,从癌前病变到癌组织的过渡中也检测到了ORF2p表达的增加[1,30]。尽管还需要验证其临床相关性,但这些数据提示ORF2p的表达可能发生在肿瘤形成的早期,ORF2p的过表达有潜力成为一些特定肿瘤的早期诊断标志物。
3 LINE-1作为肿瘤治疗靶点
尽管还不清楚LINE-1在肿瘤发生发展过程发挥的具体作用,但大量的研究表明LINE-1的表达和逆转录转座与肿瘤细胞的增殖[47]、形成[48]、分化[49]、上皮细胞间质转型[50]以及肿瘤侵袭[51]密切相关。LINE-1 ORF2编码的逆转录酶在分化的体细胞组织中低表达或不表达且在生殖细胞、胚胎组织中高表达,这说明LINE-1逆转录酶的表达水平与细胞的增殖潜力相关[47],提示可以利用LINE-1在肿瘤细胞和正常细胞中表达的差异来开发新的肿瘤治疗方法。
WhaKuo等[52]用靶向ORF2编码的逆转录酶的反义寡核苷酸处理人肝癌细胞Hep3B能够间接抑制癌细胞的增殖。奈韦拉平、依非韦伦等非核苷类逆转录酶抑制剂(nonnucleoside RT inhibitors, NNRTIs)在抑制LINE-1逆转录酶活性的同时,也能强烈抑制结直肠癌、胰腺癌和前列腺癌细胞的增殖并促进细胞死亡[53,54]。其中,依非韦伦是已获美国FDA批准,用于临床艾滋病治疗的抗逆转录病毒的药物之一,具有抗病毒效果较强、耐受性良好、口服吸收好和单剂量给药的终点半衰期相对较长等优点。最新的研究结果显示依非韦伦作用于癌细胞后可以通过损伤基因组和破坏核纤层诱导染色体结构重塑,改变细胞核功能,触发细胞自噬途径并促进细胞凋亡。同时,依非韦伦几乎不能抑制非致瘤性细胞PNT2和WI-38增殖能力,依非韦伦作用后的PNT2和WI-38的核纤层结构不发生改变,且不能触发细胞的自噬机制[54]。依非韦伦还被证明能够通过影响脂肪酸代谢途径抑制三阴性乳腺癌细胞系的细胞增殖活性[32]。目前,依非韦伦已在转移性去势抵抗性前列腺癌患者中进行了二期临床试验,与血药浓度低的受试者相比,血药浓度高的受试者出现癌症转移的概率相对更低[55]。除此之外,一些以LINE-1 ORF2p为靶点的核苷类逆转录酶抑制剂(nucleoside RT inhibitors, NRTIs)具有与NNRTIs相似的抗癌作用[56,57]。有研究表明,NRTIs阿巴卡韦能够通过诱导前列腺癌细胞的抗增殖活性触发细胞的衰老机制[56]。
4 LINE-1作为肿瘤预后的生物标志物
LINE-1启动子的甲基化状态是支持LINE-1有潜力作为预后的分子标志物的主要证据,目前已经在多种癌症中证明了LINE-1启动子的低甲基化与不良预后显著相关。一项研究收集了643例结直肠癌患者的临床病理资料,对患者进行了为期4年的随访调查。结果显示,LINE-1低甲基化与结直肠癌特异性死亡显著相关;结直肠癌患者甲基化水平降低30%,死亡风险增高2.37倍(HR:1.37;95%CI= 1.42~3.94);将患者按甲基化程度分为四组(≥75%,60%~75%,45%~60%和<45%),LINE-1甲基化程度低于45%的患者的死亡风险比LINE-1甲基化程度高于75%的患者高5倍(HR:5;95%CI=1.92~13.1)[58]。还有研究在检测了40名晚期结直肠癌患者的原发肿瘤组织中LINE-1启动子的甲基化水平后发现,在都进行过化疗的背景下,LINE-1启动子甲基化水平高的患者,无进展生存期和总生存期要显著高于LINE-1甲基化水平低的患者。一项针对食管鳞状细胞癌的研究表明:LINE-1甲基化指数<0.78的患者的平均生存期为34个月,而LINE-1甲基化指数> 0.78的患者的平均生存期则为43个月[59]。之后,研究人员在肝细胞癌、食管癌、膀胱癌和肺癌的研究中也得到了相似的结果[41,60]。LINE-1启动子的甲基化状态可能深刻影响着肿瘤细胞的增殖、分化和侵袭,有望成为独立的肿瘤预后指标。
ORF1p在多类癌症中过表达已成为癌症的生物标志物之一,ORF2p被证明能够影响癌症的发生发展,这两条重要的证据支持ORF1p和ORF2p具有成为肿瘤预后评价生物标志物的潜力。一项研究表明,脑胶质瘤组织中ORF1p高表达的患者生存率要显著低于ORF1p低表达的患者[61]。ORF1p大多定位在细胞质中,但也有少量的ORF1p定位在细胞核中。一项LINE-1与乳腺癌预后关系的研究结果显示,ORF1p在核内的表达量与乳腺癌预后相关,与ORF1p大多定位在细胞质的乳腺肿瘤患者相比,ORF1p定位在细胞核内的患者的局部复发率和远端转移率更高,其无病生存率和总体生存率相对较低[62]。同样地,ORF2p的核定位情况也具有预后价值,有研究发现在乳腺导管内原位癌中,ORF1p和ORF2p都定位在细胞质,且定位在细胞质中的ORF1p和ORF2p的表达量与患者的生存率无关,而在恶化程度更高的浸润性导管癌的细胞核和细胞质中均检测到了ORF1p和ORF2p,且细胞核中ORF1p和ORF2p表达量高的患者的淋巴结转移率要显著高于细胞质中ORF1p和ORF2p表达量高的患者,细胞核中ORF1p和ORF2p表达量高的患者的生存期也更短[63],这可能是由于大量表达且定位在细胞核内的ORF1p和ORF2p增加了LINE-1的逆转录转座进而增加了基因组的不稳定。
5 结语与展望
越来越多的证据表明LINE-1的表达与肿瘤的发生发展存在密切的关联,从LINE-1的角度出发寻找诊断、预后和治疗癌症的方法已成为新的方向。比如ORF1p和ORF2p可以作为癌症诊断的生物标志物,癌组织中LINE-1启动子的甲基化状态有望成为独立的癌症预后指标,ORF2p也逐渐表现出成为治疗靶点的潜力,一些逆转录酶抑制剂已在临床试验中表现出治疗癌症的希望。尽管已有相当多的证据,但LINE-1的表达与肿瘤发生发展之间具体的影响机制仍未阐明,支持LINE-1作为癌症治疗靶点的实验主要集中在细胞水平上,临床试验信息仍十分有限。与此同时,还需要开发更多能够适应临床的、有效、灵敏和微创的检测手段,比如ORF2p虽然有潜力成为一些特定肿瘤的早期诊断标志物,但由于其表达较少,所以很难检测,可以尝试开发特定的逆转录酶活性测定法用于肿瘤的早期诊断。同时,LINE-1对于癌症治疗的潜在用途还未发掘,如LINE-1的表达与癌症的化疗、放疗和免疫治疗过程中出现的肿瘤耐药性是否具有相关性,如果能够证明化疗、放疗或免疫治疗促进了肿瘤中LINE-1的异常激活从而引起基因突变最终导致肿瘤耐药性的形成,那么LINE-1就可以帮助临床医生提供更多、更有效的治疗方案。
[1] Burns KH. Transposable elements in cancer., 2017, 17(7): 415–424.
[2] Goodier JL, Kazazian HH. Retrotransposons revisited: the restraint and rehabilitation of parasites., 2008, 135(1): 23–35.
[3] De Cecco M, Ito T, Petrashen AP, Elias AE, Skvir NJ, Criscione SW, Caligiana A, Brocculi G, Adney EM, Boeke JD, Le O, Beauséjour C, Ambati J, Ambati K, Simon M, Seluanov A, Gorbunova V, Slagboom PE, Helfand SL, Neretti N, Sedivy JM. L1 drives IFN in senescent cells and promotes age-associated inflammation., 2019, 566(7742): 73–78.
[4] Hancks DC, Kazazian HH. Roles for retrotransposon insertions in human disease., 2016, 7: 9.
[5] Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation., 2011, 144(5): 646–674.
[6] Liu Q, Wang JH, Li XY, Cen S. The connection between LINE-1 retrotransposition and human tumorigenesis., 2016, 38(2): 93–102.
刘茜, 王瑾晖, 李晓宇, 岑山. 逆转录转座子LINE-1与肿瘤的发生和发展. 遗传, 2016, 38(2): 93–102.
[7] Zhang X, Zhang R, Yu JP. New understanding of the relevant role of LINE-1 retrotransposition in human disease and immune modulation., 2020, 8: 657.
[8] Bodak M, Yu J, Ciaudo C. Regulation of LINE-1 in mammals., 2014, 5(5): 409–428.
[9] Deniz Ö, Frost JM, Branco MR. Regulation of transposable elements by DNA modifications., 2019, 20(7): 417–431.
[10] Elbarbary RA, Lucas BA, Maquat LE. Retrotransposons as regulators of gene expression., 2016, 351(6274): aac7247.
[11] Terasaki N, Goodier JL, Cheung LE, Wang YJ, Kajikawa M, Kazazian HH, Okada N.screening for compounds that enhance human L1 mobilization., 2013, 8(9): e74629.
[12] Grundy EE, Diab N, Chiappinelli KB. Transposable element regulation and expression in cancer., 2021, doi: 10.1111/febs.15722.
[13] Dolci M, Favero C, Toumi W, Favi E, Tarantini L, Signorini L, Basile G, Bollati V, D'Alessandro S, Bagnoli P, Ferrante P, Delbue S. Human endogenous retroviruses long terminal repeat methylation, transcription, and protein expression in human colon cancer., 2020, 10: 569015.
[14] Ko EJ, Oh YL, Kim HY, Eo WK, Kim H, Ock MS, Kim HS, Kim KH, Cha HJ. Correlation of long interspersed element-1 open reading frame 1 and c-Met proto-oncogene protein expression in ovarian cancer., 2019, 41(11): 1293–1299.
[15] Daskalos A, Nikolaidis G, Xinarianos G, Savvari P, Cassidy A, Zakopoulou R, Kotsinas A, Gorgoulis V, Field JK, Liloglou T. Hypomethylation of retrotransposable elements correlates with genomic instability in non-small cell lung cancer., 2009, 124(1): 81–87.
[16] Harada K, Baba Y, Ishimoto T, Chikamoto A, Kosumi K, Hayashi H, Nitta H, Hashimoto D, Beppu T, Baba H. LINE-1 methylation level and patient prognosis in a database of 208 hepatocellular carcinomas., 2015, 22(4): 1280–1287.
[17] Iwagami S, Baba Y, Watanabe M, Shigaki H, Miyake K, Ishimoto T, Iwatsuki M, Sakamaki K, Ohashi Y, Baba H. LINE-1 hypomethylation is associated with a poor prognosis among patients with curatively resected esophageal squamous cell carcinoma., 2013, 257(3): 449–455.
[18] van Hoesel AQ, van de Velde CJH, Kuppen PJK, Liefers GJ, Putter H, Sato Y, Elashoff DA, Turner RR, Shamonki JM, de Kruijf EM, van Nes JGH, Giuliano AE, Hoon DSB. Hypomethylation of LINE-1 in primary tumor has poor prognosis in young breast cancer patients: a retrospective cohort study., 2012, 134(3): 1103–1114.
[19] Weber B, Kimhi S, Howard G, Eden A, Lyko F. Demethylation of a LINE-1 antisense promoter in the cMet locus impairs Met signalling through induction of illegitimate transcription., 2010, 29(43): 5775–5784.
[20] Wolff EM, Byun HM, Han HF, Sharma S, Nichols PW, Siegmund KD, Yang AS, Jones PA, Liang GN. Hypomethylation of a LINE-1 promoter activates an alternate transcript of the MET oncogene in bladders with cancer., 2010, 6(4): e1000917.
[21] Roman-Gomez J, Jimenez-Velasco A, Agirre X, Cervantes F, Sanchez J, Garate L, Barrios M, Castillejo JA, Navarro G, Colomer D, Prosper F, Heiniger A, Torres A. Promoter hypomethylation of the LINE-1 retrotransposable elements activates sense/antisense transcription and marks the progression of chronic myeloid leukemia., 2005, 24(48): 7213–7223.
[22] Lin L, Wang ZW, Prescott MS, van Dekken H, Thomas DG, Giordano TJ, Chang AC, Orringer MB, Gruber SB, Moran JV, Glover TW, Beer DG. Multiple forms of genetic instability within a 2-Mb chromosomal segment of 3q26.3-q27 are associated with development of esophageal adenocarcinoma., 2006, 45(4): 319–331.
[23] Miret N, Zappia CD, Altamirano G, Pontillo C, Zárate L, Gómez A, Lasagna M, Cocca C, Kass L, Monczor F, Randi A. AhR ligands reactivate LINE-1 retrotransposon in triple-negative breast cancer cells MDA-MB-231 and non-tumorigenic mammary epithelial cells NMuMG., 2020, 175: 113904.
[24] Tharp ME, Malki S, Bortvin A. Maximizing the ovarian reserve in mice by evading LINE-1 genotoxicity., 2020, 11(1): 330.
[25] Rodić N, Sharma R, Zampella J, Dai LX, Taylor MS, Hruban RH, Iacobuzio-Donahue CA, Maitra A, Torbenson MS, Goggins M, Shih IM, Duffield AS, Montgomery EA, Gabrielson E, Netto GJ, Lotan TL, De Marzo AM, Westra W, Binder ZA, Orr BA, Gallia GL, Eberhart CG, Boeke JD, Harris CR, Burns KH. Long interspersed element-1 protein expression is a hallmark of many human cancers., 2014, 184(5): 1280–1286.
[26] Rodić N, Steranka JP, Makohon-Moore A, Moyer A, Shen PL, Sharma R, Kohutek ZA, Huang CR, Ahn D, Mita P, Taylor MS, Barker NJ, Hruban RH, Iacobuzio-Donahue CA, Boeke JD, Burns KH. Retrotransposon insertions in the clonal evolution of pancreatic ductal adenocarcinoma., 2015, 21(9): 1060–1064.
[27] Doucet-O'Hare TT, Rodić N, Sharma R, Darbari I, Abril G, Choi JA, Young Ahn J, Cheng YL, Anders RA, Burns KH, Meltzer SJ, Kazazian HH. LINE-1 expression and retrotransposition in Barrett's esophagus and esophageal carcinoma., 2015, 112(35): E4894–E4900.
[28] Papasotiriou I, Pantopikou K, Apostolou P. L1 retrotransposon expression in circulating tumor cells., 2017, 12(2): e0171466.
[29] Tristán-Ramos P, Rubio-Roldan A, Peris G, Sánchez L, Amador-Cubero S, Viollet S, Cristofari G, Heras SR. The tumor suppressor microRNA let-7 inhibits human LINE-1 retrotransposition., 2020, 11(1): 5712.
[30] De Luca C, Guadagni F, Sinibaldi-Vallebona P, Sentinelli S, Gallucci M, Hoffmann A, Schumann GG, Spadafora C, Sciamanna I. Enhanced expression of LINE-1-encoded ORF2 protein in early stages of colon and prostate transformation., 2016, 7(4): 4048–4061.
[31] Briggs EM, Spadafora C, Logan SK. A re-evaluation of LINE-1 ORF2 expression in LNCaP prostate cancer cells., 2019, 10: 51.
[32] Chiou PT, Ohms S, Board PG, Dahlstrom JE, Rangasamy D, Casarotto MG. Efavirenz as a potential drug for the treatment of triple-negative breast cancers., 2021, 23(2): 353–363.
[33] Shademan M, Zare K, Zahedi M, Mosannen Mozaffari H, Bagheri Hosseini H, Ghaffarzadegan K, Goshayeshi L, Dehghani H. Promoter methylation, transcription, and retrotransposition of LINE-1 in colorectal adenomas and adenocarcinomas., 2020, 20: 426.
[34] Kerachian MA, Kerachian M. Long interspersed nucleotide element-1 (LINE-1) methylation in colorectal cancer., 2019, 488: 209–214.
[35] Wang XY, Zhang Y, Yang N, Cheng H, Sun YJ. DNMT3a mediates paclitaxel-induced abnormal expression of LINE-1 by increasing the intragenic methylation., 2020, 42(1): 100–111.
王昕源, 张雨, 杨楠, 程禾, 孙玉洁. DNMT3a通过提升基因内部甲基化介导紫杉醇诱导的LINE-1异常表达. 遗传, 2020, 42(1): 100–111.
[36] Chalitchagorn K, Shuangshoti S, Hourpai N, Kongruttanachok N, Tangkijvanich P, Thong-ngam D, Voravud N, Sriuranpong V, Mutirangura A. Distinctive pattern of LINE-1 methylation level in normal tissues and the association with carcinogenesis., 2004, 23(54): 8841–8846.
[37] Belancio VP, Roy-Engel AM, Pochampally RR, Deininger P. Somatic expression of LINE-1 elements in human tissues., 2010, 38(12): 3909–3922.
[38] Pattamadilok J, Huapai N, Rattanatanyong P, Vasurattana A, Triratanachat S, Tresukosol D, Mutirangura A. LINE-1 hypomethylation level as a potential prognostic factor for epithelial ovarian cancer., 2008, 18(4): 711–717.
[39] Barchitta M, Quattrocchi A, Maugeri A, Vinciguerra M, Agodi A. LINE-1 hypomethylation in blood and tissue samples as an epigenetic marker for cancer risk: a systematic review and meta-analysis., 2014, 9(10): e109478.
[40] Rodić N, Sharma R, Sharma R, Zampella J, Dai LX, Taylor MS, Hruban RH, Iacobuzio-Donahue CA, Maitra A, Torbenson MS, Goggins M, Shih IM, Duffield AS, Montgomery EA, Gabrielson E, Netto GJ, Lotan TL, De Marzo AM, Westra W, Binder ZA, Orr BA, Gallia GL, Eberhart CG, Boeke JD, Harris CR, Burns KH. Long interspersed element-1 protein expression is a hallmark of many human cancers., 2014, 184(5): 1280– 1286.
[41] Lavasanifar A, Sharp CN, Korte EA, Yin T, Hosseinnejad K, Jortani SA. Long interspersed nuclear element-1 mobilization as a target in cancer diagnostics, prognostics and therapeutics., 2019, 493: 52–62.
[42] Hosseinnejad K, Yin T, Gaskins JT, Bailen JL, Jortani SA. Discovery of the long interspersed nuclear element-1 activation product [open reading frame-1 (ORF1) protein] in human blood., 2018, 487: 228–232.
[43] Gasior SL, Wakeman TP, Xu B, Deininger PL. The human LINE-1 retrotransposon creates DNA double-strand breaks., 2006, 357(5): 1383–1393.
[44] Alisch RS, Garcia-Perez JL, Muotri AR, Gage FH, Moran JV. Unconventional translation of mammalian LINE-1 retrotransposons., 2006, 20(2): 210–224.
[45] Gualtieri A, Andreola F, Sciamanna I, Sinibaldi-Vallebona P, Serafino A, Spadafora C. Increased expression and copy number amplification of LINE-1 and SINE B1 retrotransposable elements in murine mammary carcinoma progression., 2013, 4(11): 1882–1893.
[46] Sciamanna I, De Luca C, Spadafora C. The reverse transcriptase encoded by LINE-1 retrotransposons in the genesis, progression, and therapy of cancer., 2016, 4: 6.
[47] Sciamanna I, Landriscina M, Pittoggi C, Quirino M, Mearelli C, Beraldi R, Mattei E, Serafino A, Cassano A, Sinibaldi-Vallebona P, Garaci E, Barone C, Spadafora C. Inhibition of endogenous reverse transcriptase antagonizes human tumor growth., 2005, 24(24): 3923–3931.
[48] Oricchio E, Sciamanna I, Beraldi R, Tolstonog GV, Schumann GG, Spadafora C. Distinct roles for LINE-1 and HERV-K retroelements in cell proliferation, differentiation and tumor progression., 2007, 26(29): 4226–4233.
[49] Patnala R, Lee SH, Dahlstrom JE, Ohms S, Chen L, Dheen ST, Rangasamy D. Inhibition of LINE-1 retrotransposon- encoded reverse transcriptase modulates the expression of cell differentiation genes in breast cancer cells., 2014, 143(2): 239–253.
[50] Zhu YF, Feng F, Yu JY, Song B, Hu MM, Gao XD, Wang Y, Zhang Q. L1-ORF1p, a Smad4 interaction protein, promotes proliferation of HepG2 cells and tumorigenesis in mice., 2013, 32(9): 531–540.
[51] Hur K, Cejas P, Feliu J, Moreno-Rubio J, Burgos E, Boland CR, Goel A. Hypomethylation of long interspersed nuclear element-1 (LINE-1) leads to activation of proto- oncogenes in human colorectal cancer metastasis., 2014, 63(4): 635–646.
[52] Kuo KW, Sheu HM, Huang YS, Leung WC. Expression of transposon LINE-1 is relatively human-specific and function of the transcripts may be proliferation-essential., 1998, 253(3): 566–570.
[53] Banuelos-Sanchez G, Sanchez L, Benitez-Guijarro M, Sanchez-Carnerero V, Salvador-Palomeque C, Tristan- Ramos P, Benkaddour-Boumzaouad M, Morell S, Garcia- Puche JL, Heras SR, Franco-Montalban F, Tamayo JA, Garcia-Perez JL. Synthesis and characterization of specific reverse transcriptase inhibitors for mammalian LINE-1 retrotransposons., 2019, 26(8): 1095– 1109.e14.
[54] Bellisai C, Sciamanna I, Rovella P, Giovannini D, Baranzini M, Pugliese GM, Zeya Ansari MS, Milite C, Sinibaldi-Vallebona P, Cirilli R, Sbardella G, Pichierri P, Trisciuoglio D, Lavia P, Serafino A, Spadafora C. Reverse transcriptase inhibitors promote the remodelling of nuclear architecture and induce autophagy in prostate cancer cells., 2020, 478: 133–145.
[55] Houédé N, Pulido M, Mourey L, Joly F, Ferrero JM, Bellera C, Priou F, Lalet C, Laroche-Clary A, Raffin MC, Ichas F, Puech A, Piazza PV. A phase II trial evaluating the efficacy and safety of efavirenz in metastatic castration- resistant prostate cancer., 2014, 19(12): 1227– 1228.
[56] Carlini F, Ridolfi B, Molinari A, Parisi C, Bozzuto G, Toccacieli L, Formisano G, De Orsi D, Paradisi S, Grober OMV, Ravo M, Weisz A, Arcieri R, Vella S, Gaudi S. The reverse transcription inhibitor abacavir shows anticancer activity in prostate cancer cell lines., 2010, 5(12): e14221.
[57] Aschacher T, Sampl S, Käser L, Bernhard D, Spittler A, Holzmann K, Bergmann M. The combined use of known antiviral reverse transcriptase inhibitors AZT and DDI induce anticancer effects at low concentrations., 2012, 14(1): 44–53.
[58] Ogino S, Nosho K, Kirkner GJ, Kawasaki T, Chan AT, Schernhammer ES, Giovannucci EL, Fuchs CS. A cohort study of tumoral LINE-1 hypomethylation and prognosis in colon cancer., 2008, 100(23): 1734– 1738.
[59] Zhu J, Ling Y, Xu Y, Lu MZ, Liu YP, Zhang CS. Elevated expression of MDR1 associated with Line-1 hypomethylation in esophageal squamous cell carcinoma., 2015, 8(11): 14392–14400.
[60] Huang Y, Wei L, Sun AM, Zhao RC, Zhang J, Yang HT, Li B, Sun CJ, Ding XQ, Gao B, Zhong YQ, Qin Y. The evaluation value of methylation status of CpG island of SFRP1 and LINE1 gene promoter area in the prognosis of hepatocellular carcinom., 2016, 47(6): 883–888.
黄元, 魏玲, 孙爱民, 赵荣策, 张静, 杨含腾, 李波, 孙成均, 丁雪琴, 高波, 钟艳琴, 覃扬. SFRP1、LINE1基因启动子区CpG岛甲基化状态在肝细胞癌预后评估的价值. 四川大学学报(医学版). 2016, 47(6): 883–888.
[61] Wang CH, Meng FK, Fei SY, Chen M, Zheng LJ. The clinical significance of retrotransposon expression in glioma., 2020, 24(8): 1308–1310.
王春辉, 孟繁凯, 费邵阳, 陈莫, 郑林杰. 逆转座子LINE-1在脑胶质瘤中异常活化的临床意义. 中国实验诊断学, 2020, 24(8): 1308–1310.
[62] Harris CR, Normart R, Yang QF, Stevenson E, Haffty BG, Ganesan S, Cordon-Cardo C, Levine AJ, Tang LH. Association of nuclear localization of a long interspersed nuclear element-1 protein in breast tumors with poor prognostic outcomes., 2010, 1(2): 115–124.
[63] Chen L, Dahlstrom JE, Chandra A, Board P, Rangasamy D. Prognostic value of LINE-1 retrotransposon expression and its subcellular localization in breast cancer., 2012, 136(1): 129–142.
Research and application on LINE-1 in diagnosis and treatment of tumorigenesis
Yanni Kou, Shan Cen, Xiaoyu Li
Long interspersed element-1 (LINE-1), which is the only autonomous retrotransposon in human genome, makes up about 17% of the human genome. For LINE-1 retrotransposition may result in genome instability, it was strictly restricted by organisms, and its expression was therefore barely detected in normal somatic cells. However, the expression of LINE-1 is a common phenomenon in most tumor or cancer tissues, suggesting a close relationship between LINE-1 expression and cancer development. Differentially expressed LINE-1 in cancer tissues can be used as a biomarker for tumor diagnosis and an important indicator of prognosis after cancer therapeutics. Meanwhile, the feasibility of LINE-1 as a potential drug target for tumor treatment has also been evaluating and verifying in clinicals. In this review, we introduce the application of LINE-1 as a biomarker in tumor diagnosis and prognostic, as well as the research progress in LINE-1 as potential drug target for tumor treatment, in order to provide some references for clinical application in cancer treatment.
long interspersed element-1; tumor; diagnosis; treatment; prognosis
2021-02-10;
2021-05-07
国家科技重大专项(编号:2018ZX10301408-004)和国家自然科学基金(编号:31870164)资助[Supported by the National Science and Technology Major Project of the Ministry of Science and Technology of China (No. 2018ZX10301408-004) and the National Natural Science Foundation of China (No. 31870164)]
寇艳妮,在读硕士研究生,专业方向:LINE-1与肿瘤维持的内在机制研究。E-mail: yanni_1995@126.com
李晓宇,博士,研究员,研究方向:病毒学。E-mail: xiaoyulik@hotmail.com
岑山,博士,研究员,研究方向:病毒学。E-mail: shancen@imb.pumc.edu.cn
10.16288/j.yczz.21-062
2021-05-31 15:52:03
URI: https://kns.cnki.net/kcms/detail/11.1913.R.20210531.0836.002.html
(责任编委: 宋旭)

