Crystallization evolution and relaxation behavior of high entropy bulk metallic glasses using microalloying process
Danhong Li(李丹虹) Changyong Jiang(江昌勇) Hui Li(栗慧) and Mahander Pandey
1Changzhou Institute of Technology,Changzhou 213000,China
2Department of Materials Science and Metallurgical Engineering,Heydarabad 502258,India
Keywords: metallic glasses,disordered structures,amorphous materials,relaxation
1. Introduction
High entropy bulk metallic glasses (HEBMGs) are a new class of metallic alloys that simultaneously inherit the unique properties of crystalline high entropy alloys and attractive structural features of metallic glasses.[1–5]Compared to conventional BMGs, this novel metallic alloy has a more homogeneous atomic arrangement with large configuration entropy.[6–8]Moreover, the serration sizes in loaddisplacement curves,which are an indicator of shear band formation and propagation,are significantly smaller,showing the homogenous plasticity of HE-BMGs.[9,10]However,considering the same constituents, the HEBMGs possess a low glassforming ability (GFA) and marred thermal stability. Due to this fact, the design of HE-BMGs has been restricted to few chemical compositions. Therefore, the identification of the glass formation mechanism and relaxation behavior of HEBMGs is of great importance for engineering novel materials with varied alloying compositions and outstanding properties.There are several works focusing on the study of inherent features of HEBMGs.[11–15]Duanet al.[16]indicated that the shear modulus relaxation data could be employed to evaluate the effects of structural relaxation, glass transition, and crystallization in TiZrHfCuNiBe alloy. Guet al.[17]found that the relaxation enthalpy monotonically enhanced with the application of cryogenic cycling treatment. Yinet al.[18]investigated the atomic structure of GdTbCoAl alloy and found that the cryogenic environment would decrease the bond lengths of the large atom–small atom and small atom–small atom pairs,while large atom–large atom pairs unexpectedly enhanced.
In order to evaluate a septenary HEBMG,Wadaet al.[19]fabricated a ZrHfTiAlCoNiCu BMG system with excellent GFA, which makes the critical thickness of 18 mm possible. Tonget al.[20]discovered that the tiny defect concentration in the HEBMGs led to the small Newtonian viscosities compared to their conventional counterparts. Jinet al.[21]reported that the substitution of Cu by Ni in TiZrHfBeCu alloy could improve the GFA properties. However, it deteriorates the thermal stability and introduces undesired crystalline phases.Bizhanovaet al.[22]designed a near-equiatomic HEBMG with excellent GFA and revealed that the crystallization evolution shows both characteristics of traditional BMGs and equiatomic HEBMGs. In another study, it was unveiled that the low fragility in HEBMGs hampers micro-forming and thermoplastic formability.[23]The evaluation of physical properties and plasticity are other topics,which have been investigated by researchers.[24,25]
Although several works have investigated the inherent properties of HEBMGs,their crystallization evolution and relaxation behaviors are still research highlights in this field.The minor addition process is one of the main techniques providing novel BMGs with improved characteristics.[26,27]For example, Samavatianet al.[28]added different elements with negative heat of mixing into the Zr-Co-Al system and found that the minor addition improved the GFA of ZrCoAl BMGs; however, the level of improvement strongly relied on the atomic size of trace element. On the other hand,an added element with positive heat of mixing might also enhance the GFA in BMGs. It was revealed that Nb minor addition into the ZrCu-based metallic glass enhanced the GFA by strong interaction between Zr and Nb atoms causing shorter metallic bonds.[29,30]In another work, it was reported that the Sn minor addition into TiZrHfCuBe BMG led to the improvement of GFA and plasticity through the promotion of chemical heterogeneity.[7]Positive heat of mixing in Sn–Cu(Be)pairs is the main reason for the intensification of chemical heterogeneity in this system.Liet al.[31]also showed that the minor addition improved the mechanical properties of HEBMGs. Consequently,in this work,we try to show that how the minor addition of elements,including positive or negative heat of mixing,into a high entropy system alters the dynamic relaxation and crystallization evolution in a HEBMG system.
2. Materials and methods
The ingots with nominal chemical compositions of Zr20Cu20Ni20Ti20Hf20−x(Al,Nb)x=2were prepared by arc remelting process with high-purity elements under a Ti-gettered argon environment. As observed in Table 1, the Al and Nb elements were selected to induce negative and positive heat of mixing in the master alloying composition, respectively.The copper mold suction casting was then used to fabricate HEBMGs in the form of rods with 3 mm diameter and 4 cm length. X-ray diffraction (XRD) test was performed to ensure the amorphousness of samples. Using a differential scanning calorimeter (DSC), the thermal behavior of samples under a heating rate of 20 K/min and an argon atmosphere was evaluated. Applying the DMA Q800 TA instrument, the dynamic mechanical analysis (DMA) was carried out under a nitrogen atmosphere. In this test, the loss modulus (E'') and storage modulus(E')were measured in the temperature range of 350 K to 700 K at the frequency of 1 Hz and a constant heating rate of 3 K/min, where the sample was bent by the drive shaft. Moreover,a frequency range of 1 Hz to 16 Hz was applied to obtain activation energy of relaxation in HEBMGs. In the current test, BMG samples were provided by electric discharge machining with dimensions of 30 mm×2 mm×1 mm from the reference rod. To measure the viscosity changes in the super-cooled liquid region,a thermal mechanical analyzer(TMA)under purified nitrogen, a constant load of 5 N,and a heating rate 10 K/min was performed. It should be noted that the diameter and length of samples in the TMA test were 3 mm and 6 mm,respectively.
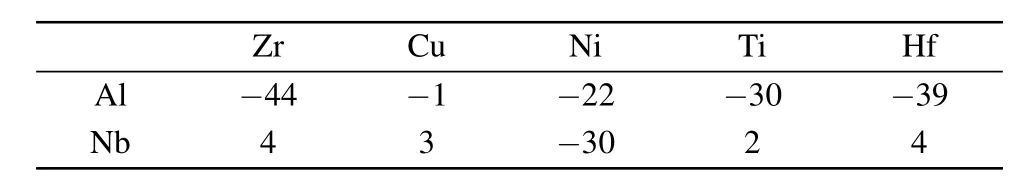
Table 1.The heat of mixing(KJ/mol)for Al,Nb–X pairs in the alloying compositions.
3. Results and discussion
3.1. Primary characterizations
The initial step in our work is to justify that the microalloying process does not alter the microstructural features of HEBMGs. As given in Fig. 1(a), the XRD patterns show a diffuse scattering trend with a broad peak in the range of 2θ=37◦–40◦for all samples. Hence, it can be concluded that the structure remains amorphous after a minor addition.Figure 1(b) shows the DSC curves for all the samples under a heating rate of 20 K/min in the temperature range of 500–900 K. According to thermal properties, it was detected that the microalloying process changed the crystallization behavior in the amorphous system. Al element with negative heat of mixing altered the crystallization evolution so that the low-temperature crystallization peak was intensified,while Nb addition with positive heat of mixing strengthened the hightemperature crystallization peak and led to the appearance of a third crystallization peak at 827 K in the DSC curves. In the following, the crystallization behaviors will be discussed in detail. As given in Table 2,it is observed that the minor addition leads to improvement of thermal stability in the HEBMGs. Although the crystallization peaks shift to the higher temperatures, the glass transition temperature shows a slight decrease, which is an indicator of thermal stability in the designed HEBMGs.
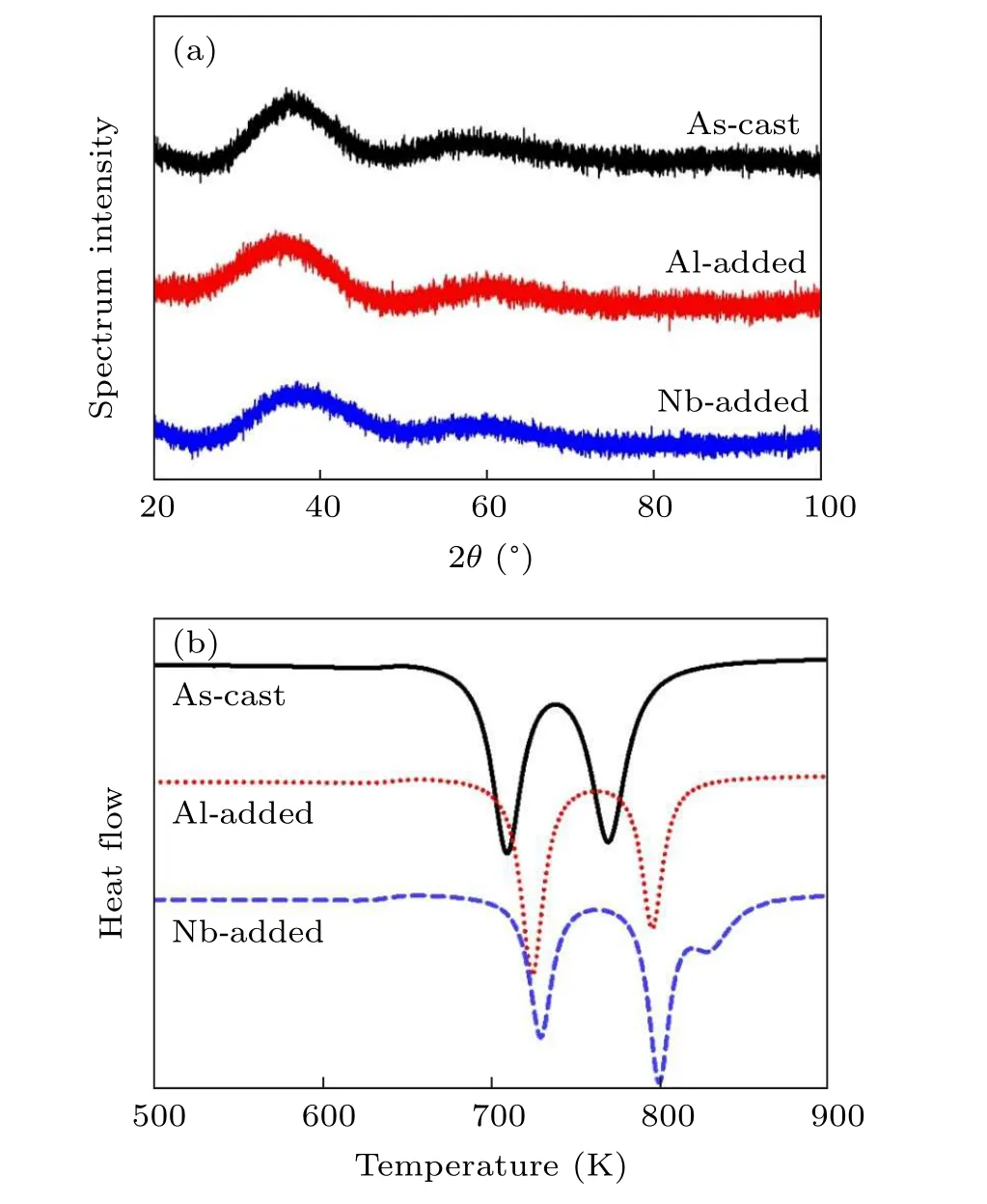
Fig. 1. (a) XRD patterns and (b) DSC curves for HEBMG samples.
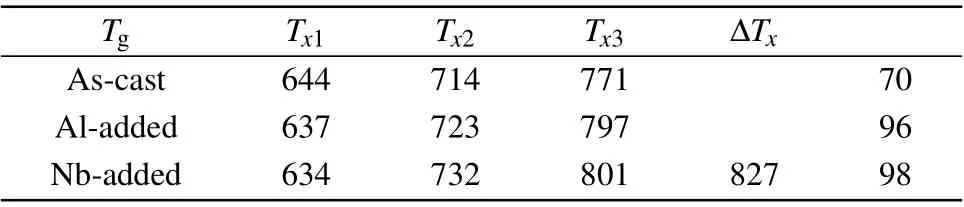
Table 2. Thermal characteristics extracted from DSC curves.
3.2. Structural relaxation features
Under a driving frequency of 1 Hz and a heating rate of 3 K/min, the DMA analysis was carried out, and the relation between energy and temperature was obtained. Figure 2(a)indicates loss modulus variations of the HEBMG as a function of temperature. The hightemperature peak is related toαrelaxation, while the lowtemperature wing, adjacent toαrelaxation, indicates slowβrelaxation. It should be noted that there are limited chemical compositions showing distinctiveβrelaxation in their thermal spectra,[32]while a huge number of glassy compositions show a slight wing as the lowtemperature relaxation. To separate the relaxation events, we used a de-convoluting technique in the MATLAB environment based on peak positions and energy distribution(see Fig.2(a)).As illustrated in Figs.2(b)–2(c),the minor addition leads to a significant change in the relaxation intensification and the corresponding temperature. When Al or Nb elements are added to the system, the maximum temperatures ofα(i.e.,Tα) andβ(i.e.,Tβ) relaxation events shift to the lower temperatures.TheTα/Tβratio also increases in minor added samples,which shows the significant effects ofβrelaxation. In other words,theβrelaxation has sharper thermal changes compared toαevent. Moreover,it is observed that the peak ofβrelaxation is intensified after the microalloying process,while there are no significant changes in the peak intensification ofαrelaxation.The results also reveal that the Nb addition with positive heat of mixing has a more pronouncing effect on the relaxation behavior of HEBMG.In general,αrelaxation is correlated to the dynamics of the supercooled liquid and the quench-in defects,whileβrelaxation energy is consistent with the nanoscale source of dynamics in the system and induces partial devitrification and deformation.[33]Moreover,βrelaxation is principally due to the localized cooperative movement of atoms in the loosely packed structures.[34]Hence, it can be concluded thatβrelaxation determines the level of structural heterogeneity and potential plastic deformation in the glassy alloys.[35]According to our results,the microalloying process intensifies the structural heterogeneity in the material. In other words,the added element is able to enhance the variety of clusters at shortrange order (SRO) and consequently manifold mediumrange order(MRO)arrangements in the atomic system.[36]This event enhances the structural confusion in the system and induces heterogeneity through atomic rearrangement.[2]On the other hand,the minor addition with positive heat of mixing provides more structural heterogeneity in the material. However,crystallization may have priority when the added element reaches a certain value.[37]In our case,the Nb addition is low enough to induce any crystallization in the microstructure. It is suggested that the Nb atoms are able to improve structural heterogeneity in the glassy systems,as that occurred under Al addition. However, the positive heat of mixing in the system also provides conditions for nanoscale glassy phase separation in the material.[38]Hence, the structural heterogeneity is more intensified andβrelaxation is more strengthened in the thermal curves. In summary,it is concluded that a certain amount of minor addition with positive heat of mixing is more effective for inducing structural heterogeneity in HEBMGs.For some BMG compositions with pronouncedβrelaxation such as La-based alloys,it was derived that the large negative heat of mixing in the system, as a chemical effect, leads to the intensification ofβrelaxation peaks in the dynamic loss spectra, while microalloying with large positive heat of mixing weakens theβrelaxation and changes theβpeak to a wing trend.[39]This result is inconsistent with studies on typical blendedα–βrelaxation spectra of BMGs, reporting that the minor addition with positive heat of mixing is able to improve the possible structural heterogeneity and plasticity behavior in the BMGs.[40]This event is due to the fact that the structural heterogeneity in a blendedα–βrelaxation BMGs is not maximum,and consequently,a modification in the alloying composition may induce the extra defects in the microstructure leading toβpeak intensification in the spectrum.
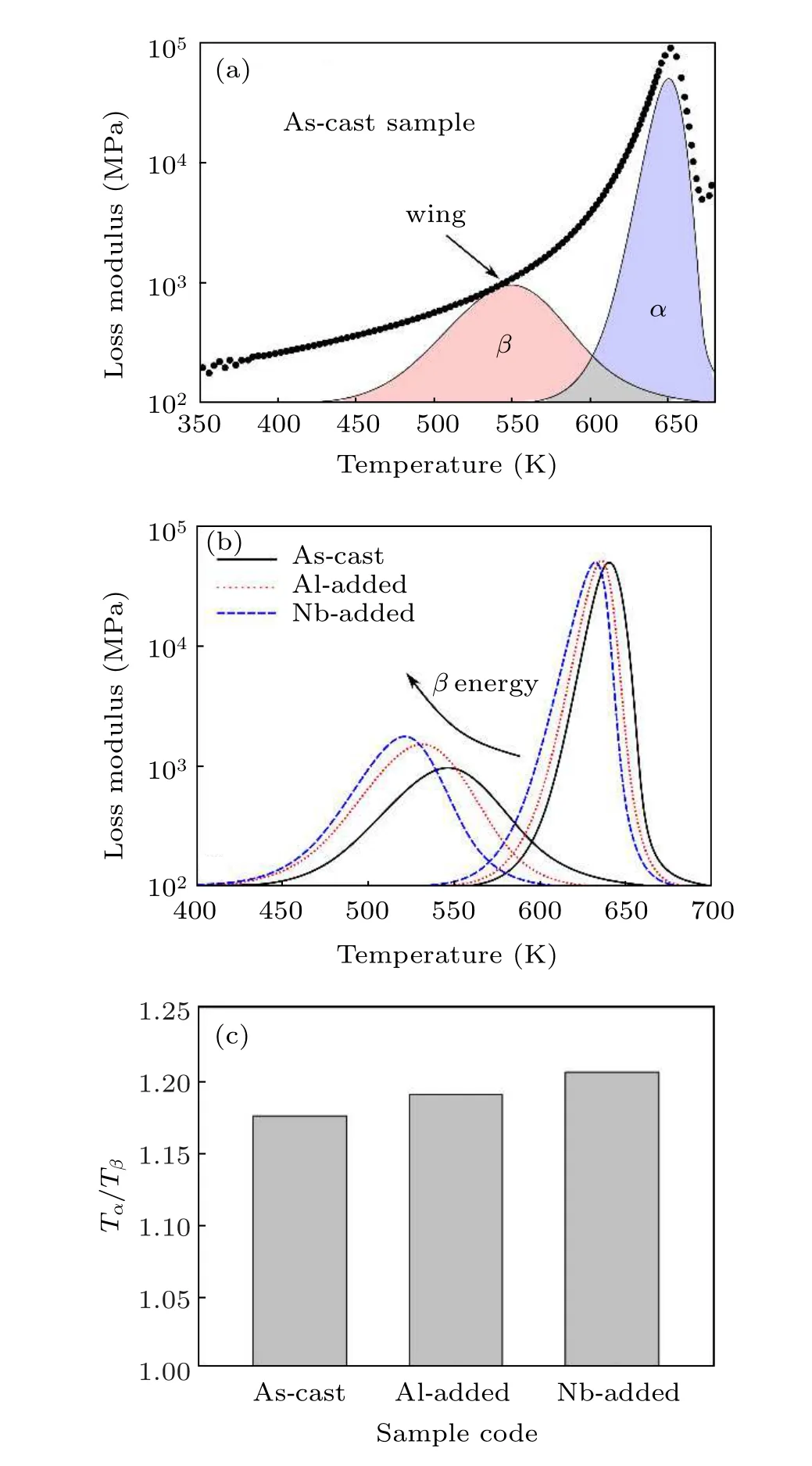
Fig.2. (a)DMA curves of the as-cast sample,(b)de-convoluted relaxation curves of samples extracted from DMA spectra, (c) Tα/Tβ ratio for the samples.
As given in Fig.3,the dynamic behavior of HEBMGs was studied under different applied frequencies, while the inset shows the fitted Arrhenius association of frequency and temperature related toβrelaxation. In general,atomic mobility is mainly dominated by thermal activation,which is the physical origin of the Arrhenius-type law.[41]It is believed that Arrhenius temperature dependence can be seen when dynamics are dominated by thermal fluctuations to prevail over the barrier.According to the results in Fig.3,the increase in applied frequency leads to the rise in the peak temperatures. The fitting of ln(f)–1/Tβcurves and their corresponding slope define the activation energy ofβrelaxation in the glassy system. According to the results,the activation energy is measured for the free-microalloying, Al-containing, and Nb-containing samples about 89±2 kJ/mol,107±3 kJ/mol,and 12±3 kJ/mol,respectively. This indicates that the HEBMG with a minor Nb addition has high structural heterogeneity. In other words,the nanoscale separation is increased in the system,and the atomic rearrangement is accompanied by the annihilation of ordered configurations in the structure.
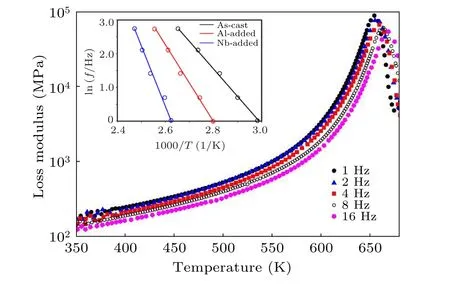
Fig.3. DMA curves of as-cast sample in different applied frequencies.Inset shows Arrhenius plots of ln(f)vs. 1000/T.
3.3. Crystallization evolution
Using thermal mechanical analysis(TMA),it is possible to evaluate the viscosity behavior of metallic glasses under the applied load. In the temperature range of the supercooled region,the crystallization evolution of BMG structures appears,which is due to their individual thermoplastic forming features. Figure 4 shows the viscosity behavior of HEBMGs as a function of temperature in the supercooled region. To obtain the viscosity changes, the sample height variation under the continuous loading was measured. According to the Stephen equation,the viscosity is attained[38]
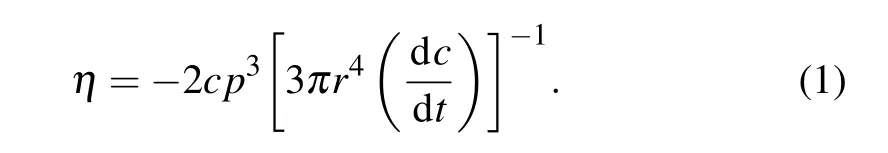
Herepdefines the applied load,randcintroduce the radius and height of the sample,respectively. As observed in Fig.4,with the temperature increasing to 700 K,the viscosity slightly decreases in all the samples, which is due to the inherent nature of glassy alloys. However, in the range of supercooled liquid region,the viscosity trend changes for each sample,owing to their crystallization behavior. For the as-cast sample,two peaks with significant viscosity alterations were detected,which are consistent with the crystallization peak temperatures in the DSC curves.

Fig.4. Viscosity curves of samples as a function of temperature in the range of the supercooled liquid region.
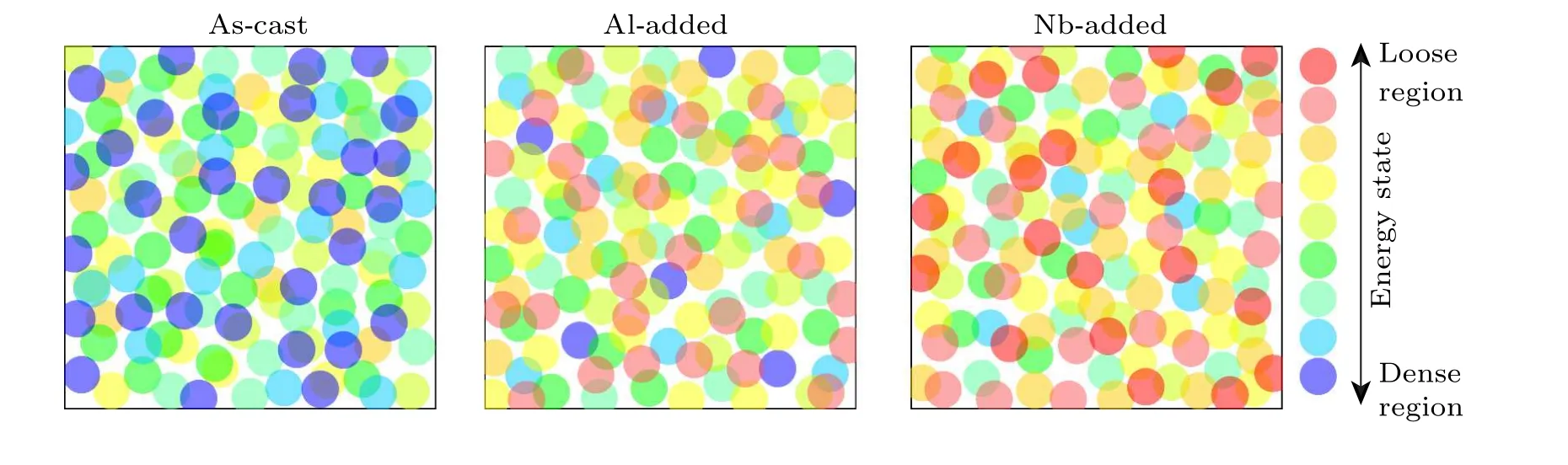
Fig.5. Schematic of energy states in HEBMG samples showing the structural heterogeneity.
The viscosities of 9.22×109Pa·s and 9.95×109Pa·s at the peaks of crystallization events show that the crystallization stages in the supercooled liquid region behave in the same trend and their energy release is at the same level. In other words, the portion of crystalline phase formation is generically and quantitatively identical in each crystallization stage.On the other side, the Al microalloying process led to the change of crystallization evolution. As observed, the Al minor addition changes the atomic structure somehow to induce the lowtemperature crystallization stage in the material. This means that the compositional heterogeneity under Al minor addition provides potential sites with lower energy barriers for crystallization at lower temperatures(see Fig.4). As schematically given in Fig. 5, Al minor addition increases the potential sites with higher energy states, i.e., free volumes, and induces more severe structural heterogeneity compared with the as-cast sample. On the other side,Nb microalloying not only intensifies the second peak but also creates a third one at the higher temperatures. According to the results, the Nb addition is accompanied by crystallization events with more varied energy levels in the structure. The intensified crystallization peaks at higher temperatures indicate that the structural change caused by Nb addition is significantly different from what happened under Al addition. In other words, the types of atomic arrangement, including SRO and MRO, are considerably affected by the certain added element. Moreover, one can see that the Nb minor addition manifold the crystallization stages in the supercooled liquid region. This means that the structural heterogeneity in this alloying composition is intensified,and consequently, several energy-level sites are provided for crystallization. In summary,one can see that the type of added element significantly affects the crystallization evolution and structural relaxation in HEBMGs, which is closely related to the change in the structural heterogeneity.
4. Conclusion
This study shows the effects of minor addition on the crystallization evolution and relaxation features of Zr20Cu20Ni20Ti20Hf20HEBMG. Al and Nb elements were separately added to the base alloying compositions to affect the heat of mixing in the master alloy negatively and positively,respectively. The results indicate that the added elements lead to a slight intensification ofβrelaxation. However, there is no obvious change inαrelaxation. The detailed DMA analysis also determines that the activation energy ofβrelaxation increases during the microalloying process,and this enhancement is optimum in the Nb-added sample with sharp structural heterogeneity. By evaluating the crystallization behavior,it is found that the microalloying process changes the crystallization enthalpy in the samples. For Nb added one,the two-peak crystallization curve changes to a three distinct peaks curve in the supercooled liquid region, which is due to the multiple energy states in the heterogeneous structure of Nb-added HEBMG.
- Chinese Physics B的其它文章
- Quantum computation and simulation with vibrational modes of trapped ions
- ℋ∞state estimation for Markov jump neural networks with transition probabilities subject to the persistent dwell-time switching rule∗
- Effect of symmetrical frequency chirp on pair production∗
- Entanglement properties of GHZ and W superposition state and its decayed states∗
- Lie transformation on shortcut to adiabaticity in parametric driving quantum systems∗
- Controlled quantum teleportation of an unknown single-qutrit state in noisy channels with memory∗

