Hepatocellular carcinoma in viral and autoimmune liver diseases: Role of CD4 + CD25 + Foxp3 + regulatory T cells in the immune microenvironment
Alessandro Granito, Luigi Muratori, Claudine Lalanne, Chiara Quarneti, Silvia Ferri, Marcello Guidi, Marco Lenzi, Paolo Muratori
Abstract More than 90 % of cases of hepatocellular carcinoma (HCC) occurs in patients with cirrhosis, of which hepatitis B virus and hepatitis C virus are the leading causes,while the tumor less frequently arises in autoimmune liver diseases. Advances in understanding tumor immunity have led to a major shift in the treatment of HCC,with the emergence of immunotherapy where therapeutic agents are used to target immune cells rather than cancer cells. Regulatory T cells (Tregs) are the most abundant suppressive cells in the tumor microenvironment and their presence has been correlated with tumor progression, invasiveness, as well as metastasis. Tregs are characterized by the expression of the transcription factor Foxp3 and various mechanisms ranging from cell-to-cell contact to secretion of inhibitory molecules have been implicated in their function. Notably, Tregs amply express checkpoint molecules such as cytotoxic T lymphocyte-associated antigen 4 and programmed cell-death 1 receptor and therefore represent a direct target of immune checkpoint inhibitor (ICI) immunotherapy. Taking into consideration the critical role of Tregs in maintenance of immune homeostasis as well as avoidance of autoimmunity, it is plausible that targeting of Tregs by ICI immunotherapy results in the development of immune-related adverse events (irAEs). Since the use of ICI becomes common in oncology, with an increasing number of new ICI currently under clinical trials for cancer treatment, the occurrence of irAEs is expected to dramatically rise. Herein, we review the current literature focusing on the role of Tregs in HCC evolution taking into account their opposite etiological function in viral and autoimmune chronic liver disease, and we discuss their involvement in irAEs due to the new immunotherapies.
Key Words: Autoimmune liver disease; Hepatitis B virus-related chronic hepatitis;Hepatitis C virus-related chronic hepatitis; Hepatocellular carcinoma; Tumor microenvironment
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third most frequent cause of cancer-related death worldwide, with more than 900000 new cases and more than 800000 deaths in 2020 [1 ].
HCC accounts for nearly 90 % of primary liver cancers and is a leading world health problem. The incidence of HCC rises sharply with age in all populations, achieving a peak at age 70 and it is increasing in most countries representing the dominant cause of mortality in cirrhotic patients[2 -4 ].
Worldwide, chronic viral hepatitis has been reported as the leading risk factors for HCC development, although in high-income areas HCC related to non-alcoholic fatty liver disease is increasing due to the rising prevalence of metabolic disorders[5 -7 ].
In contrast, vaccination and treatment for hepatitis B virus (HBV) infection,prevention campaigns for sexual and iatrogenic transmission of hepatitis B (HBV) and hepatitis C virus (HCV), and the introduction of effective HCV antiviral agents are reducing the burden of chronic viral liver disease[8 -11 ].
HCC arises almost exclusively in the setting of chronic liver inflammation and,unlike the elevated risk associated with chronic viral (HBV and HCV) infections, it has been reported that the tumor is less common in liver cirrhosis caused by autoimmune liver diseases[12 -15 ]. However, it is known that, regardless of etiology, cirrhosisper serepresents a precancerous condition leading to an increased risk of HCC[15 ,16 ].
Indeed, irrespective of etiology, a typical sequence of chronic necroinflammation,compensatory liver regeneration, induction of liver fibrosis and subsequent cirrhosis often precedes hepatocarcinogenesis. HCC is a prototypical inflammation-driven tumor arising on the backdrop of liver cirrhosis. The evidence of an immune-rich contexture of the HCC microenvironment has inspired several studies in recent years that have further defined profile and crucial pathogenetic role of immune cells in tumor development[17 ].
The liver is a central immunomodulator that ensures organ and systemic protection while maintaining immunotolerance. Deregulation of this tightly controlled liver immunological network is a hallmark of chronic liver disease and HCC[18 ].
Recently, within the cell subset characterizing the HCC immune microenvironment,a key role has been highlighted for CD4 + CD25 + regulatory T cells (Tregs), which are crucially implicated in both pathogenesis of chronic liver diseases and development and spread of HCC[19 ,20 ].
In this review we examine the evidence that has recently accumulated on the different role that these cells play in chronic viral liver diseases (HBV and HCV) and autoimmune liver diseases, and their function in the tumor microenvironment (TME)characterizing HCC. We also discuss the possible implications for emerging immunotherapies and the potential risks of immune-mediated liver toxicity from this treatment[21 ].
HCC RISK IN VIRAL AND AUTOIMMUNE LIVER DISEASES
It is well established that cirrhosis represents the most significant risk factor for the development of HCC[22 ]. Historically, it has been reported that the risk of HCC in cirrhosis due to viral causes is higher than in other non-viral etiologies, however a precise comparison of the incidence of HCC in various chronic liver diseases,especially in cirrhosis, has only recently been evaluated[23 ,24 ].
In a recent meta-analysis it was shown that the annual incidence of HCC in chronic liver diseases and the ratio of HCC incidence in non-cirrhotic/cirrhotic stages has the following etiological hierarchy: HCV-related disease 0 .68 % to 4 .81 % (7 .07 -fold,P<0 .001 ), HBV-related liver disease 0 .37 % to 3 .23 % (8 .73 fold, P < 0 .001 ), primary biliary cholangitis (PBC) (pre-cirrhoticvsScheuer’s III-IV stage) 0 .26 to 1 .79 % (6 .88 -fold,P<0 .001 ), NASH 0 .03 % to 1 .35 % (45 -fold, P < 0 .001 ), autoimmune hepatitis (AIH) 0 .19 % to 0 .53 % (2 .79 -fold, P = 0 .03 ), and that the incidence of HCC is markedly increased (2 .79 -fold to 45 -fold) in the cirrhotic stage compared with the non-cirrhotic stage, regardless of etiology[24 ]. Thus, it is confirmed that there is a significant difference in HCC incidence between viral and autoimmune liver diseases, with the lowest risk in the latter even when in the cirrhotic stage.
Worldwide, about 54 % of cases can be ascribed to HBV infection (affecting 400 million people globally) while 31 % can be associated with HCV infection (affecting 170 million people), leaving about 15 % attributable to other causes[25 ].
Incidence of HCC in autoimmune liver disease is less definitively established. In a recent systematic review of 25 published cohorts, a total of 6 .528 AIH patients with a median follow-up of 8 years were evaluated for the incidence of HCC. The pooled incidence rate was 3 .1 per 1 .000 person-years in AIH patients that tripled in those with cirrhosis[26 ,27 ].
PBC-related cirrhosis has also been reported as a potential HCC risk factor. In a study of 273 PBC-related cirrhotic patients, followed for 3 years, the incidence rate was 5 .9 %, significantly higher in males with stage III/IV disease than in females[28 ].
In a systematic review of 17 studies, including 16 .368 patients seen between 1984 and 2011 , compared with the general population, PBC patients exhibited a significantly higher risk of HCC (pooled risk ratio 18 .80 ; 95 % confidence interval:10 .81 -26 .79 )[29 ].
Factors associated with an increased risk of HCC development in chronic liver disease have been only partially defined. However, chronic inflammation has been reported as a crucial mechanism for the development of HCC[30 -32 ].
In this respect, it has been reported that in HBV and HCV cirrhotic patients, transaminase serum level is one of the predictive factors for the development of HCC[32 ].Similar findings have been reported in AIH as persistent elevation of serum transaminases was reported to be associated with development of HCC hence supporting the prominent pathogenic role of chronic inflammation. Other emerged risk factors included cirrhosis ≥ 10 years, portal hypertension, and immunosuppressive therapy ≥3 years[33 ].
The risk factors of HCC usually lead to a unresolving inflammatory response and necrosis resulting in tissue damage which in turn drives the sequential development of regeneration, fibrosis, cirrhosis, and eventually HCC[34 ,35 ]. In parallel, immune cells within the premalignant environment produce a wide range of cytokines, growth factors, chemokines, prostaglandins, and proangiogenic factors, contributing to an environment that supports hepatocyte transformation and promotes their survival through activation of anti-apoptotic pathways, neoangiogenesis and inhibition of immune surveillance[36 ].
It has been recently established that the carcinogenic process is aided by a host of immuno-related factors intrinsically linked to cell infiltrate, chemokines and their receptors that foster cell survival and proliferation[37 ].
In this regard, it has increasingly gained relevance to fully define the immunological characteristics of liver immune microenvironment.
TREGS IN VIRAL AND AUTOIMMUNE LIVER DISEASES
The liver can be considered as an “immunological” organ, housing a wide range of resident immune cells performing key functions in preserving organ homeostasis[38 ].
As a result of its intrinsic role in detoxification, the liver is repeatedly exposed to external agents, including dietary products or commensal bacteria derived from the intestineviathe portal vein, as well as infectious microorganisms arising from the systemic circulationviathe arterial vein. Therefore, immune surveillance in the organ is extremely dynamic.
Resident innate immune cells comprising macrophages or Kupffer cells, natural killer (NK) cells, NKT cells, and dendritic cells (DCs) are recognized as the most predominant sentinels in the liver[39 ]. In addition, tissue resident memory T cells,which are normally homing cells without recirculating and which readily attack pathogens at the site of infection, are also implicated[40 ]. However, a key role appears to be played by resident Tregs that are highly specialized in preserving tissue tolerance[19 ,41 ]. Tregs are a subset of T lymphocytes that regulate the immune response by suppressing the proliferation and cytokines production of effector T lymphocytes[42 ,43 ].
In 2003 , the forkhead box transcription factor foxp3 was identified as a specific marker of Tregs, and its expression was found crucial for their suppressive activity[44 -46 ].
Tregs arise in the thymus, constitutively express high levels of the interleukin (IL)-2 receptor (IL-2 R) α chain (CD25 ), cytotoxic T lymphocyte-associated antigen 4 (CTLA-4 ), and glucocorticoid-induced TNF receptor family-related gene (GITR), accounting for 5 % to 10 % of peripheral CD4 + T cells[47 ,48 ].
They play a critical role in mediating immunological self-tolerance by suppressing self-reactive T lymphocytes[44 ]. Tregs have been proposed to operate both through core mechanisms of suppression, including IL-2 deprivation and CTLA-4 –mediated downregulation of costimulatory molecules on antigen presenting cells (APCs), and by diverse context-dependent mechanisms, including the secretion of cytokines[49 -52 ].Earlier studies have confirmed that Tregs mediate suppressive effectsin vivomainly through the production of inhibitory cytokines such as IL-10 , IL-35 and transforming growth factor β (TGF-β)[53 -55 ].
With the discovery of Tregs and the understanding of their immunosuppressive role, evidences have been accumulated that this cell population is decisively implicated in the pathogenesis of various conditions such as chronic viral and autoimmune liver diseases as well as HCC[56 ].
In particular, CD4 +CD25 + Tregs are thought to contribute to the impaired immune response during chronic HBV and HCV infection. Patients with chronic HBV infection are characterized by increased percentage of CD4 +CD25 + Tregs in their peripheral blood and a significant accumulation of these cells in the liver, with a positive correlation between their frequency and serum HBV DNA load[57 -60 ]. Similarly, in patients with persistent HCV infection it has been reported an increased frequency of CD4 +CD25 + Tregs in the blood and in the liver[61 -65 ]. Taken together, these data prove that chronic HBV and HCV infections are immunologically characterized by a host immune response suppression driven by Tregs.
On the contrary, autoimmune liver diseases are related to both numerical and functional defect of CD4 +CD25 + Tregs, to the extent that therapeutic interventions aimed at restoring an adequate number and function of these cells are followed by a remission of the autoimmune inflammatory activity[66 ,67 ].
These findings have thus outlined a pattern of chronic inflammation characterized by a diametrically opposed liver immune phenotype in chronic viral and autoimmune diseases, the former being characterized by a predominance of Tregs exerting an immunosuppressive effect that hinders the antiviral response and infection eradication, the latter by a significant numerical and functional deficiency of Tregs that do not adequately suppress self-reactive lymphocytes[68 -70 ].
While it has been widely reported that TME characterizing HCC during chronic viral liver disease is dominated by a marked Treg infiltration likely in continuity with the conditions favoring chronic infection, the expression pattern of Tregs in the TME supporting HCC development in autoimmune liver disease is not as well known[71 ,72 ].
This finding would be relevant for the assessment of potential adverse events related to emerging immunotherapies that cause a decline in Treg number and function and are therefore associated with the risk of triggering autoimmune disorders, as we discuss below.
IMMUNE TME IN HCC: ROLE OF CD4 +CD25 + TREGS
Tumor infiltrating lymphocytes represent the host immune response to cancer and comprise CD8 + cytotoxic T lymphocytes (CTLs) and NK cells as favorable anti-tumor responders, and CD4 + CD25 + Tregs as immunosuppressors.
Recently, many studies have shown that the TME plays a major role in HCC initiation and progression[73 ]. Lymphocytes contribute to the TME through immunity and inflammation. CD8 + CTLs can directly kill target cells by releasing granules including membrane-lytic materials such as perforin and granzymes (granzyme A and B) in acquired immune responses, thus covering a crucial role in anti-tumor immunity.As a matter of fact, a large presence of CD8 + CTL infiltrating tumor tissue is closely associated with a better prognosis[74 ,75 ]. However, despite T cell infiltration, HCC develops and spreads as a result of a depletion of pro-inflammatory T cells and a significant accumulation of Tregs[76 -78 ].
T-cell exhaustion is characterized by reduced responses to stimulation, impaired cytokine production, decreased proliferation and reduced toxicity. Such immune profile is hallmarked by over-expression of co-inhibitory receptors such as CTLA-4 and programmed cell-death 1 receptor (PD-1 ).
An exhausted state of circulating and intratumoral CD8 + T cells is associated with a worse prognosis in HCC patients[79 ,80 ].
Exhaustion inside the TME is dominated by the inhibitory cytokine environment rich in IL-10 and TGF-β released by the Treg, both prohibiting CTLs and TH1 CD4 + T cells activation[81 ,82 ].
In addition, tumor associated neutrophils (by secreting CCL17 and CCR4 ) and M2 tumor-associated macrophages (by secreting IL-10 ) can induce CD4 + CD25 + Tregs thereby indirectly supporting tumor growth and progression[83 ,84 ] (Figure 1 ).
TARGETING CD4 +CD25 + TREGS IN HCC MICROENVIRONMENT WITH IMMUNOTHERAPY
A significant advance in the field of immunotherapy has now reached with the use of immune checkpoint inhibitors (ICIs), which are antagonistic antibodies that inhibit key immune regulatory molecules (checkpoint molecules), such as CTLA-4 , PD-1 , and its ligand PD-L1 , which suppresses T cell effector function under physiological conditions[85 ].
Tregs are among the most prevalent suppressor cells in TME and their presence has been related to tumor progression, invasiveness, and metastasis. Their regulatory function involves a broad spectrum of immune cells besides T cells, including macrophages, DCs, neutrophils, NK cells, T cells, and innate lymphoid cells[86 -88 ].
There are many factors favoring Treg enrichment in the TME. Experimental evidences implicate the Treg recruitment within the tumor mass through chemokines produced by cancer cells and, specifically, HCC cells have been shown to secrete CCL5 , CCL22 and CCL28 chemokines mediating Treg accumulation (Table 1 )[89 -100 ].

Table 1 Regulatory T cell function and recruitment in hepatocellular carcinoma
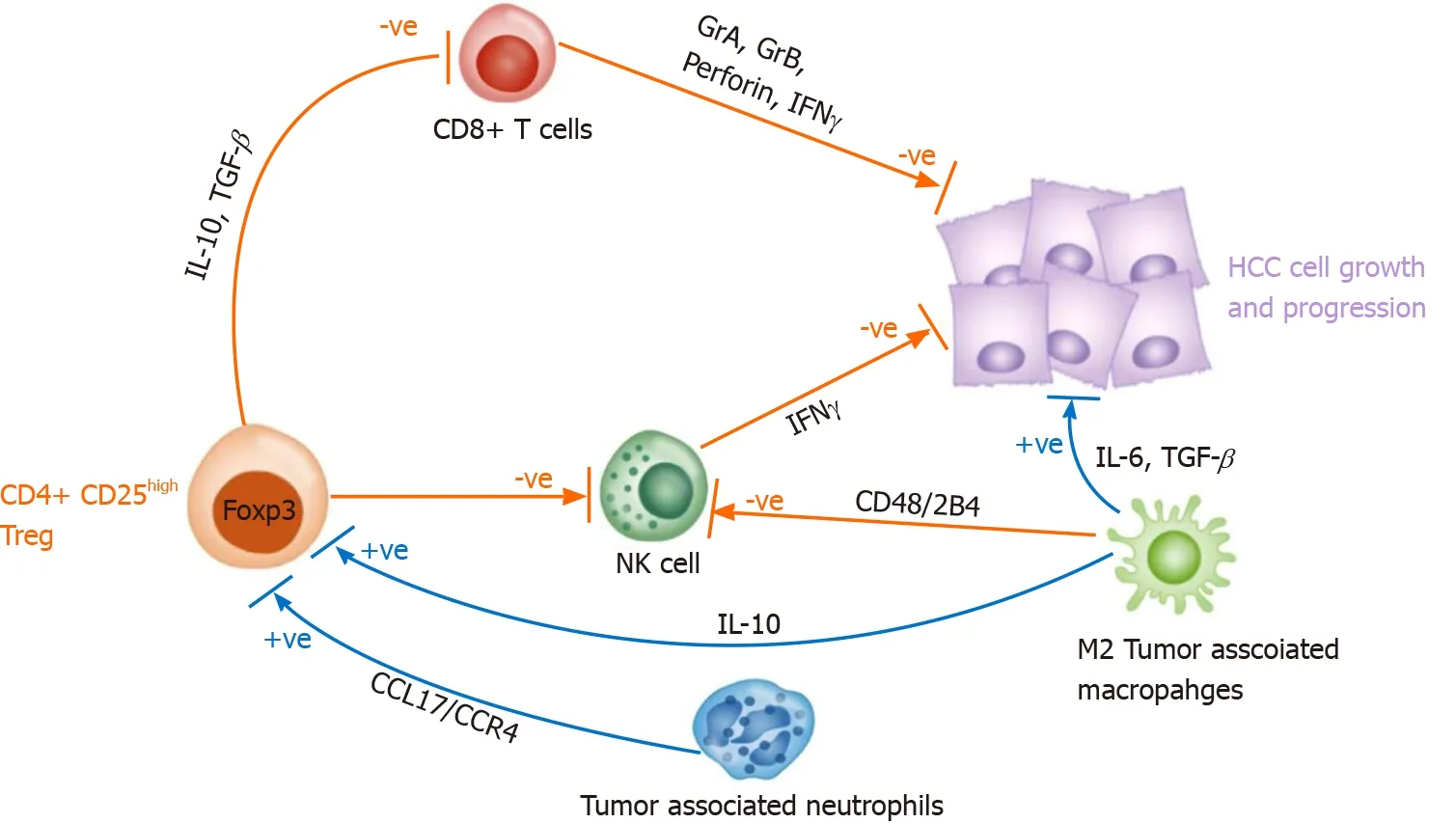
Figure 1 The hepatocellular carcinoma liver microenvironment is characterized by a large population of immune cells. Natural killer and CD8 + T cells exert antitumor effect by secreting interferon-γ, granzyme A, granzyme B and perforin, however they are mostly defective since these cells are suppressed by CD4 + CD25 regulatory T cell (Tregs). Additionally, tumor associated neutrophils (by secreting CCL17 and CCR4 ) and M2 tumor associated macrophages (by secreting interleukin-10 ) can induce CD4 + CD25 + Tregs thus supporting tumor growth and progression. NK: Natural killer; Treg: Regulatory T cell;IL: Interleukin; TGF-β: Transforming growth factor β; IFN: Interferon; HCC: Hepatocellular carcinoma.
The mechanisms through which Tregs induce suppression of proliferation,activation and function of immune effector cells have been well studied. Firstly, they modulate the activity of APCs by engaging inhibitory co-stimulatory receptors on their surface and in this way, signaling between APCs and T cells is impaired or abolished[101 ,102 ].
On a similar line, they down-regulate the expression of CD40 , CD80 and CD86 on DCs[103 ]. Second, Tregs, through the secretion of inhibitory cytokines (e.g., IL-10 , IL-35 , TGF-β), repress the activity of immune cells[104 ].
Of major interest, Tregs express a panel of chemokine receptors and surface molecules such as CTLA4 , PD-1 and others, thus potentially making them a very direct target of ICI immunotherapy (Figure 2 ).

Figure 2 Regulatory T cells are recognized as CD4 +CD25 high positive and Foxp3 + expressing cells. They feature a range of other phenotypic markers such as T-cell immunoreceptor with Ig and ITIM domains, glucocorticoid-induced tumor necrosis factor receptor-related protein, cytotoxic T lymphocyteassociated antigen 4 , programmed cell-death 1 receptor, V-domain Ig suppressor of T cell activation, lymphocyte activation gene-3 , T cell immunoglobulin mucin 3 .Upon activation, Tregs release the inhibitory cytokines interleukin (IL)-10 , transforming growth factor β and IL-35 . TCR: T-cell receptor; IL: Interleukin; TGF-β:Transforming growth factor β; TIGIT: T-cell immunoreceptor with Ig and ITIM domains; VISTA: V-domain Ig suppressor of T cell activation; LAG3 : Lymphocyte activation gene-3 ; GITR: Glucocorticoid-induced tumor necrosis factor receptor-related protein; TIM3 : T cell immunoglobulin mucin 3 ; CTLA-4 : Cytotoxic T lymphocyte-associated antigen 4 ; PD-1 : Programmed cell-death 1 receptor.
Advances in understanding tumor immunity have resulted in a significant shift in the HCC treatment, with the emergence of immunotherapy where therapeutic interventions are used to target immune cells rather than cancer cells.
Tregs abundantly express both co-inhibitory and co-stimulatory molecules at levels that are likely dependent on the TME. Modulating their function through stimulation of inhibitory receptors and inhibition of activating receptors could therefore result in a decrease of the TME immunosuppressive profile resulting ultimately in an enhanced antitumor immune response (Figure 3 ).
In support of this therapeutic approach, treatment with blocking antibodies for PD-1 (nivolumab, pembrolizumab, sintilimab, penpulimab, camrelizumab, toripalimab,spartalizumab, tislelizumab), programmed death ligand 1 (durvalumab, avelumab,atezolizumab), and CTLA-4 (tremelimumab, ipilimumab) have reported promising results in HCC treatment[105 ].
Since CTLA-4 is constitutively expressed on Tregs, its specific deletion is associated with a marked reduction of their suppressive ability potentially resulting in a severe T cell mediated autoimmune disease[106 ].
Resulting in a break of balance in the immune system, ICI treatments can give rise to a broad spectrum of serious autoimmune manifestations, including liver injury,reported in recent studies as immune-related adverse events (irAEs) and potentially limiting or precluding HCC treatments[107 ,108 ].
Unfortunately, clinical studies that include in their objectives the assessment of immune cell populations, particularly Tregs, are still too scarce, thus leaving this potentially relevant issue unresolved. Clinical trials based on ICI agents as treatment for HCC are reported in Table 2 .
LIVER TOXICITY DURING TREATMENT WITH ICIS
The development of irAEs, potentially affecting multiple organs, following loss of selftolerance, has been widely reported[109 ,110 ].
Among the possible adverse events, of particular relevance is the development of liver toxicity as it could cause worsening of liver function in patients who almost always have underlying preexisting chronic liver disease[107 ].

Table 2 Clinical trials assessing immune cell populations as study outcome

Figure 3 Distinct targeting of regulatory T cell activating and inhibitory receptor-targeted therapies in cancer and autoimmunity. Regulatory T cell (Treg) cells are equipped with a repertoire of activating and inhibitory receptors. For successful therapy of tumors and chronic infections, blockade of activating receptors and/or stimulation of inhibitory receptors shifts the balance toward inhibition of Tregs. In contrast, to achieve Treg activation in autoimmune diseases,blockade of inhibitory receptors and/or stimulation of activating receptors may be desirable. Treg: Regulatory T cell.
Hepatic toxicity associated with ICI is characterized by elevation of liver parameters values. The pattern of liver enzymes elevation is defined by the increase of alanine aminotransferase (ALT) or alkaline phosphatase (ALP) alone above a specific threshold or by the ratio of serum ALT to ALP levels [R value = [ALT/upper normal level (UNL)]/(ALP/UNL)] and can be categorized as hepatocellular (ALT ≥ 5 -fold above UNL or R > 5 ), mixed (R > 2 to < 5 ), or cholestatic (ALP ≥ 2 fold above UNL or R< 2 )[111 ].
The pattern of ICI-related liver toxicity is heterogenous since it may be cytolytic,cholestatic or mixed, although ICI-related cholestasis seems to be rarer[107 ].
In HCC patients receiving ICIs the incidence of liver toxicity ranges according to the type of drug and the dose received. It has been reported that liver toxicity is more frequent in HCC patients receiving anti-CTLA-4 therapies. In HCC patients receiving the anti-PD-1 antibody nivolumab (CHECKMATE 040 trial) and in those receiving another anti-PD-1 antibody pembrolizumab (KEYNOTE-224 trial), ALT elevation of any grade and of grade ≥ 3 was found in 15 % and 6 % (nivolumab) vs 9 % and 4 %(pembrolizumab), respectively[112 ,113 ].
Differently, therapy with the anti-CTLA-4 antibody tremelimumab was associated with an ALT elevation of any grade and of grade ≥ 3 in 19 % and 9 % of patients,respectively[114 ].
Interestingly, during HCC clinical studies, HCV and HBV positive patients exhibited a reduction in viral load during immunotherapy, more pronounced with anti-CTLA-4 agents, thus suggesting that the treatment induced immunological shift has favorable effect on antiviral response[112 -116 ].
No definitive data has been reported about patients with autoimmune liver diseases treated with ICIs. However, in most of the HCC clinical trial, pre-existing autoimmune diseases was a contraindication for enrollment in light of previous data demonstrating that immunotherapies might trigger a flare-up of pre-existing autoimmune disease or the onset of additional immune-related disease[117 -119 ].
No data are available concerning genetic and autoantibody profile of patients before starting ICI treatments, however autoantibodies such as antinuclear and anti-smooth muscle have been reported in patients after ICI-induced liver toxicity onset.
A management protocol for patients experiencing liver toxicity due to ICI treatment administered for non-liver tumors has been proposed and is based on corticosteroids or mycophenolate mofetil/tacrolimus (for patients not improving under corticosteroids), and ursodeoxycholic acid for those with a predominant cholestasis[120 ].
CONCLUSION
Increasing understanding of the immunologic mechanisms that characterize the TME of HCC has led to a better insight into the pathogenesis of HCC and its link to chronic inflammation and cirrhosis. As well as, the different prevalence in viralvsnon-viral liver diseases, particularly autoimmune, confirms the key role of the liver immune microenvironment.
Although the precise pathogenetic mechanisms of irAEs remain largely undefined,several processes have been proposed to be involved in the development of irAEs such as genetic factors, gut microbiome, epitope spreading, and cross-presentation of neoantigens[121 ,122 ]. A thorough evaluation on the role of Tregs in the pathogenesis of irAEs is crucial. Since cancer and autoimmunity constitute two sides of the same coin, it is perhaps not surprising that when we manipulate the immune system to treat cancer through the use of checkpoint therapy, we inevitably unbalance the vital mechanisms that regulate self-tolerance, inducing a number of irAEs. This is, at least in part, related to the impairment of Treg homeostasis, which is crucial for maintaining immune tolerance[123 ].
Currently used ICIs may also target Tregs, since several checkpoint molecules including CTLA4 and PD-1 are highly expressed on their surface, therefore it is possible that the development of irAEs may be in part attributed to the Treg destabilization. Consistent with this line, it has been demonstrated that anti-CTLA4 disrupts the crosstalk between Foxp3 Tregs and antigen-presenting cells to promote autoimmunity[124 ]. In light of the effects of immunotherapy on the enhancement of the immune response, it should be investigated whether in cases of HCC occurring in patients with pre-existing autoimmune liver and non-liver diseases these therapies are safe and not potentially hepatotoxic, since presently this information is lacking due to the exclusion of patients with autoimmune diseases from clinical trials. Of interest,other approaches to target the immunosuppressive effect of Tregs are ongoing. Given the TGF-β mediated inhibitory role in HCC development and progression, studies are ongoing to assess how to target TGF-β. Galunisertib, an inhibitor of TGF-β, is currently in a phase II clinical trial for HCC patients[125 ,126 ].
Due to the potential effect of immunotherapies on Tregs and the possible effect of triggering autoimmune disorders, it is desirable that in future HCC trials the autoantibody profile, as well as genetic background (HLA) and change in the percentage of peripheral Treg during therapy be monitored to assess whether these variables predict the risk of hepatotoxicity or extrahepatic autoimmune disorders development.
 World Journal of Gastroenterology2021年22期
World Journal of Gastroenterology2021年22期
- World Journal of Gastroenterology的其它文章
- Fecal microbiota transplantation for irritable bowel syndrome: An intervention for the 21 st century
- Application of artificial intelligence-driven endoscopic screening and diagnosis of gastric cancer
- Mucosal lesions of the upper gastrointestinal tract in patients with ulcerative colitis: A review
- Preservation of superior rectal artery in laparoscopically assisted subtotal colectomy with ileorectal anastomosis for slow transit constipation
- Early serum albumin changes in patients with ulcerative colitis treated with tacrolimus will predict clinical outcome
- Idiopathic mesenteric phlebosclerosis associated with long-term oral intake of geniposide
