Fecal microbiota transplantation for irritable bowel syndrome: An intervention for the 21 st century
Magdy EI-Salhy, Tanisa Patcharatrakul, Sutep Gonlachanvit
Abstract Irritable bowel syndrome (IBS) affects about 12 % of the global population.Although IBS does not develop into a serious disease or increase mortality, it results in a considerable reduction in the quality of life. The etiology of IBS is not known, but the intestinal microbiota appears to play a pivotal role in its pathophysiology. There is no effective treatment for IBS, and so the applied treatments clinically focus on symptom relief. Fecal microbiota transplantation(FMT), an old Chinese treatment, has been applied to IBS patients in seven randomized controlled trials (RCTs). Positive effects on IBS symptoms in various degrees were obtained in four of these RCTs, while there was no effect in the remaining three. Across the seven RCTs there were marked differences in the selection processes for the donor and treated patients, the transplant dose, the route of administration, and the methods used to measure how the patients responded to FMT. The present frontier discusses these differences and proposes:(1 ) criteria for selecting an effective donor (superdonor); (2 ) selection criteria for patients that are suitable for FMT; (3 ) the optimal FMT dose; and (4 ) the route of transplant administration. FMT appears to be safe, with only mild, self-limiting side effects of abdominal pain, cramping, tenderness, diarrhea, and constipation.Although it is early to speculate about the mechanisms underlying the effects of FMT, the available data suggest that changes in the intestinal bacteria accompanied by changes in fermentation patterns and fermentation products(specifically short-chain fatty acids) play an important role in improving the IBS symptoms seen after FMT. FMT appears to be a promising treatment for IBS, but further studies are needed before it can be applied in everyday clinical practice.
Key Words: Butyric acid; Enteroendocrine cells; Etiology; Microbiota; Short-chain fatty acids; Superdonor; Therapy
INTRODUCTION
Irritable bowel syndrome (IBS) is a common gastrointestinal disorder with a prevalence of 12 .1 % worldwide[1 ,2 ]. Although IBS does not increase mortality, it considerably reduces the quality of life[3 ]. The etiology of IBS is not known, but several factors appear to be involved in its pathophysiology, including genetics, diet,the intestinal microbiota, enteroendocrine cells, low-grade inflammation, and stress[2 ,4 ]. An effective treatment for IBS is lacking, and so the interventions applied to IBS patients clinically are aimed at symptom relief[5 ].
The human intestine is colonized by more than 10 microorganisms, including archaea, fungi, viruses, and bacteria[6 ,7 ], spanning 2172 bacteria species belonging to 12 different bacteria phyla[8 ]. Bacteria belonging toFirmicutesandBacteroidetesphyla dominate the bacterial population in the intestine of healthy subjects, and they combine with a few members from theProteobacteriaandActinobacteriaphyla[8 ,9 ]. The intestinal bacterial composition varies between individuals due to both genetic and environmental factors[6 ,10 ]. These environmental factors include diet, the frequency of antibiotic treatment, the intake of nonantibiotic drugs, geographical location, surgery,smoking, and depression[6 ,10 ,11 ]. A low intestinal bacterial diversity (dysbiosis) is found in several diseases[10 ,12 ].
The intestinal bacterial composition in IBS patients deviates from that of healthy subjects[10 ,13 -15 ]. Compared with healthy subjects, IBS patients have a lower abundance of butyrate-producing bacteria (Erysipelotrichaceae,Ruminococcaceae,Bifidobacterium,Faecalibacterium, andErysipelotrichaceaespp.) and methanogenic bacteria, and a higher abundance of bacteria belonging toProteobacteria,Veillonella, and Firmicutes such asLactobacillusandRuminococcusspp.[7 ,16 ]. In addition, the diversity of intestinal bacteria is lower (dysbiosis) in IBS patients than in healthy sub-jects[10 ,13 -15 ,17 ].
Transplanting the intestinal microbiome (viafeces) from a healthy subject with normal bowel function to patients — so-called fecal microbiota transplantation (FMT)— was applied for the first time by the Chinese physician Ge Hong in the fourth century for treating severe diarrhea and malaria[18 ]. In the present era, FMT represents an effective treatment forClostridium difficileinfection (CDI) and is also a promising invention for other diseases[19 -21 ].
The use of FMT as a treatment for IBS has been investigated in seven randomized controlled trials (RCTs)[11 ,22 -27 ], which have produced different outcomes. The present frontier aimed at clarifying these differences, highlighting the gaps in our knowledge that need to be filled, and proposing a guideline for successful FMT in IBS patients.
FACTORS THAT AFFECT THE OUTCOME OF FMT
FMT reduced the symptoms and improved the quality of life of the treated IBS patients in four of the seven RCTs[11 ,22 ,26 ,27 ], but had no effect in the remaining three RCTs[23 -25 ]. These differences in the outcomes of RCTs of FMT in IBS could be explained by differences in the protocols, donor selection, included IBS patients, dose of fecal transplant used, and administration route (Table 1 )[11 ,22 -27 ]. Several suggestions have been made about how to improve the efficacy of FMT in IBS[28 ,29 ].
Donor selection
The outcomes of FMT in inflammatory bowel disease (IBD) have varied considerably between studies[20 ], which has been attributed to differences in the donor used[10 ,30 ]. A donor that produces a large response to FMT in IBD patients is called a superdonor[10 ]. Since it was not possible to predict who is a superdonor, the feces from several donors were pooled to increase the likelihood of patients receiving superdonor feces[31 ]. However, this approach was not successful, which was probably due to the dilution of the eventual superdonor feces resulting in an insufficient dose from the superdonor to the recipients[32 ].
Similar to IBD, discrepancies in the results of the RCTs of FMT in IBS could be mainly attributed to differences in the criteria used for donor selection (Table 1 )[11 ,22 -27 ]. An RCT performed by El-Salhy et al[11 ] that obtained good responses to FMT(Figure 1 ) established clinical criteria and the bacterial profile for selecting the superdonor. In contrast, the less-successful RCTs did not establish either clinical criteria or a bacterial profile for the donor[22 -27 ] (Table 1 ). The stability of the donor intestinal bacterial composition over time is also an important factor to consider when selecting a superdonor[27 ].
The clinical criteria used to select a superdonor have been based on the factors known to affect the intestinal microbiota (Table 2 ). Aging (> 50 years),smoking/smoking cessation, being born by cesarean section, consuming formula as a baby, frequent treatment with antibiotics, and regular intake of nonantibiotic drugs are known to reduce the bacterial diversity[33 -42 ], whereas regular exercise combined with consuming a sport-specific diet are associated with a favorable intestinal microbiota[43 -45 ]. The genetic composition also affects the intestinal microbiota, and hence the superdonor should not be a first-degree relative of any recipient[46 ,47 ].Thus, the superdonor selected in the RCT by El-Salhyet al[11 ] was a healthy young male with a normal body mass index, bornviaa vaginal delivery, breastfed, and a nonsmoker, and did take any medication, had been treated only a few times with antibiotics, exercised regularly, and consumed a sport-specific diet that was richer in protein, fiber, minerals, and vitamins than average[48 ]. Furthermore, the superdonor was not related to any of the recipients[11 ]. The analysis of the fecal microbiota of this donor showed that he had a high microbial diversity (normobiotic), and his fecal bacterial composition deviated from the normal abundance of 165 healthy subjects in 14 of 39 tested bacteria markers (Figure 2 )[11 ]. Twelve of these deviated bacteria were in the Firmicutes phylum, with one each in the Proteobacteria and Verrucomicrobia phyla[11 ]. This deviation included an increased abundance of favorable bacteria such asStreptococcus,Dorea,Lactobacillus, andRuminococcaceaespp.[10 ,49 -51 ]. The fecal bacterial composition of the superdonor was stable over the 18 -mo period during which he donated his feces (Figure 3 )[11 ].
Like in IBD, pooling the feces from several donors to ensure the presence of superdonor feces resulted in no response or only a transient improvement[22 ,23 ].
Patient inclusion
The patients included in RCTs of FMT for IBS have included subsets of IBS patients who do not represent the entire IBS population, and so caution should be exercised when generalizing the outcomes of these RCTs[11 ,22 -27 ]. Four of the RCTs only included patients with the diarrhea-predominant IBS (IBS-D) and mixed-diarrhea-andconstipation IBS (IBS-M)[22 ,24 ,26 ,27 ], while the other three RCTs included three IBS subtypes: IBS-D, constipation-predominant IBS (IBS-C), and IBS-M[11 ,23 ,25 ].Moreover, the patients included in the RCT of El-Salhyet al[11 ] had participated in a 2 -d course related to living with IBS, and for at least the past 3 mo they had moderate-tosevere IBS symptoms despite adhering to a diet consistent with the National Institute for Health and Care Excellence (NICE)-modified diet. The RCT of Holsteret al[25 ]included patients with low amounts of fecal butyrate-producing bacteria. Holvoetet al[27 ] only included refractory patients with severe bloating who had failed to respond to at least three conventional therapies for IBS.
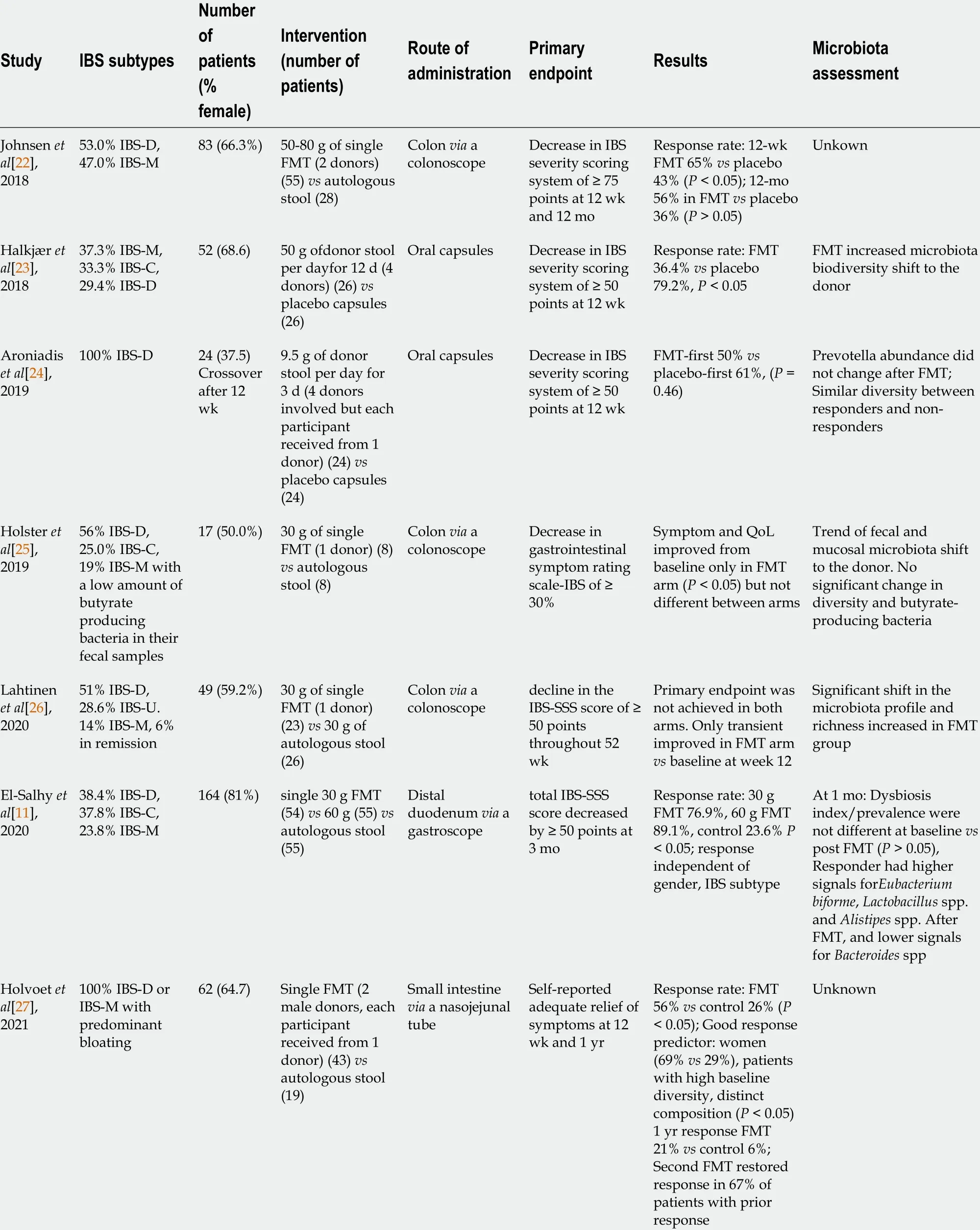
Table 1 Summary of seven randomized controlled trials of fecal microbiota transplantation for irritable bowel syndrome
Route of administration and dose of the fecal transplant

Figure 1 Responses of irritable bowel syndrome patients to placebo, 30 -g fecal microbiota transplantation, and 60 -g fecal microbiota transplantation at different intervals after transplantation. **P < 0 .001 ; ****P < 0 .0001 compared with placebo. **P < 0 .001 ; ****P < 0 .0001 for 30 -g fecal microbiota transplantation (FMT) compared with 60 -g FMT. Citation: El-Salhy M, Hatlebakk JG, Gilja OH, Bråthen Kristoffersen A, Hausken T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut 2020 ; 69 : 859 -867 . Copyright @BMJ Publishing Group Ltd 2020 . Published by BMJ Publishing Group Ltd[11 ].
The fecal transplant can be administered either to the small intestineviathe working channel of a gastroscope or a nasojejunal probe, or to the large intestineviathe working channel of a colonoscope (Figures 4 and 5 )[11 ,22 ,25 -27 ]. Administering the fecal transplant to either the small or large intestine seems to be effective[11 ,22 ,25 -27 ].However, a placebo effect was evident in 43 %-44 % and 23 .6 %-26 % of the patients that received the fecal transplant into the large and small intestine, respect-ively[11 ,22 ,25 -27 ]. This placebo effect might have been greater in patients that received fecal transplant into the colon due to the favorable effect of the bowel preparation needed for a colonoscopy on IBS symptoms[52 ].
While administering fecal transplantsviacapsule ingestion has been effective in CDI, it was unsuccessful in IBS[23 ,24 ,53 ]. This is could be due to the selection of the donor, a low transplant dose, or pooling of the donors rather than the mode of administration[23 ,24 ].
The dose of the fecal transplant seems to significantly affect the outcome of FMT,with a dose–dependent response appearing to be present (Figure 1 )[11 ]. This was confirmed by 70 % of patients that did not respond to 30 -g FMT responding to 60 -g FMT (Figure 6 )[54 ]. All but two of the RCTs of FMT for IBS used a dose of at least 30 g[11 ,22 ,23 ,25 ,26 ]; in the remaining two studies the dose was either lower than 30 g or not specified[24 ,27 ]. Further investigations are needed to evaluate the efficacy singlevsrepeated transplantation.

Figure 3 Scaled principal component analysis plot of fecal samples from the superdonor and patients before transplantation. The patient samples are indicated by small gray circles. The superdonor samples are indicated by the larger circles of different colors reflecting the sampling times: black, 3 mo;red, 6 mo; green, 9 mo; blue, 12 mo; light blue, 15 mo; and pink, 18 mo. All of the superdonor samples are grouped closely together and remain in very similar positions over time. Citation: El-Salhy M, Hatlebakk JG, Gilja OH, Bråthen Kristoffersen A, Hausken T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut 2020 ; 69 : 859 -867 . Copyright @BMJ Publishing Group Ltd 2020 . Published by BMJ Publishing Group Ltd[11 ].
CHANGES IN BACTERIAL AND FERMENTATION PROFILES AFTER FMT
In the RCT of El-Salhyet al[11 ,55 ] in which FMT was most effective, the severity of dysbiosis did not differ significantly between the placebo and active treated groups after 1 mo, but it was reduced significantly after 1 year. One month after FMT, the bacterial profile changed significantly in the active treated groups compared with the placebo group (Figure 7 )[11 ]. Higher signals forEubacterium biforme,Lactobacillusspp.,andAlistipesspp., and lower signals forBacteroidesspp. were observed at 1 mo after FMT in the active treated groups (Figure 8 ) but not in the placebo group (Figures 9 –12 )[11 ]. The IBS severity scoring system (IBS-SSS) score was significantly correlated with the signals ofLactobacillusspp. (P= 0 .002 , r = –0 .3 ) and Alistipes spp. (P = 0 .001 ,r=–0 .3 ) but not with those of Eubacterium biforme(P = 0 .754 , r = 0 .03 ) orBacteroidesspp. (P= 0 .458 , r = 0 .06 )[11 ]. The signals of nine bacteria had changed at 1 year after FMT(Figure 13 )(unpublished data): Whereas the signals ofBacteroidesspp.,Prevotellaspp.,Alistipesspp.,Bacteroides stercoris,Parabacteroidesspp., andBacteroides zoogleoformanswere significantly higher than those at the baseline, the signals ofStreptococcus salivariusssp.thermophilus, Shigellaspp.,Escherichiaspp.,Eubacterium hallii, andDoreaspp. were significantly lower than those at the baseline. The signals of the following six of these bacteria were correlated with IBS-SSS total scores:Bacteroidesspp. AndPrevotellaspp. (P< 0 .0001 , r = -0 .4 ), Alistipes spp. (P < 0 .001 , r = -0 .3 ),Bacteroides stercorisandStreptococcus salivariusssp.thermophilus(P< 0 .0001 , r = 0 .3 ),Bacteroides zoogleoformans(P< 0 .002 , r = -0 .3 ), and Eubacterium hallii (P < 0 .04 , r = -0 .2 )(unpublished data). Changes in the bacterial profiles in IBS patients that received FMT have been recorded, but which bacteria had changed was not described in detail[23 ,25 -27 ].
The main products of the bacterial fermentation of intestinal undigested and unabsorbed carbohydrates are short-chain fatty acids (SCFAs)[55 ]. It has been reported that the fecal level of propionic acid was significantly higher in IBS patients[13 ].Moreover, the level of butyric acid has been found to be lower in IBS-C patients and higher in IBS-D patients than in healthy subjects[13 ]. The levels of total SCFAs at 1 mo after FMT were significantly higher in IBS patients that received 60 -g FMT but not in those that received 30 -g FMT[55 ]. The levels of butyric acid at 1 mo after FMT were also significantly higher in IBS patients that received either 30 -g or 60 -g FMT[55 ].There were no significant differences in the levels of total SCFAs and in level of individual SCFAs in the placebo group at 1 mo after FMT[55 ]. The butyric acid level was inversely correlated with the scores on the IBS-SSS and fatigue assessment scale(Figure 14 )[55 ].

Figure 4 Nonresponders to 30 -g donor`s fecal transplant were retransplanted with 60 -g donor´s transplant. A-E: Irritable bowel syndromeseverity scoring system (SSS) total score (A) and IBS-SSS scores for abdominal pain (B), abdominal distension (C), dissatisfaction with bowel habits (D), and interference with quality of life (E) at different intervals after receiving 30 -g and 60 -g fecal microbiota transplantation. *P < 0 .05 compared with baseline. Citation: El-Salhy M, Hausken T, Hatlebakk JG. Increasing the Dose and/or Repeating Faecal Microbiota Transplantation (FMT) Increases the Response in Patients with Irritable Bowel Syndrome (IBS). Nutrients 2019 ; 11 [PMID: 31238507 DOI: 10 .3390 /nu11061415 ]54 ].
The fecal levels of total SCFAs and butyric acid remained elevated in the active treated group with FMT at 1 year after FMT (unpublished data). Furthermore, the fecal levels of isobutyric and isovaleric acids were significantly higher while that of acetic acid was significantly lower (unpublished data).

Figure 5 Fecal transplant can be administered to the small intestine (distal duodenum) or to the large intestine (colon).

Figure 6 Although the fecal transplant can be administered to the small intestine via a nasojejunal probe and to the colon via an enema,administering a fecal transplant via the working channel of a gastroscope or colonoscope is faster and more acceptable to patients.
DIFFERENCES IN RESPONSES BETWEEN IBS SUBTYPES AND SEX
The response to FMT did not differ between the IBS-D, IBS-C, and IBS-M subtypes of IBS[11 ], whereas it was not clear whether there was a difference between females and males. In the subgroup of IBS-D and IBS-M patients with refractory IBS and severe bloating who had not responded to at least three conventional therapies for IBS, the response to FMT was better for females than for males[27 ]. On the other hand, the response to FMT did not differ between female and male IBS patients with moderateto-severe symptoms belonging to the IBS-D, IBS-C, and IBS-M subtypes who had previously not responded to the NICE-modified diet[56 ].

Figure 7 Scaled principal component analysis plot of fecal samples before and 1 mo after transplantation for placebo, 30 -g and 60 -g fecal microbiota transplantation, responders and nonresponders. A: Placebo; B: 30 -g fecal microbiota transplantation (FMT); C: 60 -g FMT; D: Responders; E:Nonresponders. Fecal samples before and after transplantation are indicated by pink circles and blue triangles, respectively. The ellipses cover 80 % of the samples within a group. Citation: El-Salhy M, Hatlebakk JG, Gilja OH, Bråthen Kristoffersen A, Hausken T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut 2020 ; 69 : 859 -867 . Copyright @BMJ Publishing Group Ltd 2020 . Published by BMJ Publishing Group Ltd[11 ].
SAFETY OF FMT
A recently published consensus report from a multidisciplinary United European Gastroenterology working group did not classify voluntarily donated material (feces)as a drug. Instead, it was recommended to be collected, handled, and used according to the standards defined by the EU Commission in EU Tissue and Cells Directive 2004 /23 /EC[21 ,57 ].
FMT appears to be safe, with limited side effects[58 ,59 ]. The short-term adverse events for FMT in IBS patients have been reported to be mild, self-limiting, and occurring during the first few days following transplantation: Abdominal pain,cramping, tenderness, diarrhea, and constipation[11 ,22 -27 ]. However, infectious complications have been described in two patients that received FMT for diseases other than IBS, which resulted in one fatality[60 ,61 ]. While these patients were old,severely ill, and immunocompromised, such events raised questions about safety issues regarding FMT for IBS, especially given that IBS is considered to be a benign gastrointestinal condition[3 ,62 ,63 ]. It has therefore been suggested that the screening of FMT donors should include testing of their feces for extended-spectrum-beta-lactaseproducingE. coliand severe acute respiratory syndrome coronavirus 2 . This would reduce the risks of infection and restrict the selection of IBS patients for FMT to those who are not immunocompromised and do not have systemic disease or severe illness[3 ].
No adverse events were observed 1 -year after FMT for IBS patients (unpublished data). However, long-term follow-ups of patients that received FMT because of CDI raised some concerns about weight gain, the development or worsening of IBD,cancer, autoimmune diseases, allergies, and neurological diseases[64 ].
POSSIBLE MECHANISMS UNDERLYING THE EFFECTS OF FMT

Figure 8 Box plots of the logarithm of the difference in signals between baseline and 1 mo after fecal microbiota transplantation for the responders in both the 30 -g and 60 -g fecal microbiota transplantation groups. Red stippled line indicates 0 , where no change occurred between before and after fecal microbiota transplantation (FMT). Signals after FMT in responders were higher for Eubacterium biforme, Lactobacillus spp., and Alistipes spp.,and lower for Bacteroides spp. Citation: El-Salhy M, Hatlebakk JG, Gilja OH, Bråthen Kristoffersen A, Hausken T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut 2020 ; 69 : 859 -867 . Copyright @BMJ Publishing Group Ltd 2020 .Published by BMJ Publishing Group Ltd[11 ].
The enteroendocrine cells and fecal SCFAs in IBS patients differ from those of healthy subjects, and these differences are believed to play a central role in the pathophysiology of IBS[2 ,65 -70 ]. Although it is early to speculate about the mechanisms underlying the effects of FMT, there are data suggesting that the improvement in IBS symptoms by FMT is caused by changes in the enteroendocrine cells and in SCFAs.
Most of the body serotonin (95 %) is localized to the gastrointestinal tract, of which only 10 % is present in the neurons of the enteric nervous system and the rest is in the serotonin-containing enterochromaffin (EC) cells that are scattered between the epithelial cells lining the gastrointestinal lumen[71 -74 ]. Serotonin plays an important role in gastrointestinal motility by inhibiting gastric emptying and stimulating colonic motility, and accelerating transit through the small and large intestines. Serotonin also activates the submucosal sensory branch of the enteric nervous system that conveys sensation from the gut to the central nervous system[70 ]. When serotonin exerts its effects at serotonin receptors, it is transported by the serotoninselective reuptake transporter (SERT) into intestinal epithelial cells, where it is degraded[75 ,76 ]. The epithelial cells lining the luminal surface of the intestine express SERT[70 ,77 ], and a reduction in SERT results in impaired intracellular uptake of serotonin and its degradation in the intestinal epithelial cells[78 ,79 ]. In IBS patients, the density of EC cells in the colon and the SERT immune-intensity in the large intestine have been reported to be lower than those in healthy subjects (Figures 15 and 16 )[80 -82 ]. Several bacteria includingCorynebacterium,Streptococcus, andEnterococcusspp. produce serotonin and indigenous spore-forming bacteria from the microbiota of mice and humans that promote serotonin biosynthesis by colonic EC cells[83 ,84 ]. Furthermore,Clostridium ramosumregulates the release of serotonin from EC cells[85 ]. It is possible that the changes in the intestinal bacterial composition induced by FMT affect the serotonin-regulating system.
Peptide YY (PYY) occurs in enteroendocrine cells of the ileum, colon, and rectum[86 ,87 ]. PYY binds to and activates receptors Y1 and Y2 , which are localized to the epithelial cells lining the intestinal lumen and in the submucosal and myenteric plexus neurons of the small intestine and colon[88 -100 ]. PYY acts as a mediator of the ileal brake and stimulates the absorption of water and electrolytes in the large intestine[87 ,101 ,102 ]. The density of colonic PYY cells is reduced in IBS patients (Figure 17 )[80 -82 ].The total levels of fecal SCFAs increased significantly in IBS patients at 1 mo after FMT and remained higher than those at the baseline at 1 year after transplantation(unpublished data)[55 ]. Intestinal SCFAs increase the secretion of peptide PYY by up-regulating the gene expression of PYY[102 -105 ]. It is likely that the improvement in IBS symptoms following FMT is due to the effect of SCFAs on PYY secretion.

Figure 9 Differences in signals for Eubacterium biforme in the responder, nonresponder, and placebo groups between before and 1 mo after transplantation. There were significant changes in the signal levels in responders (P < 0 .001 ). The nonresponders also exhibited increased signal levels,though the changes were smaller than those in the responders. A: The signal level did not change in the placebo group; B: Signal levels at baseline were similar in the responder, nonresponder, and placebo groups, but they were higher for the superdonor; C: The signal levels in the responders and nonresponders became as high as that for the superdonor after fecal microbiota transplantation, while that in the placebo group did not change.
In the patients that received FMT, the fecal levels of butyric acid increased at 1 mo after FMT and remained elevated at 1 year after transplantation(unpublished data)[56 ]. Butyrate provides colonic epithelial cells with energy[105 ], modulates the immune response and oxidative stress, and decreases intestinal motility and the permeability of intestinal cells[87 ]. Butyrate also modulates colonic hypersensitivity,and an intake of butyrate was found to reduce the abdominal pain in patients with IBS[106 -108 ]. An increase in butyric acid could be one of the factors underlying the improvement seen after FMT.
The fecal level of the straight SCFA acetic acid was reduced at 1 year after FMT(unpublished data). This reduction could be significant since acetic acid has been found to induce visceral hypersensitivity in rodents[109 ]. The levels of the branched SCFAs isobutyric and isovaleric acids were significantly increased at 1 year after FMT(unpublished data). These increases indicate a shift in the microbial metabolism from a saccharolytic to a proteolytic fermentation pattern, which could be of pathophysiological relevance[110 ]. It is particularly interesting that similar changes in the profiles of isobutyric and isovaleric acids have been found in patients with IBS following adherence to a low-FODMAPs (fermentable oligosaccharides, disaccharides,monosaccharides, and polyols) diet[111 ].
CONCLUSION
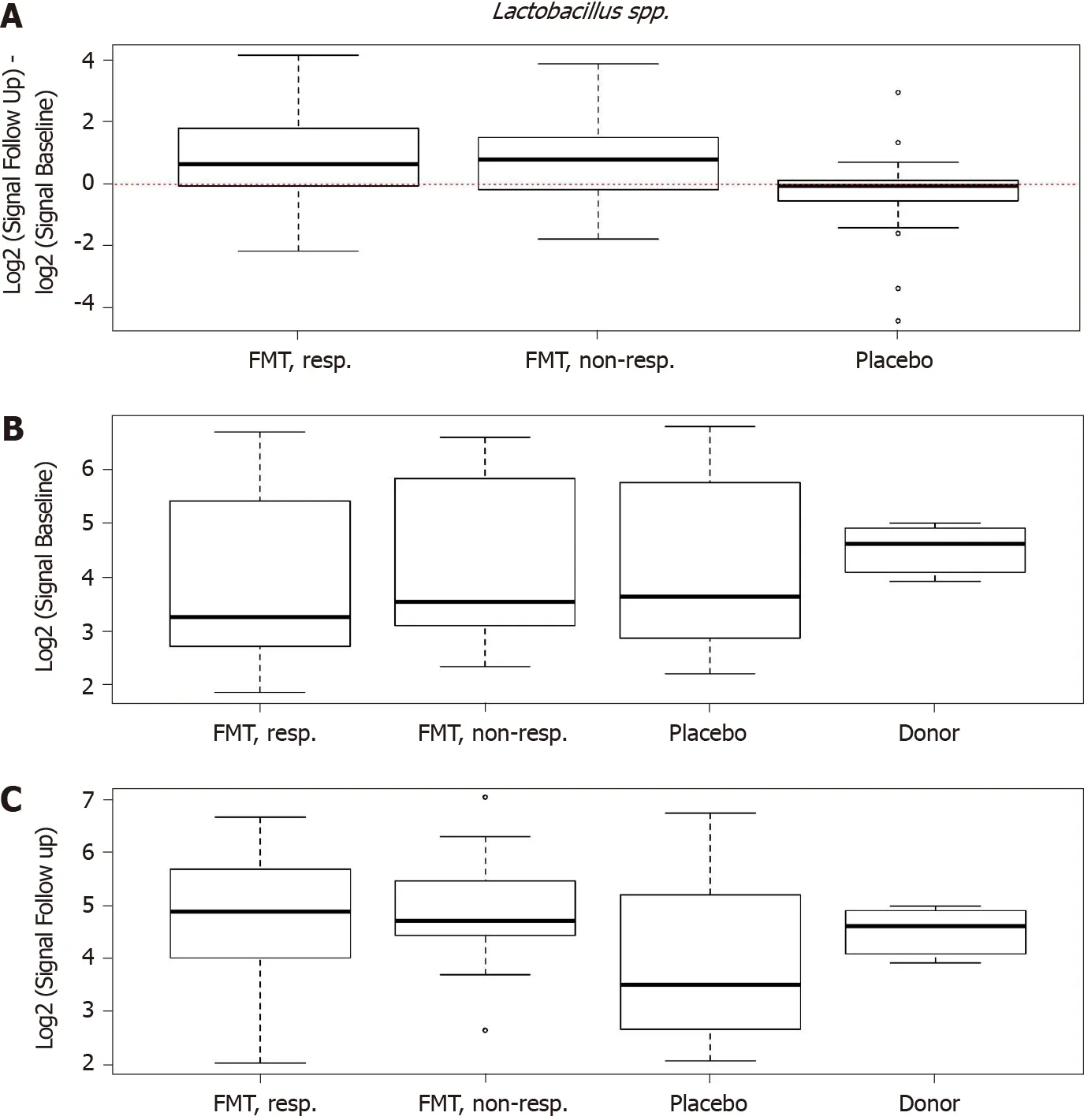
Figure 10 Differences in signals for Lactobacillus spp. in the responder, nonresponder, and placebo groups between before and 1 mo after transplantation. There were significant changes in the signal levels in responders (P < 0 .001 ). A: The nonresponders exhibited similar signal levels to those in the responders, while there was no change in the placebo group; B: The signal levels in the responder, nonresponder, and placebo groups were similar, but it was higher for the superdonor; C: After transplantation, the signal levels in responders and nonresponders became similar to that for the superdonor, while that in the placebo group did not differ from the baselin. Citation: El-Salhy M, Hatlebakk JG, Gilja OH, Bråthen Kristoffersen A, Hausken T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut 2020 ; 69 : 859 -867 . Copyright @BMJ Publishing Group Ltd 2020 . Published by BMJ Publishing Group Ltd[11 ].
FMT is a longstanding Chinese treatment applied to both gastrointestinal and nongastrointestinal diseases[18 ], and is now also a promising treatment for IBS patients since it improves the IBS symptoms, fatigue, and quality of life in about 90 %of patients in a RCT[11 ]. However, several questions remain to be answered, and further investigations are needed before FMT can be applied in everyday clinical practice. The criteria to apply when selecting an effective (superdonor) for FMT remain unclear, as do the optimal dose, administration route, and frequency of treatment. Moreover, it is not clear whether FMT is effective for all IBS patients, or whether it should be restricted to certain subsets of IBS patients. There is also some concern regarding the long-term side effects of FMT[21 ].
There is an exciting task in front of us to modernize an effective treatment for IBS that was first used more than a thousand years ago.

Table 2 Criteria of a super-donor and how the 7 published randomized clinical trials met them

Figure 11 Changes in signals for Alistipes spp. in responders, nonresponders, and placebo groups as well as in the donor. A: Differences in signals for Alistipes spp. between before and 1 mo after transplantation in the responder, nonresponder, and placebo groups; B: There were significant changes in the signals level in responders (P = 0 .004 ). The signal levels also changed in nonresponders, but they did not change in the placebo group. Signal levels at baseline in the responder, nonresponder, and placebo groups as well as the superdonor; C: The baseline signal levels were similar in the responder, nonresponder, and placebo groups, while that for the superdonor was higher. Signal levels in the responder, nonresponder, and placebo groups and the superdonor after transplantation.The signal levels were similar in responders, nonresponders and the superdonor, while it was lower in the placebo group. Citation: El-Salhy M, Hatlebakk JG, Gilja OH, Bråthen Kristoffersen A, Hausken T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind,placebo-controlled study. Gut 2020 ; 69 : 859 -867 . Copyright @BMJ Publishing Group Ltd 2020 . Published by BMJ Publishing Group Ltd[11 ].

Figure 12 Changes in signals for Bacteroides spp. in responders, nonresponders, and placebo groups as well as in the donor. A:Differences in signals for Bacteroides spp. between before and 1 mo after fecal microbiota transplantation in the responder, nonresponder, and placebo groups; B:There were significant changes in the signal levels in responders (P < 0 .034 ). The nonresponders showed similar changes, while there were no changes observed in the placebo group. Signal levels at baseline in the responder, nonresponder, and placebo groups and for the superdonor; C: The baseline signal levels were similar in the responder, nonresponder, and placebo groups, and both higher and lower than those for the superdonor. Signal levels in the same groups after transplantation.Citation: El-Salhy M, Hatlebakk JG, Gilja OH, Bråthen Kristoffersen A, Hausken T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut 2020 ; 69 : 859 -867 . Copyright @BMJ Publishing Group Ltd 2020 . Published by BMJ Publishing Group Ltd[11 ].
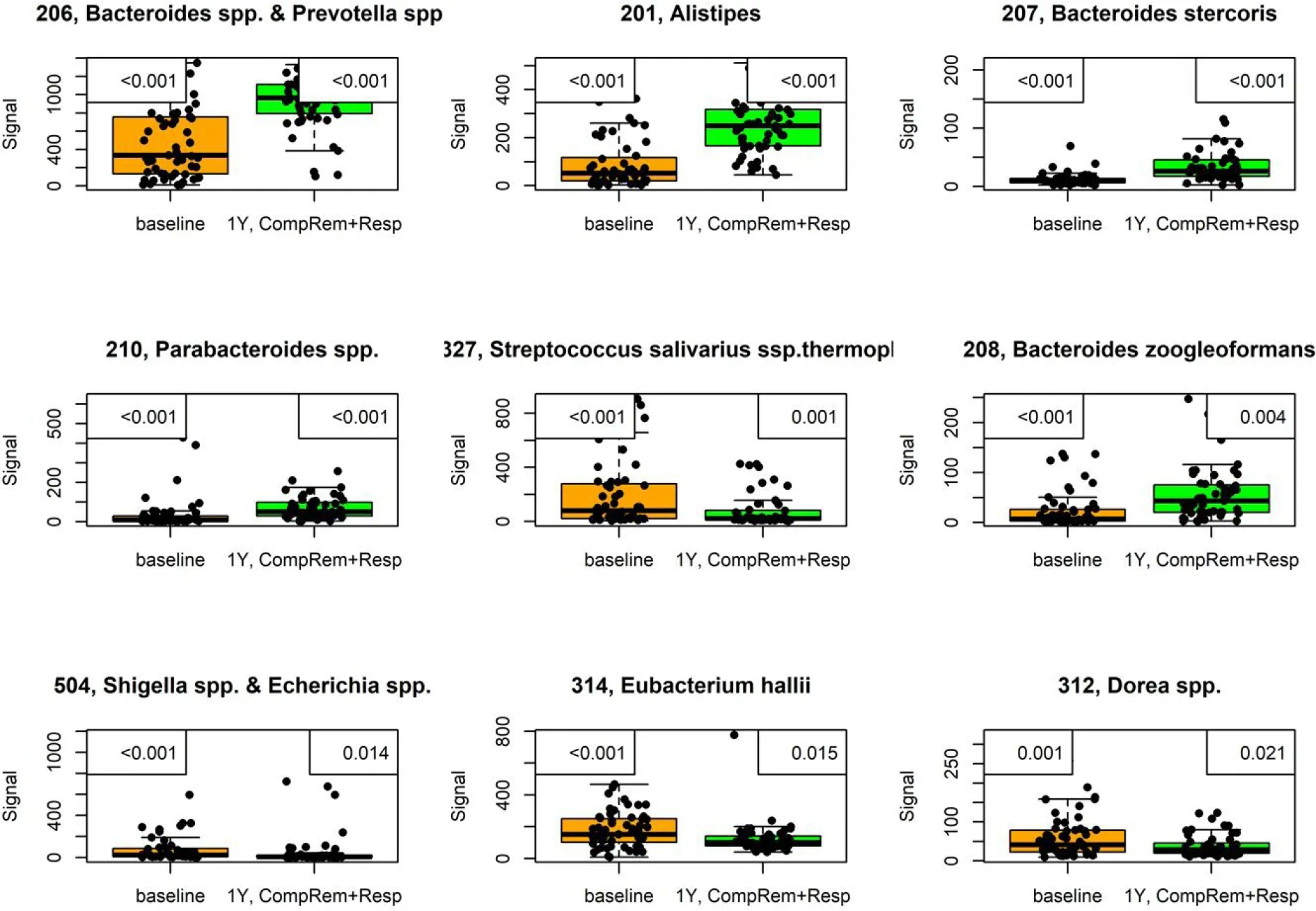
Figure 13 Signals of nine bacteria had changed at 1 year after fecal microbiota transplantation in irritable bowel syndrome patients that either maintained a response or experienced complete remission. P values are in the upper-left corner, with corrected P values in the upper-right corner. This figure is based on unpublished data from El-Salhy et al.

Figure 14 The correlation between butyric acid levels and IBS symptoms and fatigue 1 mo after fecal transplation. A and B: Correlations between butyric acid levels and irritable bowel syndrome-severity scoring system total scores (A) and fatigue assessment total score (B). Citation: El-Salhy M,Hausken T, Hatlebakk JG. Increasing the Dose and/or Repeating Faecal Microbiota Transplantation (FMT) Increases the Response in Patients with Irritable Bowel Syndrome (IBS). El-Salhy M, Valeur J, Hausken T, Gunnar Hatlebakk J. Changes in fecal short-chain fatty acids following fecal microbiota transplantation in patients with irritable bowel syndrome. Neurogastroenterol Motil 2021 ; 33 : e13983 [PMID: 32945066 DOI: 10 .1111 /nmo.13983 ][55 ].

Figure 15 Serotonin-immunoreactive cells (arrows) in the colon of a healthy subject and in a patient with irritable bowel syndrome. A:Healthy subject; B: Patient with irritable bowel syndrome.

Figure 16 Difference in serotonin selective reuptake transporter immunoreactivity between a healthy subject and a patient with irritable bowel syndrome. A and B: Serotonin selective reuptake transporter immunoreactivity in the cytoplasm of the epithelial cells lining the rectal lumen (arrows) of a healthy subject (A) and of an irritable bowel syndrome patient (B).
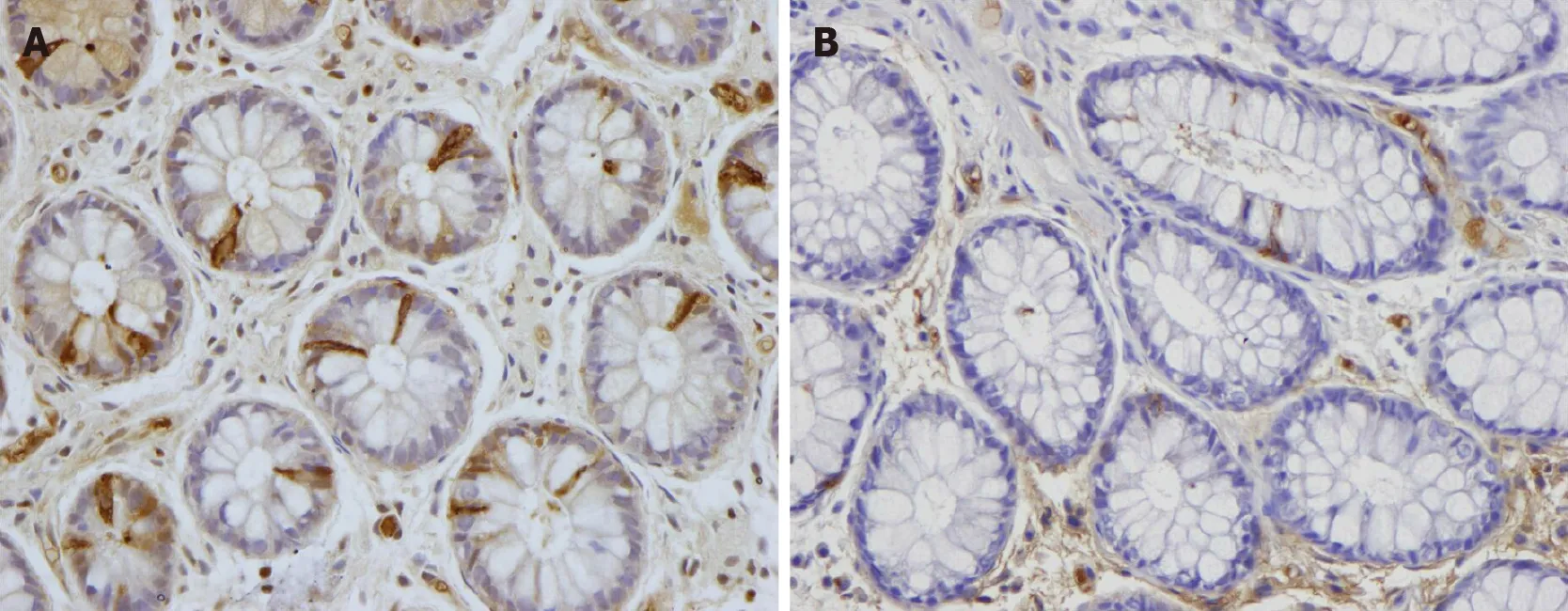
Figure 17 Peptide YY immunoreactivity (arrows) in the colon of a healthy subject and in a patient with irritable bowel syndrome. A: Healthy subject; B: Patient with irritable bowel syndrome.
 World Journal of Gastroenterology2021年22期
World Journal of Gastroenterology2021年22期
- World Journal of Gastroenterology的其它文章
- Role of imaging in evaluating the response after neoadjuvant treatment for pancreatic ductal adenocarcinoma
- High fecal calprotectin levels are associated with SARS-CoV-2 intestinal shedding in COVID-19 patients: A proof-of-concept study
- Liver injury in COVID-19 : Detection, pathogenesis, and treatment
- Enhancer of zeste homolog 2 contributes to apoptosis by inactivating janus kinase 2 / signal transducer and activator of transcription signaling in inflammatory bowel disease
- Interplay between nuclear factor erythroid 2 -related factor 2 and inflammatory mediators in COVID-19 -related liver injury
- Helicobacter pylori promotes invasion and metastasis of gastric cancer by enhancing heparanase expression
