Anti-tumor activity of rice bran hydrolysates on migration, invasion and angiogenesis
Suphanthip Phusrisom, Laddawan Senggunprai, Auemduan Prawan, Sarinya Kongpetch, Upa Kukongviriyapan,Supawan Thawornchinsombut, Sirithon Siriamornpun, Theeraphan Chumroenphat, Ronnachai Changsri,Veerapol Kukongviriyapan✉
1Department of Pharmacology, Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand
2Department of Physiology, Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand
3Department of Food Technology, Faculty of Technology, Khon Kaen University, Khon Kaen 40002, Thailand
4Department of Food Technology and Nutrition, Faculty of Technology, Mahasarakham University, Mahasarakham, 44150, Thailand
5Chumphae Rice Research Center, Ministry of Agriculture and Cooperatives, Khon Kaen 40130, Thailand
ABSTRACT
KEYWORDS: Rice bran hydrolysates; Ferulic acid; CCA cells; Proliferation; Migration; Invasion; Adhesion; Metastasis;Angiogenesis; NF-κB
1. Introduction
Rice bran is a byproduct of the rice milling process, which is derived from the outer layer of the rice grain. Preparations from rice bran have recently received much attention because they show several pharmacological activities. Rice bran is rich in dietary fiber,protein, lipid[1], and a significant amount of phytochemicals with biological activities, such as ferulate esters, γ-oryzanols, various small phenolic acids, and flavonoids[2,3]. Rice bran hydrolysates(RBH) show antihypertensive effect in rats via upregulation of endothelial nitric oxide synthase and suppressions of nicotinamide adenine dinucleotide phosphate-oxidases and angiotensin Ⅱformation[4]. RBH preparation rich in protein prevents diabetic nephropathy in diabetic db/db mice[5], improves insulin sensitivity,and suppresses inflammatory cytokines[6,7]. Moreover, RBH has shown various beneficial effects, including anti-inflammatory activity and immunomodulatory effect on monocytes, NK cells, and T cells[8,9].
Compounds in rice bran possess interesting antitumor activity.Polyphenolic compounds such as ferulic acid and ferulate esters,quercetin, and isoquercitrin which are found in rice bran have several activities such as induction of apoptosis, antiproliferation, and antiangiogenesis in cancer cells[3,9,10]. Small peptide hydrolysates and hemicellulose (arabinoxylans) prepared from rice bran could inhibit several cancer cells including colon and breast cancer[11], and enhance NK cell-mediated cytotoxicity against neuroblastoma in vitro and in vivo[12]. However, the mechanisms of anticancer effects of rice bran are still poorly defined.
Cancer of the bile duct or cholangiocarcinoma (CCA) is notoriously difficult to diagnose and treat[13]. The disease usually has a very poor prognosis due to modest response to chemotherapy and high recurrence even after curative resection of tumor[13]. Currently,many strategies have been devised for better control and prevention of CCA[14]. Tumor proliferation, migration, and invasion are characteristics of aggressive cancers, and the molecular processes may involve several signaling pathways[15-17]. NF-κB and PI3K/Akt signalings are involved in inflammation, cancer cell survival,metastasis, and angiogenesis[15]. During metastatic progression,epithelial cancer cells undergo the phenotypic change, which is known as epithelial-to-mesenchymal transition (EMT) and endows cancer cells with enhanced migration and invasion properties[18].Phytochemicals could inhibit these signaling pathways and could be potential therapies for cancers. This study was conducted using RBH from a non-glutinous color Thai rice, a strain with many promising characteristics, to evaluate RBH for antitumor effects on cell proliferation, migration, invasion, and angiogenesis in CCA cells,and elucidate the underlying mechanisms.
2. Materials and methods
2.1. Materials
Chemicals used in studies were as follows. MCDB131 media,endothelial cell growth supplement, heparin and sulforhodamine B were obtained from Sigma Aldrich (St. Louis, MO, USA). Fetal bovine serum and Ham’s F-12 media were purchased from Life Technologies (Grand Island, NY, USA). Matrigel was obtained from Corning (Lowell, MA, USA). Protease inhibitor cocktails and RIPA Lysis buffer were products of VWR International (Solon, OH, USA).Halt Phosphatase Inhibitor cocktail and nuclear and cytoplasmic extraction kit were purchased from Thermo Scientific (Rockford, IL,USA). Protease G6 was purchased from Genencor (Palo Alto, CA,USA). Fibronectin and Luminata Forte Western HRP substrate were obtained from Merck Millipore (Billerica, MA, USA). Insulin-like growth factor-1 (IGF-1) was purchased from Prospec (Ness-Ziona,Israel).
2.2. Preparation and analysis of RBH
Rice bran was prepared from rice (Oryza sativa) variety Tubtim Chumphae (RD69), obtained from Chumphae Rice Research Center,Rice Department, Thailand. RBH was prepared according to a method previously described with some modifications[9]. Briefly, rice bran was defatted by the screw press method. Defatted rice bran was dispersed in distilled water, pH 9.5, subjected to hydrothermolysis by autoclaving at 126.5℃ for 40 min to demobilize the complex structure of rice bran,and digested with 2% protease G6 at pH 8.0, 60℃ for 8 h. The reaction was terminated by heating at 90℃ for 2 min. The supernatant was ultrafiltered through a membrane with a molecular weight cut-off of 50 kDa. The solution was freeze-dried into powder and kept in an air-tight container. Phenolic acids in RBH were analyzed by HPLC method described previously[9]. Flavonoid content was analyzed by Central Laboratory (Thailand) Co., Ltd with detection limits of 5 ppm.
2.3. Cell culture
Three CCA cell lines, KKU-100, KKU-156, and KKU-452,established in our institute[19,20] were used in the studies. Human umbilical vein endothelial cells (HUVECs) (JCRB1459) were obtained from JCRB Cell Bank, Japan. All cells were free from mycoplasma contamination, verified by using a test kit of MycoAlert Plus (Lonza, Walkersville, MD, USA). CCA cells were cultured in Ham’s F12 media supplemented with 10% fetal bovine serum, 50 mg/L gentamicin sulfate, 100 mU/L penicillin, and 10 mM N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid at pH 7.35. The cultured cells were maintained under an atmosphere of 5% COin air at 37℃ as described previously[21]. The cells were subcultured every 2 days using 0.25% trypsin-EDTA. HUVECs were cultured in MCDB131 media, supplemented with NaHCO1.18 g/L, 30 µg/L endothelial cell growth supplement, 2 500 µ/L heparin sulfate, and 10% fetal bovine serum. Cells were subcultured every 2 days using 0.25% trypsin-EDTA.
2.4. Cell proliferation assay
CCA cultured cells were incubated with RBH at concentrations based on our recent report[9]. The sulforhodamine B assay was used to determine proliferation of CCA cells. After treatment of CCA cells with RBH for 24 h, cultured cells were fixed with ice-cold 10% trichloroacetic acid and stained with 0.4% sulforhodamine B in 1% acetic acid. Excess dye was removed with 1% acetic acid, and protein-bound dye was solubilized with 10 mM Tris for determining the absorbance at 540 nm with a microplate reader (Sunrise,TECAN Austria GmbH Austria).
2.5. Wound healing assay
KKU100, KKU-156, and KKU-452 cells were grown to a monolayer in a 24 well-plate. IGF-1 (50 nM) was used to stimulate KKU-100 cell migration. Migration of CCA cells was assessed by wound healing method as previously described[22]. Briefly, a scratch wound was made using a sterile pipette tip. Cultured cells were washed with PBS to remove the detached cells then the wound was photographed as a baseline. The cells were treated with RBH, then series of images of the scratched wound were taken at 6 h for KKU-452 cells, at 18, 24, and 36 h for KKU-156 cells, and 24, 36, and 48 h for KKU-100 cells. The migration of CCA cells was calculated from the closing of the wound distance.
2.6. Cell invasion assay
The invasiveness of KKU-156 and KKU-452 cells was analyzed using the Transwell chamber assay as the method previously described[22]. In brief, for transwell inserts with polycarbonate membrane, the upper suface of membrane was coated with matrigel(0.3 mg/mL) overnight. CCA cells suspended in serum-free medium containing RBH or vehicle were seeded onto the inserts at a density of 2×10cells in 100 μL. The lower part of the chamber of transwell insert was filled with completed medium containing 10% fetal bovine serum. After incubation for 6 h for KKU-452 cells or 36 h for KKU-156 cells, cells remaining on upper surface of inserts were gently removed by scraping with a cotton swab. The invaded cells on the other side of membrane inserts were fixed with ice-cold methanol, stained with crystal violet, and then photographed. The assessment was made by using ImageJ software (version 1.47, NIH,USA).
2.7. Cell adhesion assay
Cell adhesion assay was performed in 48 well-plate pre-coated with fibronectin 0.2 µg/mL for 2 h at 37 ℃, followed by blocking with 3% bovine serum albumin for 30 min, and then washing once with PBS. To measure adhesion, KKU-156 cells were pre-treated with RBH at concentrations of 200-1 600 µg/mL for 18 h. After trypsinization, the cells were adjusted to a density at 4×10cells/well and plated on the pre-coated 48 well-plate. After incubation for 30 min, non-adherent cells were removed by gently washing two times with PBS containing 1 mM CaCland 1 mM MgCl. The adhered cells were fixed with freezer-cold methanol for 10 min at room temperature. After removal of methanol and washing with PBS,the adhered cells were stained with crystal violet. Cell number was quantified by solubilizing the cells with 110 µL of 5% acetic acid in 10% methanol. Absorbance was read by a microplate reader with a 570 nm filter.
2.8. Preparations of nuclear protein extract and whole-cell lysates
KKU-156 cells were cultured at a density of 1×10cells/well in a 100 mm culture dish overnight. After treatment with RBH(400-1 600 µg/mL), the cells were trypsinized and washed with cold PBS at pH 7.4. Nuclear extraction was performed using an extraction kit according to manufacturer instructions. Whole-cell lysates were prepared using RIPA cell lysis buffer according to manufacturer instructions. Protein concentration in nuclear and cytosol fractions was assayed by Bradford dye-binding method.
2.9. Western blot analysis
A total of 25 µg of protein was analyzed using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After electrophoresis,the separated proteins were transferred to a polyvinylidene fluoride membrane, blocked with 5% bovine serum albumin, and incubated overnight with primary antibodies against p-FAK (Tyr, #3283),p-Akt (Thr, #9275), Akt (pan #4685s) from Cell Signaling(Danvers, MA, USA), and p-PI3K (Tyr, sc-12929), FAK (sc 271195), vimentin (sc-6260), ICAM-1 (sc-8439), E-cadherin (sc-21791), vascular endothelium growth factor (VEGF) (sc-7269), NF-κB (sc-109), and β-actin (sc-1616) from Santa Cruz Biotechnology(Dallas, TX, USA). The dilution of antibodies was 1:1 000, except β-actin, which was 1:5 000. After washing with Tris buffer containing 0.1% Tween-20, the membranes were incubated with the appropriate horseradish peroxidase-conjugated secondary antibodies for 2 h.The specific protein bands were detected by the chemiluminescent method using the ChemiDoc MP imaging system (BioRad). The protein bands were quantified using Gel-Pro Analyzer (ver 3.1,Media Cybernatics, USA), and the relative intensity of the protein band was normalized using β-actin as a loading control.
2.10. Tube formation assay
The tube formation assay was performed as described previously[23]. In brief, 100 µL of 1 mg/mL matrigel were coated on 48 well-plate and incubated at 37℃, 5% COin the cell culture incubator. HUVECs at a density of 3.5×10cells/well were plated on top of the matrigel, then incubated for 1 h for attachment,then RBH at a concentration of 400-1 200 µg/mL or vehicle was added and incubated for 18 h. The images of tube formation were captured under an inverted microscope, Nikon Eclipse Ts2-FL(Nikon Corp, Tokyo, Japan). Images of tube structure were taken with Nikon Coolpix digital camera. The tube length and number of junctions (points where several nodes or branches of endothelial cell processes are joined) were quantified with the ImageJ software with angiogenesis analysis plug-in.
2.11. Statistical analysis
All values were presented as the mean ± SEM of three experiments.Comparison among the control and treatment groups was performed with an analysis of variance with Student-Newman-Keuls posthoc test. Analysis was performed using SigmaStat ver 3.5 (Systat Software, CA, USA). Results were considered significant when P<0.05.
3. Results
3.1. Composition of RBH
RBH comprised mainly carbohydrates, followed by fiber,protein, and lipid. The main content of phenolic compounds and flavonoids in RBH was analyzed and reported previously[9]. The most abundant phenolic acids were ferulic acid, p-hydroxybenzoic acid, and protocatechuic acid, and main flavonoid compounds were rutin, isoquercitrin, and catechin. When RBH was used at the highest concentration, concentrations of phenolic compounds in the culture medium were calculated to be 4.8 µM ferulic acid, 2.2 µM protocatechuic acid, 3.2 µM p-hydroxybenzoic acid, 1.2 µM caffeic acid, and 1.3 µM p-coumaric acid, and concentrations of flavonoids were 2.9 µM catechin, 1.7 µM isoquercitrin, 1.2 µM quercetin, and 1.1 µM rutin[9].
3.2. Effects of RBH on proliferation, migration, and invasion of CCA cells
To determine whether RBH possessed antitumor effects on CCA cells, we first assessed the effect of RBH on cell proliferation in two CCA cell lines, KKU-100 and KKU-156. Results showed that RBH at concentrations up to 1 600 µg/mL had no significant antiproliferative effects in either cell type (data are not shown). We further investigated the effect of RBH on migration and invasion by wound healing assay and Transwell chamber assay, respectively.Three cell types, including KKU-100, KKU-156, and KKU-452 cells, were used in a wound-healing assay (Figure 1A, B, and C).KKU-100 was induced by IGF-1 to stimulate cell motility. Results showed that RBH markedly suppressed migration in the three CCA cell types (Figure 1D, E, and F). In the most motile cells KKU-452,migration was markedly suppressed (Figure 1C and F). In the cell invasion assay, the aggressive CCA cells KKU-452 and KKU-156 were used. KKU-452 and KKU-156 cells were treated with RBH and allowed to invade through matrigel for 6 h and 36 h, respectively(Figure 2A and C). The results showed that both cell lines were significantly inhibited by RBH in a concentration-dependent manner(Figure 2B and D).
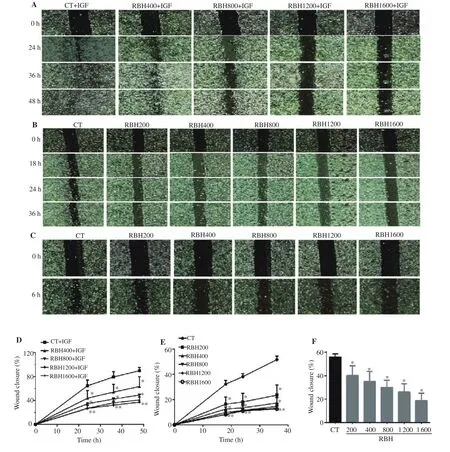
Figure 1. Effect of rice bran hydrolysates (RBH) on cell migration. Three cell lines were treated with RBH and wound gap distance was measured at indicated time points. Images of wound healing assay and graphs representing closure of the wound gap (%) in three cell lines: KKU-100 (A, D), KKU-156(B, E), and KKU-452 (C, F). CT: control; IGF: insulin-like growth factor-1; RBH200: RBH at 200 µg/mL; RBH400: RBH at 400 µg/mL; RBH800: RBH at 800 µg/mL; RBH1200: RBH at 1 200 µg/mL; RBH1600: RBH at 1 600 µg/mL; Each value represents the mean ± SEM of three experiments. *P < 0.05 vs. the corresponding time 0 as control.
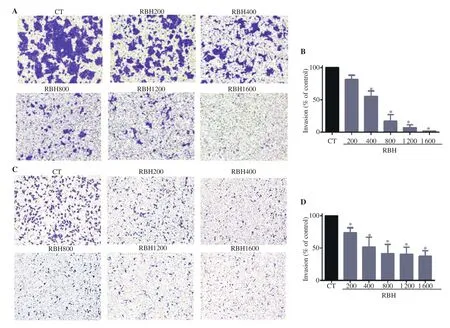
Figure 2. Effect of RBH on cell invasion. KKU-156 and KKU-452 cells were seeded onto coated Transwell inserts and incubated with medium containing RBH. Images of invading cells and bar graphs representing the number of invading cells (%) after 36 h incubation for KKU-156 (A, B) and 6 h incubation for KKU-452 (C, D) are shown. Each bar represents the mean ± SEM of three experiments. *P < 0.05 vs. the control group.
3.3. RBH inhibits CCA cells adhesion on fibronectin-coated surface
Cell adhesion is a critical step in the metastatic process. Cancer cells that detach from the primary tumor site may adhere and grow in remote tissues. We used the fibronectin cell adhesion assay to evaluate whether RBH can suppress adhesion process of metastatic cells to extracellular matrix. It was shown that 18 h of pre-treatment with RBH significantly decreased the ability of KKU-156 cells to adhere to fibronectin surface (Figure 3A and B).
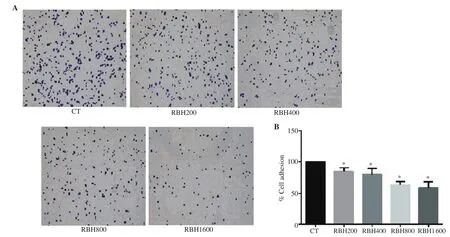
Figure 3. RBH suppresses cell adhesion. KKU-156 cells were pre-treated with RBH for 18 h before seeding onto the fibronectin pre-coated plate. (A)Images of adhered cells on fibronectin coating plate, and (B) analysis of adhesion of KKU-156 cells. Each bar represents the mean ± SEM of three experiments. *P < 0.05 vs. the control group.
3.4. RBH suppresses protein expression involving in cell motility
To evaluate molecular mechanism by which RBH affects cell migration, invasion, and adhesion, Western blot analysis was performed with RBH treated KKU-156 cells and KKU-452 cells(Figure 4A and B). The results showed that expression of p-FAK,p-PI3K, and p-Akt in KKU-156 cells treated with RBH for 6 h was strongly suppressed (Figure 4C-E). Similarly, treatment with RBH in KKU-452 cells decreased expression of p-FAK and p-PI3K, while p-Akt was significantly decreased at the highest concentration of RBH (Figure 4F-H).
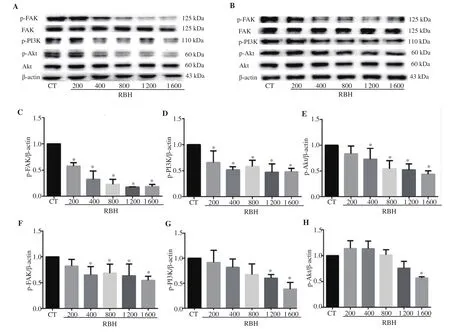
Figure 4. Effect of RBH on expression of proteins associated with cell migration and invasion. Cells were treated with RBH for 6 h and cell lysates were subjected to Western blot analysis. Representative immunoblots from KKU-156 cells (A) and analysis of p-FAK (C), p-PI3K (D), p-Akt (E), and immunoblots from KKU-452 cells (B), and analysis of p-FAK (F), p-PI3K (G), and p-Akt (H) are shown. Each bar represents the mean ± SEM of three experiments. *P < 0.05 vs. the control group.
3.5. RBH suppresses activation of NF-κB in association with EMT and its regulated proteins
EMT is an important process that endows cancer cells with invasion and metastasis. We investigated a key transcription factor of the process, i.e. NF-κB, and its downstream proteins. RBH inhibited nuclear translocation of NF-κB (Figure 5A), decreased expression of ICAM-1 (Figure 5B) and mesenchymal marker, vimentin, and increased expression of epithelium marker, E-cadherin (Figure 5C-E).
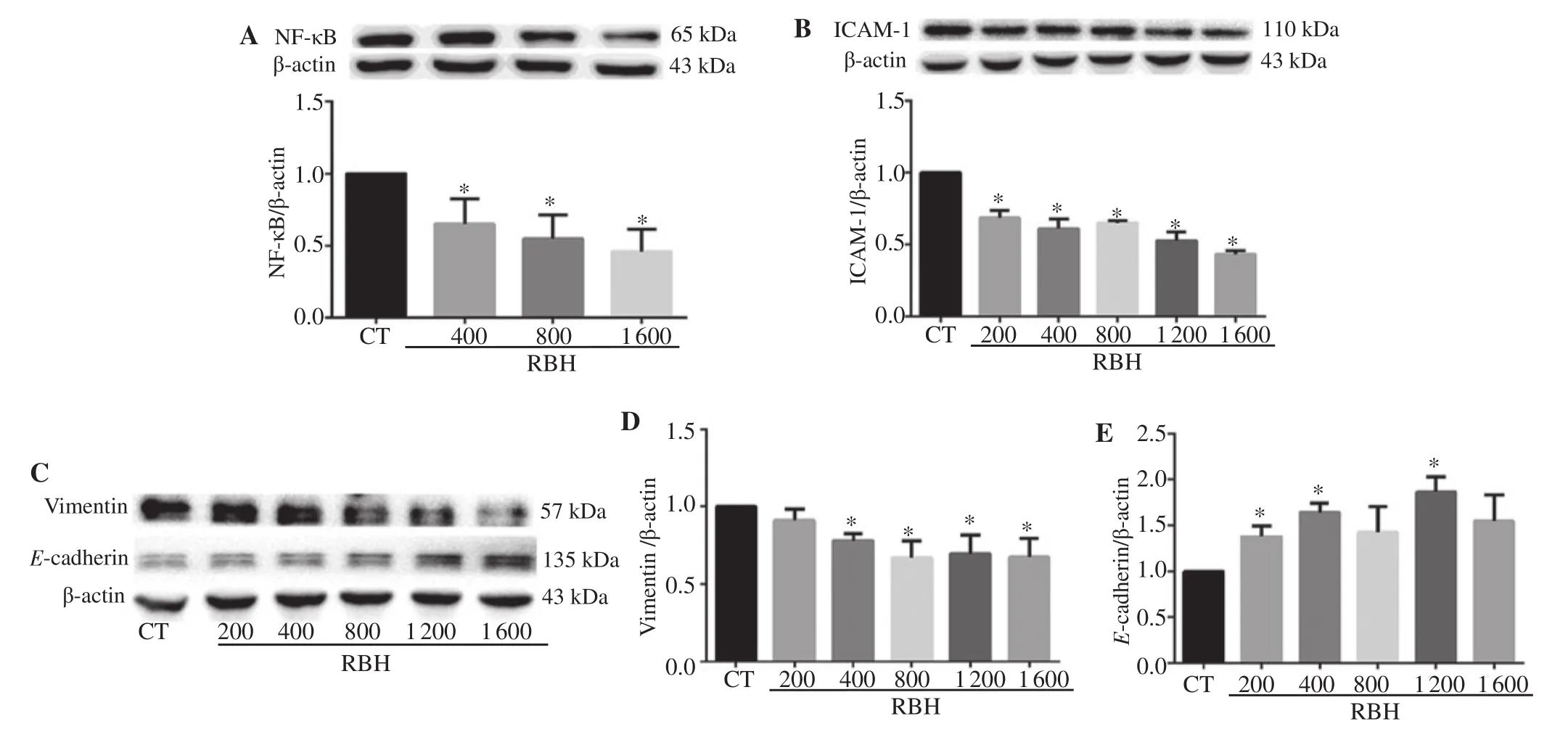
Figure 5. RBH suppresses NF-κB nuclear translocation and its regulated proteins. KKU-156 cells were incubated with RBH for 6 h, and nuclear protein was extracted and subjected to Western blot assay (A). Whole-cell protein extract was prepared and analyzed for ICAM-1 (B), vimentin (C, D) and E-cadherin(C, E). Each bar represents the mean ± SEM of three experiments. *P < 0.05 vs. the control group.
3.6. Effect of RBH on tube formation of HUVECs
This study investigated effect of RBH on tube formation by HUVECs. Results showed that the reticular network of HUVECs was apparent within 2 h. RBH at concentrations of 400 to 1 200µg/mL significantly inhibited tube network formation as shown in Figure 6A. The number of junctions was greatly reduced (Figure 6B)and the total length of tubes was significantly decreased at treatment concentrations of 800 and 1 200 µg/mL (Figure 6C). VEGF, a proangiogenic factor, obtained from cell lysates of HUVECs were analyzed and found to be significantly decreased by treatment with RBH (Figure 6D).
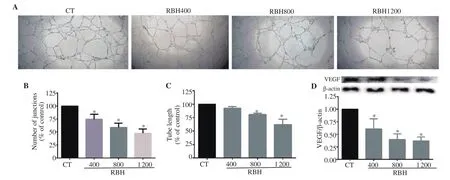
Figure 6. RBH suppresses tube formation by human umbilical vein endothelial cells (HUVECs). HUVECs were cultured on matrigel-coated 48 wellplates and treated with or without RBH for 18 h. Tube formation networks were observed under a microscope (A). Analysis of the number of junctions (B)and total tube length (C). Expression of vascular endothelium growth factor (VEGF) in HUVECs was analyzed by Western blotting assays (D). Each bar represents the mean ± SEM of three experiments. *P<0.05 vs. the control group.
4. Discussion
Rice bran has shown several biological activities. Many dietary products made from rice bran are currently available, such as rice bran oil and rice bran food supplements. Hydrolysates of rice bran in our studies are preparations in which the structure and function of carbohydrate and protein in rice bran have been modified to improve water solubility and increase applicability[1,24]. The present study investigated the antitumor properties of hydrolysates from rice bran. We demonstrated that RBH suppressed cancer cell migration,invasion, adherence to cellular matrix, and the formation of capillarylike structure. These effects are associated with down-regulation of NF-κB and proteins associated with cell motility, and EMT process.The composition of rice bran varies depending on rice varieties and the method of preparation. Our RBH from Tubtim Chumphae rice contains small peptides, hemicellulose, arabinoxylans from partial hydrolysis of protein and carbohydrate, and phenolic compounds[9].Some previous studies showed that small peptides from hydrolysates of rice bran suppressed cell growth in colon and liver cancer cells[11].However, our RBH preparation did not show antiproliferation effect on CCA cells. It is probably due to relatively lower abundance of protein present in our RBH preparation as compared to the previous report using an enriched protein fraction[11].
RBH contains a significant amount of phenolic compounds.Some compounds, such as ferulic acid, protocatechuic acid,p-hydroxybenzoic acid, isoquercitrin, rutin, and catechins, are known to possess anti-tumor activity[3,25,26]. Although individual concentrations of these phenolic compounds do not seem to be sufficiently cytotoxic to cancer cells, they suppress cell motility and invasion at sub-cytotoxic concentrations. Compounds, such as protocatechuic acid, rutin, catechin, and ferulic acid have been shown to inhibit cell migration, invasion, and metastasis at concentrations that are below the cytotoxic concentrations[26-29].Furthermore, various phenolic compounds in rice bran in the forms of esters or glycosides such as cycloartenyl ferulate and cyanin glucoside have shown to be very potent in inhibiting colorectal and prostate cancer cells[3,30]. The phenolic compounds present in RBH probably significantly contribute to its anticancer activity.
The anti-migration activity of RBH is remarkable and the effect is consistent in all three cell lines we tested, including the most motile KKU-452 cells[19]. Moreover, in an invasion assay, migration of the two highly invasive cell lines is also suppressed. The result is consistent with previous reports, which demonstrated that ferulic acid found in our RBH preparation could inhibit cell migration and invasion[29]. Metastasis is one of the hallmarks of cancer, and aggressive cancer usually shows widespread metastasis. Metastasis is correlated with a poor prognosis. RBH conferred inhibitory effects on migration, invasion, and adhesion of CCA cells. It is consistent with recent studies on protocatechuic acid, rutin, and catechin, which were found to inhibit invasion and metastasis of lung, colon, and skin cancer cells[26-28].
The process of metastasis involves several signaling pathways,including FAK, PI3K, and Akt pathways. RBH shows antimetastatic properties, and these effects are associated with suppression of activation of FAK, PI3K, and AKT. FAK, an intracellular nonreceptor tyrosine kinase, is activated in many cancers and promotes cancer progression. FAK activates downstream effectors including PI3K/Akt pathway, which confers survival and metastasis[16].Furthermore, RBH has been shown to suppress the occurrence of EMT, one of the principal mechanisms of cancer metastasis[18]. RBH down-regulated a mesenchymal marker (vimentin) and up-regulated an epithelial marker (E-cadherin). These effects are supported by recent reports, which demonstrated that anthocyanins in color rice bran suppress EMT process[30]. Suppression of several signaling pathways, i.e. FAK, PI3K/Akt, and EMT, maybe the mechanism by which RBH suppresses cell motility and invasion.
NF-κB is a critical transcription factor that plays an important role in inflammation and cancer progression. It promotes cancer survival, EMT, invasion, adhesion, and neovascularization by the up-regulation of PI3K-Akt, FAK, ICAM-1, and VEGF signaling pathways[15]. RBH suppressed NF-κB activation in CCA cells,which is correlated with decreased cell motility, invasion, and adhesion. Our results are consistent with previous studies, which showed that ferulic acid at low micromolar concentrations inhibits angiogenesis in association with down-regulation of NF-κB, PI3K/Akt pathways[29]. Moreover, key molecules participated in metastasis include adhesion molecules. And ICAM-1, an important adhesive molecules, appears to have a central role in the metastatic cascade by initiating the cell-cell contacts leading to cancer cell extravasation,colonization, and growth in distant organs[31].
Angiogenesis is one of important characteristics of cancer. It results in outgrowing vascularized tumors and leads to tumor progression[32]. Various growth factors stimulate the release of proangiogenic factors such as VEGF from cancer and endothelial cells,leading to formation and stabilization of new blood vessels[32]. RBH inhibits growth factor induced-angiogenesis in tube formation assay of HUVECs. The effect was associated with down-regulation of VEGF. It is suggested that the inhibition of angiogenesis could be another potential mechanism in suppression of cancer progression.The study is limited as it is an in vitro study performing in cell culture models. Further proofs of effect of RBH on cancer development are needed in animal studies.
In summary, RBH suppresses cancer cell motility, invasion through cellular matrix, cell adhesion, and formation of tube-like capillary structures. The effects are associated with down-regulation of FAK,PI3K-Akt, and NF-κB signaling pathways in association with inhibition of the EMT process. RBH contains multiple compounds that show a potential nutraceutical for cancer prevention.
Conflict of interest statement
We declare that there is no conflict of interest.
Funding
This work is supported by Bureau of Rice Research &Development, Thailand, and Grant-in-aid from Faculty of Medicine(IN62133), Khon Kaen University, Thailand.
Authors’ contributions
VK conceived the study. SP, LS, AP and SK designed and performed the experiments. ST and RC prepared rice bran hydrolysates. UK, SP, SS, TC and RC analyzed rice bran compositions, VK, SP, UK, and SS analyzed the data. SP, VK, and UK wrote manuscript. All authors approved the final manuscript.
 Asian Pacific Journal of Tropical Biomedicine2021年7期
Asian Pacific Journal of Tropical Biomedicine2021年7期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- New trends in pharmacological treatment of acute kidney injury
- Synergistic effect of flavonoids combined with antivenom on neutralisation of Naja naja venom
- Antioxidative, cytotoxic, and anti-metastatic potentials of Laurencia obtusa and Ulva lactuca seaweeds
