New trends in pharmacological treatment of acute kidney injury
Heba M.I. Abdallah
Department of Pharmacology, Medical Research Division, National Research Centre, Giza 12622, Egypt
ABSTRACT
KEYWORDS: Acute kidney injury; Recent therapy; Promising pharmacological targets
1. Definition and epidemiology of acute kidney injury(AKI)
AKI affects both structure and functions of the kidney. It is defined as a sudden decrease in kidney function which occurs within hours or days resulting in azotemia, and irregularities in fluid, electrolyte,metabolic homeostasis, and acid-base balance. AKI is considered as an independent cause of admission to intensive care unit. Its mortality may reach 50% in elderly patients[1]. Patients with noncomplicated disease have a 10% mortality rate; however, mortality rate reaches up to 80% in patients with other organs’ failure[2]. In Egypt, AKI occurs in 40% of patients admitted to surgical intensive care[3].
The old nomenclature of acute renal failure (ARF) is now replaced by AKI. Kidney failure is one stage of AKI and is developed when glomerular filtration rate (GFR) is less than 15 mL/min per 1.73 mof body surface area or if there is a need for renal replacement therapy[4].Renal failure, unlike AKI, is not a reversible stage. In most cases, AKI is caused by acute ischemic or toxic insults affecting mainly proximal convoluted tubule (PCT). Thus, the term acute tubular necrosis (ATN)is a recently used synonym.
2. Diagnosis of AKI
Filtration and excretion of nitrogenous waste products (urea and creatinine) are the major functions of the kidney. Therefore, their elevated level is an indicator of diminished renal function and decreased GFR. Based on the RIFLE criteria (the first international consensus criteria describing AKI)[5], AKI can be determined by one of the following:
1. Serum creatinine increases to ≥ 0.3 mg/d in 48 h, or
2. Serum creatinine increases by ≥ 1.5 times baseline in the preceding 7 days, or
3. Urine volume reaches less than 0.5 mL/kg/h for 6 h.
3. Etiologies and pathological outcomes
The kidney is a fairly conservative organ that tolerates various toxic insults and it has a remarked capability to regenerate.Generally, pathogenesis of AKI is identified by the injury to renal epithelial cells and vasculature which is associated with widespread inflammatory response. The damage may spread to the glomerulus and interstitium leading to established disease[6].
Reactive oxygen species and metabolic products produced upon oxidative stress act as ligands for toll-like receptors potentiating the harmful process in AKI. These circulating “toxins” are considered as inflammatory mediators that trigger the expansion of injury and hemodynamic imbalance. In critically ill patients, AKI and oxidative stress preserve a bidirectional relationship[7]. Numerous experimental and some clinical data showed the important role of oxidative stress during the injury phase of AKI. Vinpocetine inhibited diclofenac-induced AKI consequences by ameliorating oxidative stress, apoptosis, and NF-κB activation[8]. In another study, pyrrolidine dithiocarbamate decreased oxidative stress, proinflammatory cytokine production, and NF-κB pathway activation.Moreover, it increased antioxidant defenses and anti-inflammatory cytokine IL-10[9]. Antioxidant activity, inhibition of COX-2, and renal PGEinduction suggested possible mechanisms mediating the nephroprotective effect of one of the natural products such as the artichoke extract[10].
Accumulating evidence shows that inflammation plays a key role in the pathophysiology of AKI. Firstly, microvascular permeability is increased due to endothelial injury resulting in recruitment of inflammatory cells to the injured kidney. Then, under hypoxic conditions, proximal tubular cells produce pro-inflammatory and pro-fibrotic factors accompanied by inflammatory cell infiltration[11].Injury is progressed to interstitial fibrosis and/or chronic kidney disease if tubular epithelial and endothelial injury exceeds the regenerative potential. Therefore, keeping the kidney away from inflammation and promoting epithelial/endothelial repair is crucial to treat AKI. Currently, anti-inflammatory therapies targeting different inflammatory pathways have been used for prevention and treatment of AKI[12]. However, a better understanding of the putative cell types involved in injury and repair is required before new targets are developed. Also, the signals that amplify injurious response or repair and the detailed interaction between both parenchymal and immune cell types should be well studied.
Pathogenic features and clinical picture of AKI differ according to the etiologic factor. However, the pathophysiology of AKI generally shares some common pathogenic denominators including inflammation, cell injury/death, and fibrosis. AKI encompasses a variety of etiologic or risk factors that are exacerbated by the presence of susceptibility factors such as dehydration or volume depletion as manifested in chronic diseases e.g. cardiac, pulmonary,hepatic diseases; advanced age, and anemia. The different etiologies of AKI are mainly classified into 3 main categories, namely, prerenal, intrinsic, and post-renal causes of AKI (summarized in Table 1)[13].
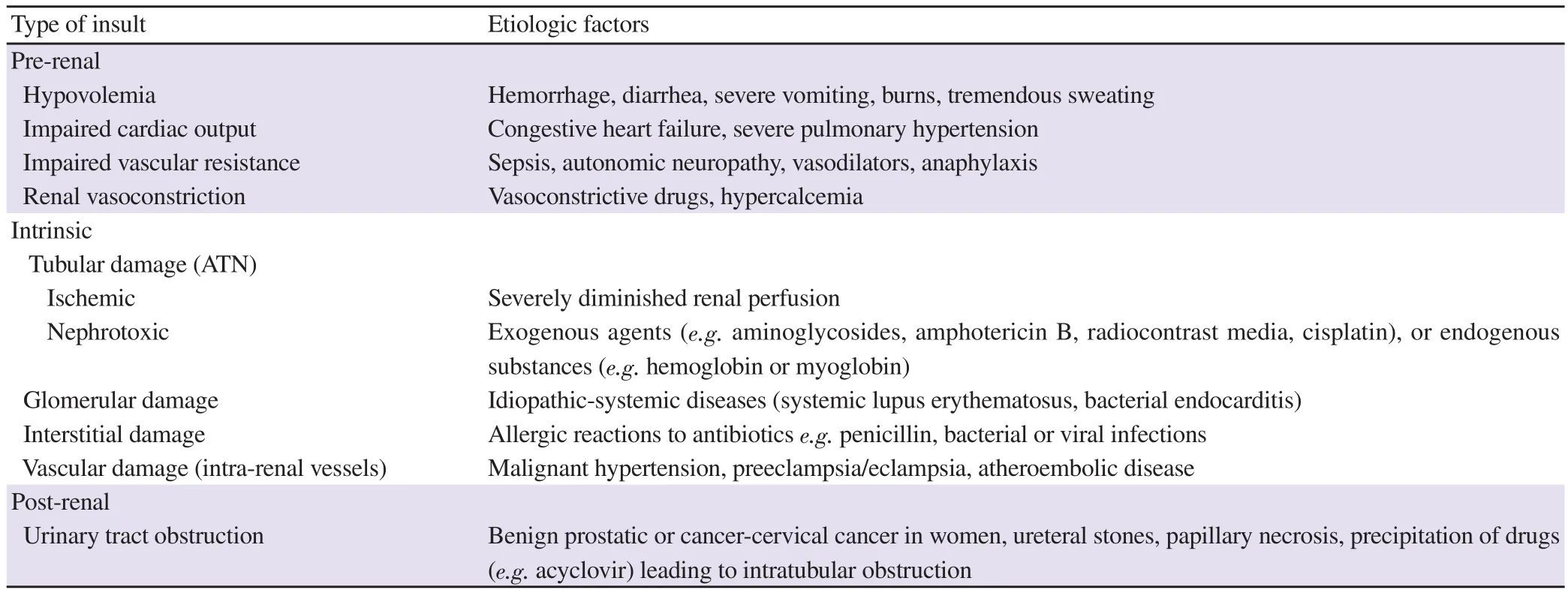
Table 1. Etiology of acute kidney injury due to different insults.
AKI is mostly converted later to chronic kidney disease especially if complicated with other comorbid factors. Recent observations have shown that even a single episode of AKI could lead to subsequent chronic kidney disease if not treated properly[14]. Patients who are discharged from hospital after resolution of AKI episode are at increased risk of end-stage renal disease (ESRD)[15].
4. Barriers to effective treatment
To date, no drug has completely prevented, treated AKI, or enhanced complete recovery from kidney failure despite the extensive experimental and clinical studies. Failure of pharmacotherapy until now can be illustrated by the presence of many barriers for treatment of this multi-faceted disease, including the following aspects:
4.1. Complexity of disease pathogenesis
A number of pathogenic factors arise due to single insult and interact with each other such as vascular congestion, vasoconstriction,leukocyte infiltration, immune modulation, and activation of growth factors. Management of the late events in AKI or only a single pathway under the umbrella of disease pathogenesis does not gain success. On the contrary, compounds that target multiple pathways or combination therapies could be more effective[16].
4.2. Presence of comorbid factors and involvement of other systems
Incidence of AKI increases when it is associated with the presence of extra-renal complications such as coronary artery disease, chronic liver disease, and diabetes. AKI also leads to systemic effects that involve other distant organs e.g. cardiopulmonary dysfunction[17].
4.3. Disconnection between animal and human studies
Indeed, experimental models don’t translate correctly to the actual clinical picture of AKI. The majority of experimental animal studies are investigating mainly “prophylactic” not “therapeutic”effect of drugs, while patients are diagnosed after development of kidney disease. These studies also rarely consider the presence of comorbid factors and involvement of other organs. They also lack sex heterogeneity, randomization, pathological features resembling human and combination therapy that targets more than one pathophysiological pathway[18].
In addition, designing a proper clinical study is difficult due to the absence of definite early end points as a pathophysiological signature. Up to 60% in Europe develop ESRD with unknown cause[19].
5. Pharmacological management
Recent studies have considered the above barriers as a way to develop new drugs that can gain success in clinical application or to find new renoprotective effects of clinically approved drugs.Herein, investigated drugs or natural products will be reviewed in the following section and summarized in Table 2.
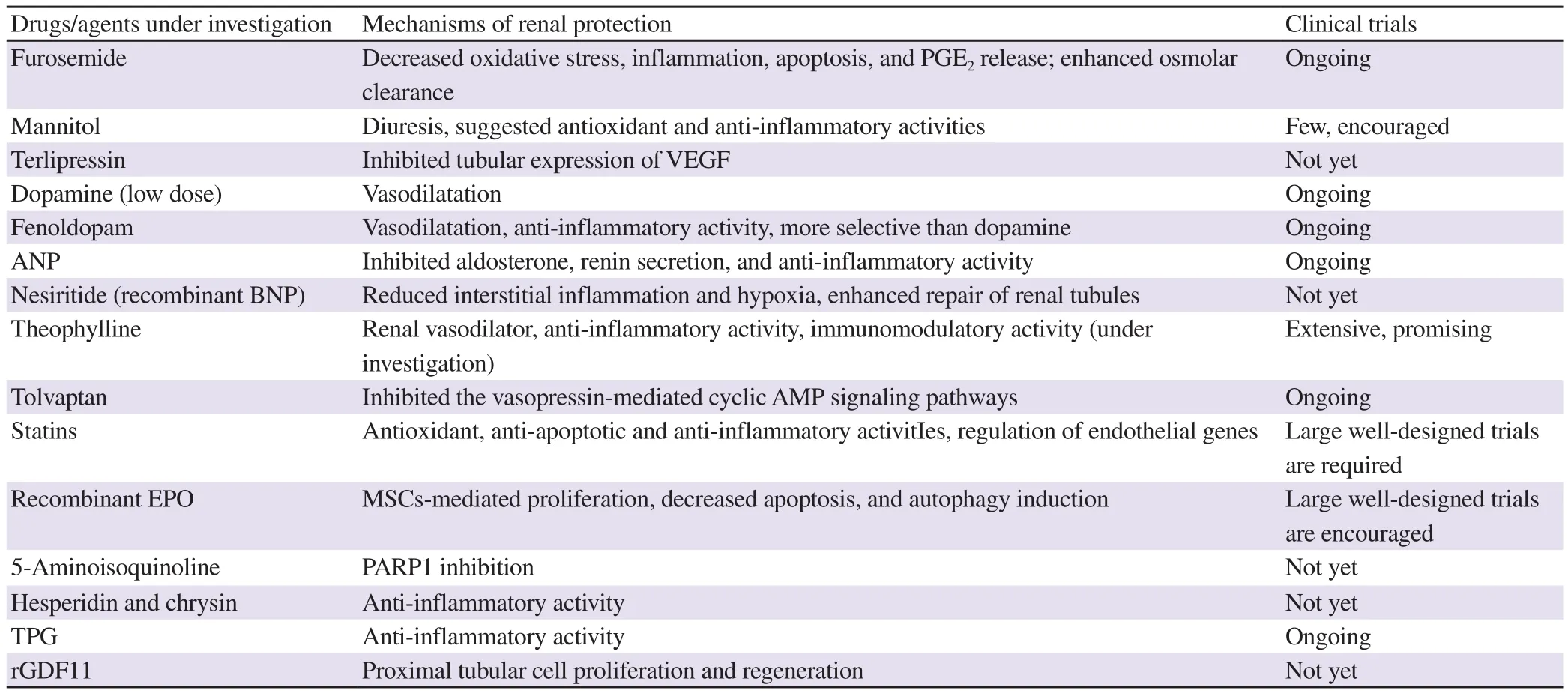
Table 2. Summary of agents having new pieces of evidence on treating potential of acute kidney injury.
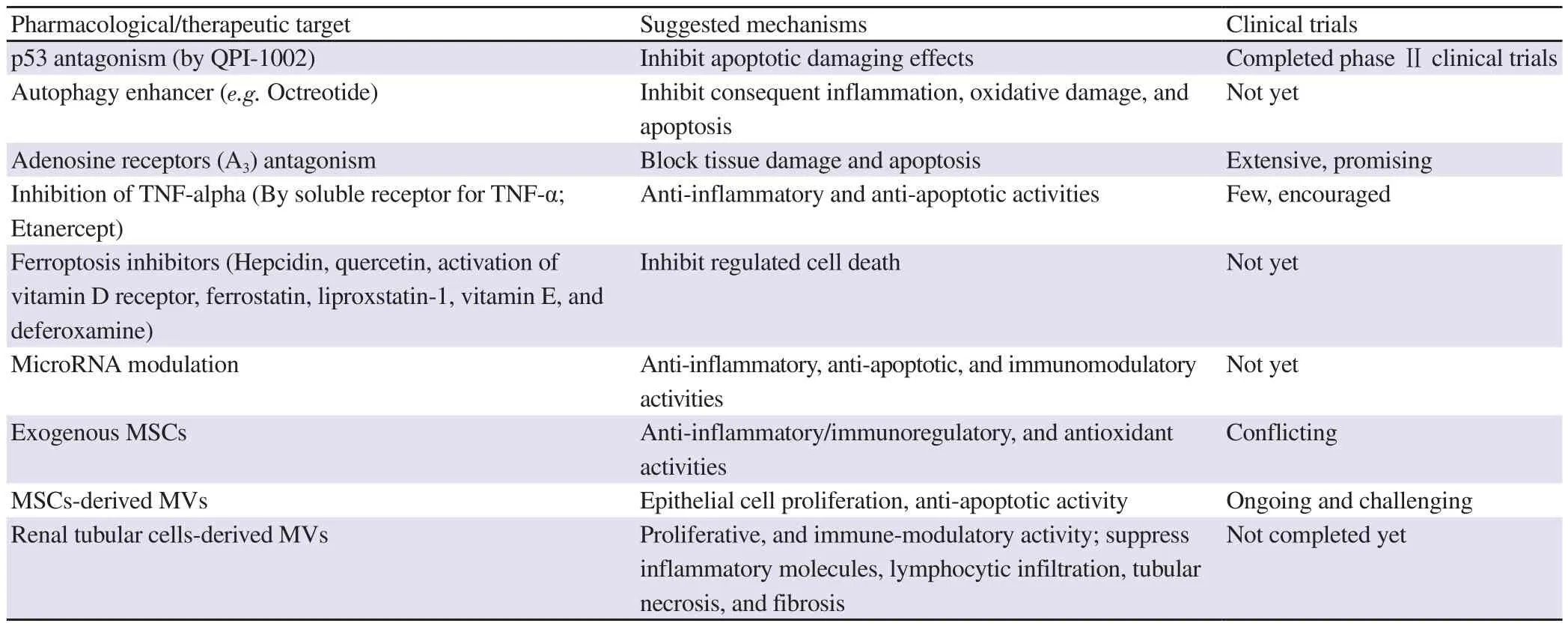
Table 3. Promising therapeutic targets for AKI treatment.
5.1. Commonly used drugs for patients with AKI
The first pharmacological approach in the treatment of AKI is medications that are commonly used in intensive care for supporting AKI. However, new pieces of evidence have been explored for their therapeutic efficacy in preventing renal disease.
5.1.1. Loop diuretics
This class of diuretics such as furosemide, bumetanide,and torasemide acts mainly at the loop of Henle (where Nareabsorption reaches up to 40%) and inhibits Na-K-2Clcotransporter preventing HO and Naretention. They are effective diuretics that are approved by Food and Drug Administration for edema accompanied congestive heart failure, renal failure, and cirrhosis. Loop diuretics could protect against hypoxic conditions in juxtamedullary regions due to oxidative stress. This action was achieved by decreasing GFR and tubular workload resulting in decreased Oconsumption combined with increased medullary oxygenation. They also improved renal blood flow (RBF) by decreasing renal vascular resistance[20].
Infusion of furosemide blocked renal ischemia/reperfusion (I/R)-induced apoptosis[21], prevented tubular obstruction after ischemia through enhanced tubular flow with flushing debris in ATN. Furosemide also increased RBF via enhanced prostaglandin (PG)Erelease in renal vein. Other notions support that furosemide acts independent of RBF manipulation, probably via triggered osmolar clearance.
In a recent study, Youssef and coworkers found that furosemide administration alone or combined with sitagliptin (a dipeptidyl peptidase-4) has conferred protection against both renal and cardiac deterioration due to I/R in rats through decreasing oxidative stress,inflammation, and apoptosis in both kidney and cardiac tissues. The combination, however, showed superior benefit in preventing AKI and produced a new therapeutic intervention strategy to improve the poor clinical outcome observed with individual furosemide therapy[22].
Treatment with furosemide in patients undergoing cardiac surgery also decreased susceptibility to AKI by increasing urine flow and fractional excretion of Naaccompanied by decreased renal Oconsumption[23].
5.1.2. Mannitol
Mannitol is one of osmotic diuretics that is mainly used in situations that need increased urine volume such as hemolysis or rhabdomyolysis. It prevents anuria resulting from loading of large compounds in the kidney[24]. The main diuretic action of mannitol is exerted on the PCT and descending limb of Henle loop.
Intravenous mannitol is the first and to date the only approved medication by FDA since 1964 to treat AKI. It was mainly used to promote diuresis for treatment of the oliguric phase of AKI before the establishment of irreversible kidney failure especially that follows rhabdomyolysis. This effect can be due to its capability to reduce tubular toxicity through increasing urinary flow, minimize tubular cell swelling, and dilate glomerular capillaries.
Such a mechanism renders mannitol an effective treatment for ATN as it washes away material that would plug the tubules. Additionally,mannitol acts through extra-renal mechanisms including free radical scavenging, decreasing skeletal muscle oedema, and stimulating prostaglandin (PG) release. It is frequently added to the cardiopulmonary bypass pump prime to reduce the incidence of renal dysfunction[25].
When patients were given 250 mL of 20% mannitol before vessel clamp removal, they were unlikely to produce post-transplant dialysis. However, such an effect was maintained for only three months after transplantation[26].
The protocol of mannitol administration has been recently modified to be infused 15 min before clamping the renal artery. Accordingly,mannitol could achieve better protection[27]. Being an osmotic agent, mannitol opposes the small molecules inside the ischemic cells after transplantation by creating a hypertonic medium around the cells[28]. Another mechanism was proposed for the protective effect of mannitol against I/R that was induced in rats by testicular torsion. Mannitol also showed antioxidant and anti-apoptotic effects.However, these mechanisms are still to be tested in AKI models[29].
Importantly, mannitol should be used with caution because when patients are treated with mannitol at a dose higher than 200 g/day,AKI may get worse and it should be discontinued if osmolality gap is increased[30].
5.1.3. Vasoactive drugs
They are drugs used to attain normal perfusion pressure for the kidneys and mainly used for hypotensive AKI.
Vasopressin [arginine vasopressin (AVP); antidiuretic hormone(ADH)] is a potent vasoconstrictor and antidiuretic neuropeptide hormone. AVP acts on the kidneys by increasing HO reabsorption and binding to vasopressin (V) receptors in the renal collecting duct increasing GFR and creatinine clearance. The antidiuretic effect of AVP predominates under normal conditions and it induces vasoconstriction only when it is given at a high concentration[31].
The data obtained from clinical studies that compared the beneficial effects of AVP and norepinephrine showed different results. AVP had more efficacy in inhibiting the progression to renal failure and reduced mortality in patients who had septic shock than norepinephrine[32]. In contrast, a more recent study showed that AVP could not inhibit the progression of kidney failure compared with norepinephrine. The plausible explanation for this observation is that AVP might attenuate kidney failure in high-risk patients but it had a little effect when AKI is established. Harmful interaction between vasopressin and norepinephrine at a high dose would also contribute to that effect[33].
Terlipressin (triglycyl lysine vasopressin) is a synthetic analog of AVP with superior advantages over AVP because of its higher selectivity for Vreceptors and possessing of longer half-life.Terlipressin improved survival in AKI patients complicated with liver failure and high-risk populations who received liver transplantation[34]. In experimental studies, terlipressin readjusted renal function and decreased ATN. It also minimized the expression of vascular endothelial growth factor (VEGF); the mediator involved in excessive angiogenesis accompanied by renal injury[35].
5.1.4. Renal vasodilators
5.1.4.1. Dopamine
Dopamine is a main neurotransmitter in the central nervous system.Low-dose (renal-dose) dopamine (i.e. 1-3 μg/kg per min) has long been used for the treatment of ARF induced by ischemia, toxins,and/or sepsis. This dose was beneficial in enhancing RBF, and sodium excretion in healthy humans, and it shows renoprotective effect in several models of AKI (ischemic or nephrotoxic animal models). However, clinical trials did not support the use of dopamine at this renal dose in the prevention of ARF. It has been recommended that dopamine should not be used at a low dose to prevent or treat this disease[5].
Interestingly, a recent study showed the beneficial effects of dopamine. Infusion of dopamine (2 μg/kg/min) in combination with mannitol (1 g/kg) under the course of cardiopulmonary bypass could prevent deleterious effects on the kidney and preserve renal functions[36]. The low dose dopamine added to human atrial natriuretic peptide was not harmful to the kidney in acute heart failure patients demonstrating diuretic resistance to human atrial natriuretic peptide[37].
5.1.4.2. Fenoldopam
Fenoldopam is a dopamine-1 receptor agonist that is highly selective and preferentially exerts dilatation of the renal and splanchnic vasculature. It is mainly used for the management of hypertension by decreasing systemic vascular resistance.Simultaneously, it increases RBF and promotes natriuresis and diuresis via direct tubular action and thus it is not affected by renal impairment as other diuretics.
Despite conflicting data about the therapeutic role of fenoldopam in the management of AKI, a considerable body of evidence supports its beneficial effect[38] and favors its use over dopamine.
In contrast to dopamine, fenoldopam has no activity on α or β receptors, so consequent deleterious effects are not found after its administration. Besides, it has an anti-inflammatory activity beyond its vasodilatory effect[39].
5.1.4.3. Natriuretic peptides
Natriuretic peptides are hormones that are secreted by the heart in response to increased cardiac stretch and other stimuli due to volume overload. Atrial natriuretic peptide (ANP), a 28-amino acid peptide,is synthesized by atrial myocytes. Brain natriuretic peptide (BNP) is another 32-amino acid peptide that is synthesized in the brain and the heart. ANP and BNP are both systemic as well as renal vasorelaxants and vasodilators.
At the initial phase of AKI, ANP vasodilates the pre-glomerular artery, interferes with angiotensin axis, and prostaglandin release.It also shows natriuretic effect during the reflow period of AKI and thus prevents tubular obstruction. Studies on humans showed that ANP reduced the incidence of AKI following I/R during cardiac surgery due to inhibition of renin-angiotensin-aldosterone system[40]and had renoprotective effects as BNP and fenoldopam rather than other drugs such as dopamine, diuretics, or N-acetylcysteine[41]. ANP inhibited aldosterone secretion through direct action on adrenal and indirectly abolished renin release from the kidney. It also exhibited anti-inflammatory effects in a rat model of I/R injury[42].
5.1.4.4. Nesiritide
Nesiritide is a recombinant BNP approved by FDA for the treatment of acute heart failure which is symptomatic and associated with volume overload. Strong evidence recommends its use as a therapeutic agent for adjusting or preventing AKI. Nesiritide decreased oxygen consumption in mitochondria in response to decreased angiotensin Ⅱ and improved kidney damage via decreasing interstitial cell swelling and tubular epithelial swelling in sepsis induced by endotoxin in canines[43]. BNP delivery reduced hypoxia and enhanced repair of renal tubules[44].
5.1.5. Adenosine antagonists (theophylline)
Theophylline, also known as 1, 3-dimethylxanthine, was approved as a bronchodilator for treatment of respiratory diseases such as asthma and chronic obstructive pulmonary disease. It acts as a non-specific antagonist of adenosine receptors; A, A, and A[45].Theophylline exerts other pharmacological effects such as antiinflammatory and immunomodulatory effects[46].
Theophylline pretreatment considerably attenuated renal vasoconstriction in contrast-induced kidney injury in randomized,well-controlled, and meta-analysis studies[47]. Adenosine is a primary vasoconstrictive mediator that triggers afferent arteriolar vasoconstriction and decreases GFR. Thereby, the good effects of theophylline seem to be mediated through its adenosine antagonism in conditions at which renal vasodilation is requested. Actually, the subtypes of adenosine receptors have diverse roles in the progression of AKI, and research on them holds controversial data. Aactivation decreases necrosis,apoptosis, inflammation, and metabolic demand. The Amodulates leukocyte-mediated inflammation when stimulated. Asubtype exacerbates tissue damage and apoptosis after I/R. Hence, selective antagonism to this subtype opens the door for development of new effective therapies for AKI[48].
6. Repurposed drugs
6.1. Tolvaptan
Tolvaptan is an oral and selective non-peptide antagonist for vasopressin-2 receptors which acts mainly on the distal nephrons that oppose the actions of AVP. It is clinically applied for conditions associated with hyponatremia (low levels of Nain blood) as congestive heart failure and cirrhosis.
Tolvaptan appeared as a favorable candidate for AKI evidenced by a dose-dependent increase in urine volume, a decrease in urine osmolality, and slowing the onset of ESRD[49]. Probable mechanism involved in these renoprotective effects is inhibition of the AVP-mediated cyclic AMP signaling pathways which mainly contributes to AKI pathogenesis.
6.2. Etanercept
Etanercept, a soluble receptor for tumor necrosis factor-alpha(TNF-α), was licensed by FDA for the treatment of rheumatic diseases, particularly polyarticular juvenile, psoriatic arthritis, and ankylosing spondylitis, and plaque psoriasis.
TNF-α is a cytokine produced by macrophages and lymphocytes.It attracts more white blood cells to the site of inflammation and mediates an immune response that potentiates the inflammatory response. Through inhibition of these actions, etanercept is especially effective in the treatment of autoimmune diseases.
Etanercept action resembles the inhibitory effects of the naturally occurring soluble TNF receptors. However, being a fusion protein instead of a simple receptor for TNF, it possesses a prolonged halflife in bloodstream. Thus, its biologic efficacy is more profound and long-lasting.
Additionally, etanercept showed renoprotective effects in a number of experimental models through its anti-inflammatory and antiapoptotic effects. It lowered the expression of monocyte chemotactic protein-1 (which is involved in the pathogenesis of inflammatory diseases), caspases, and other apoptotic markers[50].
A case report has recently shown the development of renal sarcoidosis which may predispose to renal impairment due to etanercept administration[51]. Nonetheless, etanercept remains a challenging therapeutic agent for the prevention of kidney injurycomplicated with other disorders.
6.3. Statins
Statins are drugs that lower low-density lipoprotein cholesterol,and are used clinically for the prevention of coronary artery and atherosclerotic diseases such as ischemic heart attack. A number of statins were approved by FDA, e.g. simvastatin, pravastatin,cerivastatin, and atorvastatin. They inhibit cholesterol biosynthesis by blocking the action of HMG-CoA reductase.
Statins had attracted attention due to their renoprotective effects in contrast-induced, ischemic, and septic AKI in animal models[52].Simvastatin prevented kidney injury by a direct effect on renal vasculature in sepsis-induced AKI. Postoperative statins use also decreased incidence of AKI and mortality rates after cardiac surgery[53]. In fact, statins possess pleiotropic effects beyond their lipid-lowering effect such as antioxidant, anti-apoptotic, and antiinflammatory. During renal I/R injury, cerivastatin was found to induce heme oxygenase-1 expression in infiltrating macrophages[54].
One novel mechanism has been recently described for the pharmacological effect of statin. They regulate endothelial cell genes, preventing endothelial-mediated injury inflammation and vascular congestion[55]. Statins showed beneficial therapeutic effects in preventing contrast media-induced AKI. However, a large number of well-designed trials are still required due to heterogeneity of small clinical trials[56].
6.4. Erythropoietin (EPO)
EPO is a glycoprotein hormone that is produced by the kidney in response to low oxygen and it is implicated in the regulation of red blood cell development via controlling erythropoiesis. Recombinant EPO preparations have been proved to be safe medications for the treatment of chronic anemia (after receiving RBCs transfusion),particularly when it is associated with chronic kidney diseases,chemotherapy, zidovudine therapy for HIV, and preterm infants[57].A large body of evidence showed that EPO had additional protective effects on kidney tissues[58]. Recombinant EPO improved experimental AKI induced by different insults such as I/R and a number of nephrotoxic drugs through anti-inflammatory and antiapoptotic mechanisms via acting on EPO receptors. Recently,EPO has been found to increase migration of mesenchymal stem cells (MSCs) derived from bone marrow to the site of injury, thus,enhancing proliferation and decreasing apoptosis[59].
One clinical study in patients with coronary artery bypass graft reported the improvement of postoperative AKI when EPO was given at a dose of 300 U/kg i.v. before the surgery[60].
However, another study with a larger population showed that EPO(500 U/kg i.v.) exerted no renoprotective activity in intensive care patients[61]. Currently, data from larger clinical trials investigating the therapeutic effect of EPO in AKI are still awaited.
7. Novel therapeutic targets
7.1. Autophagy
Autophagy is a cytoprotective mechanism that essentially causes degradation and recycling of damaged organelles and macromolecules in response to stress conditions including hypoxia,and oxidant injury. Autophagy suppresses inflammatory and apoptotic pathways and readjusts homeostasis in the PCT[62].Therefore, autophagy is a potential therapeutic target and is important in the pathogenesis of renal injury.
Octreotide is an approved drug for the treatment of acromegaly.It mimics the action of the natural hormone somatostatin, thus inhibiting growth hormone synthesis in patients with acromegaly. It has been recently found that octreotide could attenuate renal injury by inducing autophagy after ischemic injury[63].
Autophagy is not such a single pathogenic feature of renal injury.There is a cross-talk between autophagy and oxidative stress. As discussed before, reactive oxygen species-mediated oxidative stress is one of the leading causes of AKI. Currently, studies have shown that autophagy is essentially up-regulated in response to oxidative stress in different animal models including those of AKI. There is also an interplay between Nrf2 signaling pathway (the main protectant of the kidney against oxidative stress) and components of autophagy[64].
Recent progress in identifying the interaction between autophagy,apoptosis, and regulated necrosis has received attention in examining common pathways/molecules during the pathogenesis of AKI. The study by Li et al.[65] showed that recombinant EPO suppressed sepsis-induced cell apoptosis via modulation of AMPK/SIRT1 pathway mediated autophagy, therefore preventing AKI. It should be known that AMPK/SIRT1 pathway is one of the important pathways involved in cell metabolism and apoptosis. Also, autophagy is regulated and induced via activation of phosphorylated AMPK-alpha(p-AMPKα).
Similarly, autophagic response has been linked to suppressed inflammation during AKI. Like AMPK, mTOR is from the key components of autophagy signaling pathway as well. Up-regulation of p-AMPK and down-regulation of p-mTOR attenuated AKI via induction of autophagy. Accordingly, inflammatory cytokine accumulation and tubular cell apoptosis were inhibited in the injured kidneys[66]. Enhancement of autophagic response plays a vital role in the prevention of inflammatory mediators and can protect against lipopolysaccharide-induced AKI[67].
7.2. Ferroptosis
Iron plays an essential role in physiological cell functions and is involved in erythropoiesis, DNA synthesis, and hypoxia signaling.The kidneys regulate iron metabolism to avoid toxicity that would arise from free or labile iron. Ferroptosis is a type of regulated necrosis which is an iron and reactive oxygen species-dependent form of regulated cell death. Ferroptosis or iron-mediated tissue injury is involved in some conditions of AKI where heme and nonheme iron play a key pathophysiologic role. This occurs during cardiac surgery or in renal transplant recipients who have undergone I/R. Herein, renal tubular cells were found to undergo sequential death after ischemic kidney injury[68].
Swaminathan[69] proved the role of iron homeostasis in the pathogenesis of AKI. Hepcidin, a known regulator of iron homeostasis, could protect against AKI via ferroptosis inhibition when administered 24 to 48 h before cardiac surgery-induced ischemic renal injury.
Ferroptotic cells are capable of recruiting macrophages, so they are likely to be extremely pro-inflammatory. Quercetin inhibited the chemotaxis of macrophages and modulated ferroptosis downstream signaling pathway. Therefore quercetin was suggested as a potent ferroptosis inhibitor and potential therapy for AKI[70]. Moreover,activation of vitamin D receptor has been recently found to protect against cisplatin-induced renal injury and prevent ferroptosis by trans-regulation of glutathione peroxidase-4[71].
Lipid peroxidation has a strong relationship with the development of ferroptosis and radical-trapping antioxidants are proved to be potent ferroptosis inhibitors. Ferrostatin was reported to attenuate tissue damage caused by lipid peroxidation in different diseases,including acute kidney disease. In-vivo studies showed that liproxstatin-1, a ferroptosis inhibitor, inhibited ferroptosis by lipid peroxide clearance[72].
Additionally, ferroptosis was suppressed by liproxstatin-1 in human renal proximal tubule epithelial cells, ischemia-reperfusion kidney injury, and in Gpx4−/− kidney mouse model[73]. Antioxidants and iron chelators (such as vitamin E and deferoxamine) were also observed to inhibit ferroptosis by reducing iron availability.
7.3. Cellular regeneration
In recent years, a subpopulation of tubular cells that were identified and found to become abundant in response to AKI are called scattered tubular cells and are involved in regeneration processes[74].This discovery may revolutionize the therapy of kidney diseases and understanding its pathways may open new strategies to treat or prevent AKI. Recombinant growth differentiation factor 11 promoted the proximal tubular cell proliferation and regeneration via stimulation of scattered tubular cells in aged ischemic mice[75].
7.4. MicroRNA modulation
MicroRNAs (miRNAs) are small non-coding RNAs that have gene regulatory functions. They can degrade or inhibit translation of the mRNA by targeting the 3′ untranslated region on the mRNA of a specific target gene. This process is known as post-transcriptional regulation as miRNA affects the expression of more than half of the gene-encoded and participates in nearly all cell biological processes.However, it is also involved in the pathology of several diseases.MiR-155 plays a key role in different pathophysiological processes such as inflammatory, immune responses, viral infection, and multiple cancers due to different expression profiles[76].
Recently, researchers have found that increased expression of miR-155 is observed during renal tubular injury of diabetic kidney disease and can even promote fibrosis in proximal tubule cells[77,78]. The miR155/TCF4/Wnt/β-catenin is a novel identified signal axis which was shown to promote AKI-induced renal apoptosis and provides a new direction for the treatment of AKI[79]. Similarly, miR-182 enhanced AKI by promoting apoptosis following regulation of the same pathway. Inhibition of miR-182 improved kidney function and morphology in animal models of AKI[80,81].
MiR-146a, on the other hand, was previously found to be downregulated in renal tissue samples and cell lines in response to injury. The restoration of miR-146a inhibited the activation of the NF-κB pathway, thus suppressing the cell inflammatory response and promoting cell proliferation. MiR-146a is considered a protective anti-inflammatory and anti-apoptotic molecule against lipopolysaccharide-induced AKI[82].
8. Promising therapeutic approaches
8.1. Inflammation inhibitors
Poly (ADP-ribose) polymerase 1 (PARP1) overactivation seemed to be linked to cisplatin-induced renal injury. It triggered the depletion of NAD+ and ATP which induced up-regulation of key inflammatory pathways and cellular death. 5-Aminoisoquinoline inhibited PARP1 during the recovery phase of cisplatin-induced AKI by ameliorating oxidative, nitrosative, and inflammatory cascades that were engaged in cisplatin-induced renal interstitial fibrosis[83]. Overactivation of the PARP1 pathway also plays a key role in vancomycin-induced renal impairment[84], endotoxin-induced AKI[85], and regulation of pro-inflammatory gene expression[86]. 5-Aminoisoquinoline did not enter clinical trial phase yet. However, it was found to be devoid of any genotoxic activity in both in vitro and in vivo systems substantiating its therapeutic value[87].
In addition, natural anti-inflammatory agents were found to be beneficial for AKI. Hesperidin and chrysin improved colistininduced renal injury via their anti-inflammatory activities by downregulation of interleukin-1β (IL-1β), interleukin-6 (IL-6), and TNF-α levels[88].
A novel triptolide (TP)-glucosamine conjugate (TPG) has been recently investigated for its renal protective mechanisms due to the specific property of being accumulated in kidney. It can preserve kidney function after I/R injury. The prominent underlying mechanism is suggested to be the robust down-regulation of proinflammatory cytokines (IL-1, IL-6, TNF-α, TGF-β) as well as chemotactic cytokines (MCP-1) in the kidneys with the consequent prevention of lymphocyte accumulation and intracellular adhesion molecules[89]. This conjugate, thus, merits further clinical studies as a potential treatment for immunological renal diseases.
8.2. MSCs
Exogenous MSCs are believed to possess anti-inflammatory/immunoregulatory properties for the treatment of AKI. Various preclinical studies proved their paracrine effects in regulating the inflammatory mediators during kidney injury[90].
MSCs are recently under extensive investigation as a potential remedy for AKI. When MSCs were introduced in a number of AKI models, renal function was preserved and recovery from AKI was promoted.
An array of underlying and interconnected effects were noticed such as anti-inflammatory, immune-modulating, and mitogenic mechanisms. Patients who experienced cardiac surgeries were infused with MSCs (AC607) during phase I clinical trial through suprarenal aorta and consequently showed preservation of postoperative renal function[91]. Similar findings were reported in experimental animal researches in which MSCs improved AKI through suppressing inflammatory and oxidative pathways[92].
Clinical trials have demonstrated the feasibility, safety, and efficacy of using MSCs for kidney disease therapy. There is still some doubts about the therapeutic effects of MSCs on renal injury. They have a low survival rate in states of inflammation and oxidative stress at the site of injury, so their use is still restricted. Moreover,if MSCs are derived from the same patient with kidney disease to be used as autologous MSCs, their function is compromised due to the poor health of the patient. Therefore, novel methods are still awaited to improve the therapeutic efficacy of MSCs under different pathophysiological conditions[93].
Therapy with microvesicles (MVs) derived from MSCs is a more recent approach that is suggested to alleviate AKI. MVs are from the components involved in the cell-to-cell communication network that are responsible for tissue regeneration and therefore contribute to the paracrine action of MSCs.
They are vesicles composed of exosomes, shedding vesicles, and apoptotic bodies. Studies have reported that MSCs-derived MVs might favor kidney repair in both toxic and ischemic AKI. They were found to exert an anti-apoptotic effect on renal tubular cells in vitro and in vivo[94,95]. MVs also promoted the proliferation of tubular epithelial cells that survived in a glycerol-induced model of AKI.
Internalization of MVs into the target cells takes place in response to injury and, consequently, transfers its mRNA. In this way, MVs contribute along with soluble factors to the regenerative power of MSCs resulting in cell repair and amelioration of kidney injury[96]. It was claimed that these MVs produced more advantages than MSCs.The clinical trials, however still ongoing and challenging, have provided emerging evidence on the renal tissue-protective properties of MVs.
Another promising type of MVs is the renal tubular cell-derived extracellular vesicles which showed renoprotective effects in experimental models of renal injury. Similar to MSCs, the cells that line renal tubules release extracellular vesicles (EVs) and, thus,accelerate renal regenerative processes[97].
Intravenous administration of EVs derived from renal tubular cells in an experimental model of I/R renal injury decreased renal tubular damage, fibrosis, and improved renal vasculature[98]. The mechanistic pathways include proliferative and immune-modulatory(as immunosuppressive effect on T-cells) effects. EVs also decreased the expression of inflammatory molecules, lymphocytic infiltration,tubular necrosis, fibrosis and potentiated the gene expression of antiapoptotic agents, such as Bcl2, Bcl-XL, Akt1, and TRAF2[99].
Moreover, injection of EVs increased the synthesis and production of renoprotective factors e.g. hepatocyte growth factor and macrophage-stimulating protein. However, the extensive clinical trials on the therapeutic potential of EVs in the treatment AKI have not yet started.
8.3. Apoptosis blockers
Aquaporins are transmembrane channels that maintain a normal urine concentration in the kidneys and substance metabolism.Aquaporin-1 is a highly selective water-permeable channel and its absence in endotoxemia-induced AKI leads to increased polyuria and more severe tubular injury using aquaporin-1-null mice[100]. Aquaporin-1 was found to play an anti-inflammatory and antiapoptotic functional role. It suppressed renal inflammatory immune response via downregulation of p38 MAPK activity. The pharmacological targeting of this channel may provide a novel treatment strategy for AKI[101].
Neutrophil gelatinase-associated lipocalin (NGAL) is a protein that is expressed in different human tissues, such as the uterus, prostate,lung, trachea, stomach, colon, and kidney. NGAL was highly upregulated in the rat model of renal I/R injury suggesting that participation of NGAL in kidney development[102]. Purified NGAL protected from tubular damage in mice model of I/R injury. Recently,administration of exogenous NGAL protein has been found to play a protective role against renal I/R injury[103] by suppression of apoptosis and induction of the protective autophagic response.
Oleanolic acid, a natural triterpenoid, is present in different food products and is the main constituent of the leaves and roots of Viscum album L., Olea europaea, and Aralia chinensis L. Oleanolic acid preconditioning is currently suggested to treat I/R induced renal damage via antiapoptotic activities. It also showed antioxidant and anti-inflammatory activities[104]. So, it is proposed to be a novel therapeutic modality for the treatment of AKI.
The role of PARP1 activation did not only trigger inflammation but also extend to induce tubular apoptosis during methotrexate-induced nephrotoxicity[105]. It is still to be elucidated whether this pathway is involved in renal injury pathogenesis using other experimental models for AKI.
p53 is activated due to DNA damage, hypoxia and promotes apoptosis and fibrosis. It organizes multiple genes regulating apoptotic cell death, oxidative stress, metabolism, and signal transduction[106].
Targeted deletion or silencing of the gene encoding for p53 was found to prevent renal injury from different etiologic factors.QPI-1002 is a synthetic siRNA that was designed to inhibit the expression of p53. This compound was tested in rodents and nonhuman primates and exhibited a good safety profile and interestingly achieved predominant accumulation in the kidneys[107]. QPI-1002 has completed phase Ⅱ clinical trials and is encouraged to enter phase Ⅲ.
Novel pharmacological targets for the treatment of AKI are summarized in Table 3.
9. Natural plants/constituents for treatment of AKI
Numerous research studies have described the renoprotective role of herbs in models of AKI. Particularly, some promising Egyptian plants or their isolated phytochemicals with potential therapeutic effects on kidney injury will be reviewed in this section. Rosemary(scientific name: Rosmarinus officinalis; family: Lamiaceae) is an aromatic plant used in food manufacture as a flavor to preserve meat.It has protected from kidney injury in multiple animal models. The powerful antioxidant and vasodilatory effects of this plant could contribute to the renal protection[108,109].
Carnosic acid, as one of the polyphenols, is present abundantly in rosemary. The previous study showed an anti-apoptotic effect besides free radical scavenging activity and it protected from kidney injury due to ureteral obstruction[110]. Curcumin, an orange-yellow aromatic extract from Curcuma longa Linn plant, presents many pharmacological activities including antioxidant, anti-apoptotic effects, and antagonism of N-methyl-D-aspartate receptor[111,112].This receptor activation has been recently implicated in the pathogenesis of AKI, specifically I/R-induced AKI[113]. Terminalia muelleri (family: Combretaceae) which is a traditionally used plant in Egypt and other countries also showed a nephroprotective effect due to its polyphenolic-rich fraction[114]. Recently, garlic extract have showed nephroprotective effects through anti-inflammatory and anti-apoptotic effects[115].
10. Conclusion and future research
AKI is a multifaceted disease rather than a single disorder. It commonly takes place in patients admitted to intensive care and complicated with the presence of other organ dysfunction. Therefore,seeking appropriate and effective pharmacotherapy for AKI remains a great challenge for health care professionals.
Many drugs, agents, and herbal remedies have recently met success during experimental studies in the prevention of AKI due to various etiologies. Some of them have shown novel renoprotective effects through mechanisms beyond those implicated in their original medical indication. Moreover, recent pharmacological/therapeutic targets of multiple cellular repairs or regenerative processes involved in AKI have been evolved such as p53 antagonism, enhancement of autophagy, ferroptosis inhibition, adenosine receptor (A)antagonism, and microRNA modulation. Additionally, promising antioxidant, anti-inflammatory, and anti-apoptotic strategies, e.g.MSCs-MVs and renal tubular cells-derived MVs have been recently described.
Large controlled and randomized clinical trials are still awaited to confirm the positive impact from experimental data and to solve problems we are facing currently on the use of drugs to treat AKI in common practice, e.g. adjusting doses in renal impairment,investigating combination therapies or testing the drugs in patients with other comorbid factors.
Conflict of interest statement
The author declares that there is no conflict of interest.
Author’s contribution
HMIA as the corresponding and single author takes the responsibility of collecting information for the work, and drafting or revising the article critically for important intellectual content.
 Asian Pacific Journal of Tropical Biomedicine2021年7期
Asian Pacific Journal of Tropical Biomedicine2021年7期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Synergistic effect of flavonoids combined with antivenom on neutralisation of Naja naja venom
- Antioxidative, cytotoxic, and anti-metastatic potentials of Laurencia obtusa and Ulva lactuca seaweeds
- Anti-tumor activity of rice bran hydrolysates on migration, invasion and angiogenesis
