Synergistic effect of flavonoids combined with antivenom on neutralisation of Naja naja venom
Srimathi Raghavan, Gurunathan Jayaraman
School of Biosciences and Technology, Vellore Institute of Technology, Vellore 632014, India
ABSTRACT
KEYWORDS: Flavonoid; Anti-venom; Snake bite; Combinatorial;Synergistic; Naja naja
1. Introduction
Snake bite is one of the neglected tropical diseases and faces the most challenging assignment throughout the world population[1].Though accurate statistical data is not available, about 46 000 snake bites are presumed to occur and lead to highly variable bites of approximately 140 000 annually[2]. In the longer run, snake bites also result in permanent tissue damage and several other pathological consequences, which affect social life as well as the psychological status of the victim[3]. The primary aspect of snakebite management involves proper first aid, followed by intravenous administration of high dose of antivenom (AV)[4]. Clinical symptoms of envenomation are deemed to be essential for therapeutic procedures. Additionally,the success of AV treatment is dependent on the availability and,more importantly, the quality of AV, especially in regions that are far from accessible transport[5].
AV is obtained from immunized animals and subsequent purification of immunoglobulins. Based on the type of snakes used in the production of AV, antivenom is classified as monovalent or polyvalent[6]. Currently, forty-five laboratories throughout the world produce AV. Depending on the protocol, AV components are obtained as the whole IgG or as F(ab’)2 fragment or as monovalent Fab[7].
Even though monovalent AV is considered to render high efficacy, many of the AV manufacturers produce only polyvalent AV. This is owing to the uncertainty or tedious process involved in the identification of snake species causing envenomation[4].The success of AV is based on its ability to interact with the snake venom proteins and subsequently eliminate the toxin from circulation[1]. Major drawback in the treatment with AV is related to the production cost, duration involved in the process, accessibility to remote areas, cross-reactions in-vivo, stability/shelf-life, titre value, and post-treatment regime[8]. Though pre-treatment with hydrocortisone and antihistamines is practised, these drugs have been reported to delay the reaction time. Attempts have also been made to reduce the concentration of the antivenom used to neutralise the venom by the formulation of protein-based nanoparticles[9].Therefore, the search for alternative therapies against snake bites is extremely essential.
Traditionally, extracts of medicinal plants are given as first-aid treatment in rural areas. Use of plants as alternative medicine is highly based on trials and experience. However, the medicinal value of a large number of plants is still undisclosed and not proven scientifically. Various plants are being investigated for their potential inhibitory activity of snake venom proteins. Despite all these advances, it is estimated that there are more than 1 000 plants still unexplored. This treasure of information about the medicinal plants which are used in snake bite management is generally passed orally from one generation to another, but in most cases, it is not documented. Flavonoids are one of the many classes of bioactive compounds that have been shown to inhibit the snake venom proteins. This class of compounds is produced by plants during stress conditions[10]. There exist numerous reports indicating the applicability of flavonoids as anti-oxidant[11], antiinflammation[11], and anti-tumour agents[12]. Flavonoids isolated from plants can inhibit phospholipase A(PLA) (both secretory and cytosolic form)[13]. Flavonoids can also reduce the toxic nature of enzymes by altering their conformations. Quercetin has been shown to interact with secretory PLAof Crotalus durissus terrificus[14].Further, Alves and colleagues[15] reported that rutin and 3’-4’-OH flavonoids, from Bredemeyera floribunda root extract, are potential inhibitors of PLA. Iglesias and collaborators[16] found that morin interacts with venom of Crotalus durissus cascavella. Kitagawa et al.[17] found that flavonoids are capable of oxidizing various whole proteins (hemoglobin) thereby affecting their biological activity.Multifunctional enzyme PLAhas a unique hydrophobic channel,and it has glycine and Caion located in the active site that stabilizes the oxyanion hole during the process of substrate hydrolysis.Residues such as aspartic acid and histidine are involved in the catalytic activity of PLA[18]. Hence, molecules that are capable of blocking the interaction of the substrate with the active site can act as a potential (PLA) inhibitors.
Despite these few reports on the potential use of flavonoids for inhibiting the snake venom proteins, no systematic and in-depth investigation is yet available. Furthermore, it has been established that flavonoids can be produced in large quantities using relevant biotechnological approaches. Therefore, the present investigation was carried out to investigate the possible venom inhibition potential of selected flavonoids and the possibility of reducing the commercial AV dosage, by adopting a combinatorial approach.
2. Materials and methods
2.1. Venom, AV, and flavonoids
Lyophilized snake venom was purchased from Irula Snake Catcher’s Industrial co-operative Society Limited (Chennai, India).Naringenin, quercetin dihydrate, flavone, and morin were purchased from Sigma Aldrich (Mumbai), while rutin was purchased from Hi-Media (Mumbai). AV was purchased from VINS Bioproducts Limited (Hyderabad, India). All other reagents and solvents used in the study were of high-quality analytical grade and were purchased from SD Fine chemicals/ Hi-Media, Mumbai.
2.2. PLA2 assay
The enzymatic activity of PLAwas assessed using the egg-yolk suspension method with slight modifications[19]. PLA, protease, and hemotoxic activities observed using 70 mg/mL crude snake venom were taken as reference (100% activity). Briefly, 2% (v/v) yolk suspension was prepared by dissolving 2 mL of egg yolk in phosphate buffer saline. To 200 µL of suspension, different concentrations of venom (10-100 mg/mL) were added, and the mixture was incubated at 37 ℃ for 30 min. The PLAactivity was determined from the change in the absorbance of the reaction mixture at 900 nm (SHIMADZU 1280 UV-spectrophotometer). For inhibition studies, the venom was preincubated with various concentrations of flavonoids (5-35 mg/mL) for 30 min at 37 ℃, before mixing with the substrate. AV (35 mg/mL) was used as a standard.
2.3. Protease assay
Proteolytic activity of snake venom was assessed using azo-casein suspension method with slight modifications[20]. The venom (10-100 mg/mL) was incubated with 0.2% (w/v) of azo-casein (20 mM Tris-HCl buffer, pH 8.5) at 37 ℃ for 30 min. Undigested casein was removed by precipitation using 10% (v/v) trichloroacetic acid(centrifugation at 10 000 rpm, 10 min, 4 ℃). To the supernatant, 500 mM of sodium carbonate was added, followed by the addition of 0.5 N of Follin’s reagent. Casein lysis was inferred from the absorption at 366 nm (SHIMADZU 1280 UV spectrophotometer). For inhibition studies, the venom was pre-incubated (30 min at 37 ℃) with different concentrations (5-35 mg/mL) of flavonoids, before mixing with the substrate. AV at a concentration of 35 mg/mL was used as a standard.
2.4. Protease inhibition on SDS-PAGE
Proteolytic inhibition study was carried out on SDS-PAGE using bovine serum albumin as the substrate[21]. Briefly, 2 µg of Naja naja(N. naja) venom was pre-incubated with various concentrations of naringenin for 1 h at 37 ℃. The samples were further incubated with 1% (w/v) bovine serum albumin. After 30 min, the reaction was stopped by addition of SDS loading buffer (containing 50 mM Tris HCl pH 6.8, 10% v/v glycerol, 2% w/v SDS, 100 mM β-mercaptoethanol and 0.1% w/v bromophenol blue). SDS-PAGE was performed, according to the method described by Chaia et al[22].
2.5. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) scavenging assay
Radical scavenging activity of flavonoids was quantified using the protocol described by Prathiba and Jayaraman[23], with slight modifications. Briefly, 0.1 mL flavonoid (10-30 mg/mL) was added to 3.9 mL DPPH solution (0.1 mM in methanol). DPPH in the absence of the added flavonoid was used as the control and methanol was used as blank. The reaction was maintained at 25 ℃ for 30 min,and the absorbance was read at 517 nm using SHIMADZU 1280 UV-spectrophotometer to determine the free radical scavenging activity of the individual flavonoids. Similar experiments were carried with different concentrations of ascorbic acid (10-50 mg/mL). Further, the percentage of inhibition was calculated using the following formula:
% DPPH radical scavenging activity = [(OD control − OD sample)/OD control]×100
The log concentration was plotted against the percentage of inhibition in order to determine the inhibitory concentration (IC)values of ascorbic acid and flavonoids[24].
2.6. Hemolytic assay
Hemolysis was carried out with slight modifications using the RBC suspension method[25]. Accordingly, 2% RBC (v/v) was suspended in 0.9% (w/v) saline. To 100 µL of the suspension, different concentrations of venom (10-100 mg/mL) were added and incubated at 37 ℃ for 30 min. The experiment was terminated by the addition of ice-cold saline, followed by centrifugation at 3 000 rpm for 10 min (4 ℃). The release of heme complex was detected by measuring the absorption of the supernatant at 490 nm (SHIMADZU 1280 UV-spectrophotometer). For inhibition studies, the venom was preincubated with different concentrations of flavonoids (5-35 mg/mL)and control AV (35 mg/mL) for 30 min at 37 ℃, before mixing with the substrate.
2.7. Molecular docking
Molecular docking was performed using AutoDock (v. 4.2.6),and the visualization was carried out using PyMol plugin and LigPlot+ (Thornton Software). Snake venom PLAstructure was obtained from PDB (code: 1A3D). The ligand IDs of the flavonoids(4444362-rutin, 388383-naringenin, 10230-flavone, 4444989-morin,and 12269344-quercetin) were obtained from Chemspider online database and the 2D structure was drawn using ChemDraw (v.8.0).The structures were energy minimized and converted to PDB format using Argus lab (v.1.1.0). Default parameters were used for the docking studies. The grid points were set according to the predicted volume of the substrate-binding sites (CASTp) present in the protein(x=49.68, y=10.82, z=13). The spacing was kept as 0.375 Å, and the number of GA run was set to 100[25]. The interactions were represented using LigPlot+.
2.8. In vivo toxicity
Male Swiss-albino mice [8 weeks old and weighing about (20±2)g] were divided into seven groups (n=4). Animals were maintained in polypropylene cage with soft husk under diurnal light cycle at the temperature of (25±2) ℃ and humidity of (66.5±0.5)%. Mice were fed contamination free and nutrition-rich normal diets (pellet)as well as water ad labium. Half the lethal dosage (LD) value of N. naja venom was 0.451 mg/kg, as per the previous report from our laboratory[25]. The venom neutralization study using specific flavonoids was carried out in vivo as per the protocol established by Meier et al[26]. Briefly, varying concentrations of naringenin (10-200 mg/g of animal body weight) pre-incubated with 2×LDof N.naja venom were administered intraperitoneally to mice, and the behavioral changes (lymph paralysis, heavy breathing, eye closure,and immobilization) in mice were monitored for 48 h. An effective dosage of the flavonoid was inferred from these dose-response curves. Mice injected with saline/naringenin (positive control) and mice injected with venom only (negative control) were maintained separately and monitored.
2.9. Histopathological studies
Organs including heart, liver, and kidney were harvested from the control and treated mice and were preserved in 10% formalin solution[25]. The samples were processed and fixed using the paraffin wax. The tissues were then sectioned (4-6 µm) using Spencer ‘800’microtome and subsequently stained with hematoxylin and eosin.The thin stained sections were examined under the microscope(Cilika MPT/0918CPF/MHI03/804344) and the images were captured.
2.10. Combinational inhibition studies
Two different combinatorial approaches were followed. In the first set of experiments, combinations of flavonoids (0.5-15 mg/mL) were used to find the maximum inhibition. In the second set of experiments, combination of flavonoids and AV was used based on half their effective dosage (ED) values. Based on these values,1:1 proportion was used. Accordingly, specific concentrations of flavonoids equivalent to ED[2.02 µg/µL (rutin), 1.25 µg/µL(quercetin), 1.95 µg/µL (morin), 1.71 µg/µL (naringenin), 1.93 µg/µL (flavone)] were mixed with the concentration of AV (1.12 µg/µL) that is equivalent to half its ED. In both these sets, the effect of venom PLAinhibition was evaluated (refer to the method given above). From the data, the AV equivalence (AVE) was calculated.AVE is defined as the amount of AV that is required to produce 25% inhibition of snake venom proteins, compared to that of the concentration of flavonoid to produce the same effect (25% inhibition) and is expressed as the ratio of flavonoid concentration to AV concentration. Amount of flavonoid/AV that exhibited 25% inhibition was inferred from the dose-response curves. If the resultant activity (observed) is equal to or with less than 10% change compared to the sum of the inhibitory activities (predicted) of the individual flavonoids, then the combination is deemed to produce an additive effect. However, if the difference is more than 10% then the combination is deemed to exert synergetic effect. For further classification, the effect is represented as ‘moderately synergetic’if the difference is in the range of 10%-25% and classified as‘highly synergetic effect’ if the difference is greater than 25%.Such differences were calculated based on the formula: [(Observed activity – Predicted activity)/Observed activity]×100, wherein the predicted activity was inferred from the regression equation obtained from the dose-response curves.
2.11. Statistical analysis
All experiments were carried out with proper controls and in triplicates. The values are expressed as mean±SD. Graphs were drawn using GraphPad Prism 6 software, and the analysis of variance(one-way ANOVA) was performed. Results were considered statistically significant if P-value < 0.05.
2.12. Ethical statement
The animal study was carried after animal ethical clearance with guidelines and approval by Institutional Animal Ethical Committee followed by submission of Committee for Supervision of Experiments on Animals (CPCSEA), Ministry of Environment,Forests and Climate Change, Government of India, New Delhi(Reference number VIT/IAEC/14/NOV5/31, dated 05-11-2017).
3. Results
3.1. PLA2 inhibitory activity of flavonoids
As predicted, enzyme inhibition was found to be dose-dependent with respect to the concentration of flavonoids and therefore, the activity of the snake venom enzymes reduced with increasing concentration of the flavonoids. Maximum PLAinhibition was dependent on the type of flavonoid, and the highest inhibition was observed with quercetin (56%), followed by flavone (45.5%),naringenin (45%), rutin (31%), and morin (31%) (Figure 1).
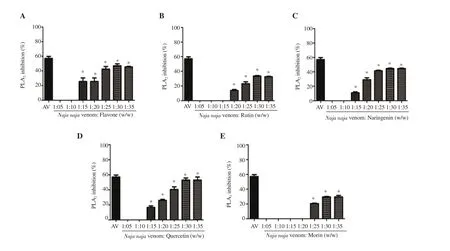
Figure 1. PLA2 inhibition of (A) flavone, (B) rutin, (C) naringenin, (D) quercetin and (E) morin. Values are expressed as mean± SD, and *P<0.05 represents significant difference. Inhibition by antivenom (AV; 35 mg/mL) is given for reference. PLA2: phospholipase A2.
3.2. Protease inhibitory activity of flavonoids
The proteolytic activity of the snake venom was evaluated in the presence of different flavonoids (Figure 2). Quercetin (71% inhibition)showed remarkable inhibitory potential followed by naringenin (64%),rutin (55%), flavone (43%), and morin (27%). As a confirmatory test,SDS-PAGE was performed for naringenin at two concentrations (20 and 35 w/w) using BSA as the substrate (Supplementary Figure 1).
3.3. Antioxidant activity of flavonoids
With regard to the effect of flavonoids on radical scavenging activity, naringenin exhibited higher (77.5%) radical scavenging activity than other flavonoids (quercetin 69.0%> morin 63.2%>flavone 53.5%>and rutin 46.0%) at its higher concentration of 30 mg/mL (Figure 3). ICvalues calculated based on linear regression analysis were 19.56 mg/mL (flavone), 32.37 mg/mL (ascorbic acid), 24.99 mg/mL (morin), 33.37 mg/mL (rutin), 22.33 mg/mL(quercetin) and 22.88 mg/mL (naringenin).
3.4. Hemolysis inhibition of flavonoids
Analysis of the hemolytic action of the venom (Figure 4) indicated that the inhibitory potential of flavonoids was in the order similar to that observed for PLAinhibition: quercetin and naringenin(70%), flavone (59%), rutin (52%), and morin (45%). On the whole,quercetin and naringenin were the potential inhibitors of snake venom compared to other flavonoids used in the study.
3.5. Molecular docking studies
In the present study, PLAwas chosen for in silico molecular interaction studies. CASTp indicated 15 possible substrate binding cavities in PLA. These sites are found to be associated with different physicochemical properties and therefore will display differential interaction with the ligands. As the objective was to unravel potential inhibitors, the flavonoids were docked at the substrate-binding site(involving amino acid residues Phe5, Ile9, Trp18, Phe21, Ala22,Tyr27, Cys28, Gly29, Arg30, Cys44, His47, Asp48, Tyr51, Tyr63,and Phe100) of PLA(Supplementary Figure 2). Of the many structures obtained, selection of appropriate PLA-flavonoid complex was based on the resultant binding energy, non-bonded interactions,and population density (preference for a given orientation) of a given cluster. Though there were several intermolecular (protein and ligand) non-bonded interactions, the amino acid residues Cys44 and Tyr63 were found to contribute significantly (Supplementary Table).Overall, hydrogen bonding interactions were inferred to be crucial.Other interactions like pi-alkyl, hydrophobic interactions were also observed. Therefore, the binding of flavonoids with the substratebinding site of PLAwas favoured by the combination of several non-bonded interactions.
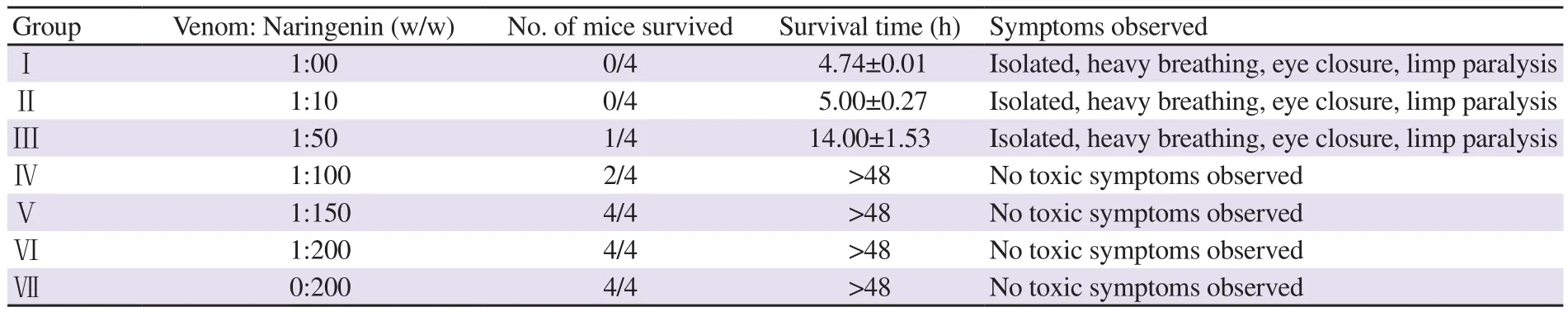
Table 1. Neutralization of venom lethality by naringenin using the model.
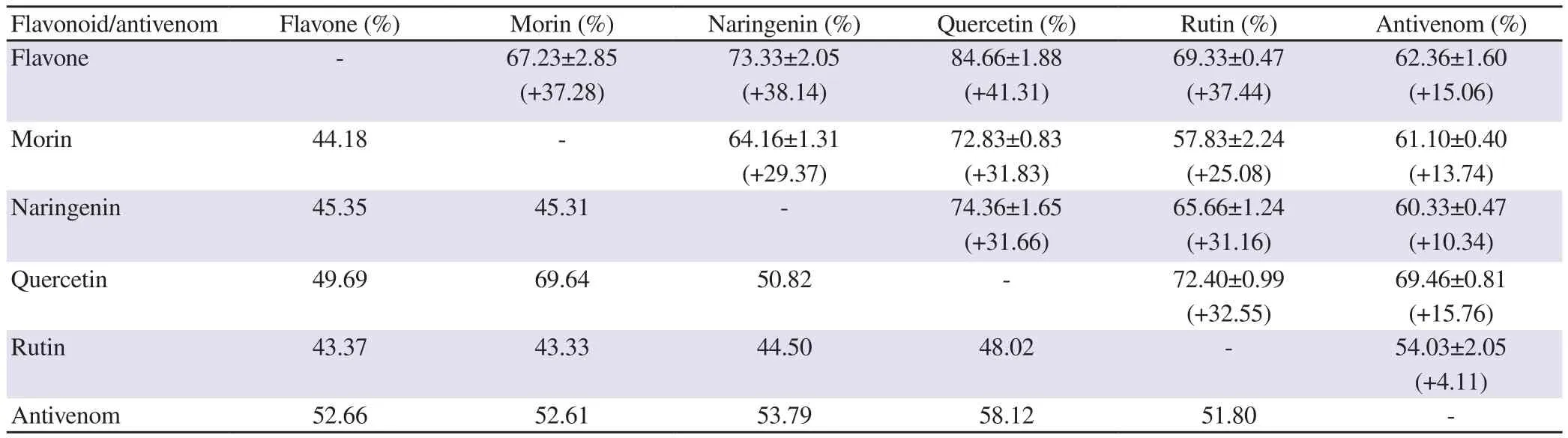
Table 2. Inhibition of PLA2 activity by a combination of flavonoids/antivenom.
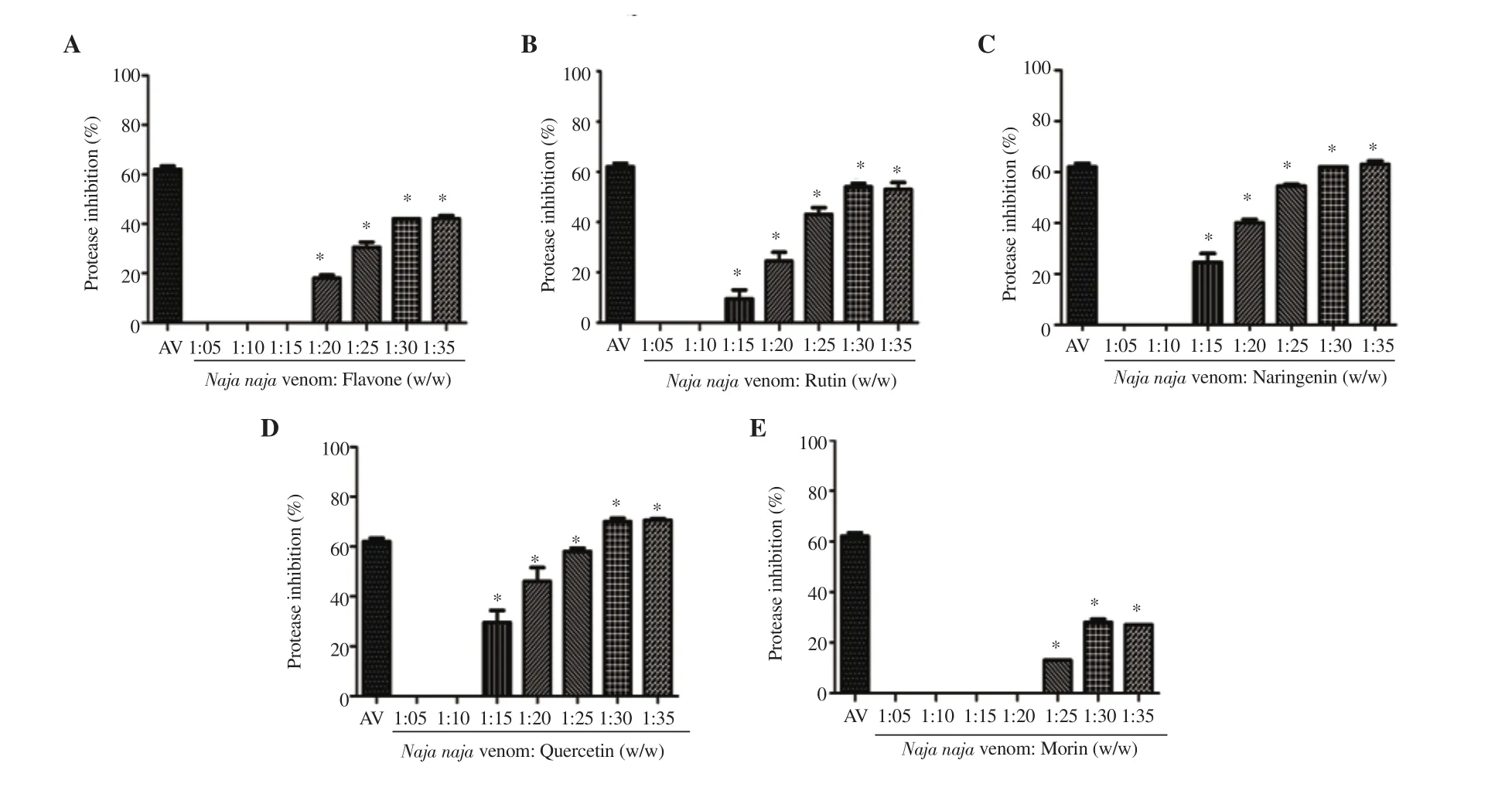
Figure 2. Venom protease inhibition of (A) flavone, (B) rutin, (C) naringenin, (D) quercetin and (E) morin. Values are expressed as mean ± SD, and *P<0.05 represents significant difference. Inhibition by antivenom (35 mg/mL; AV) is given for reference.
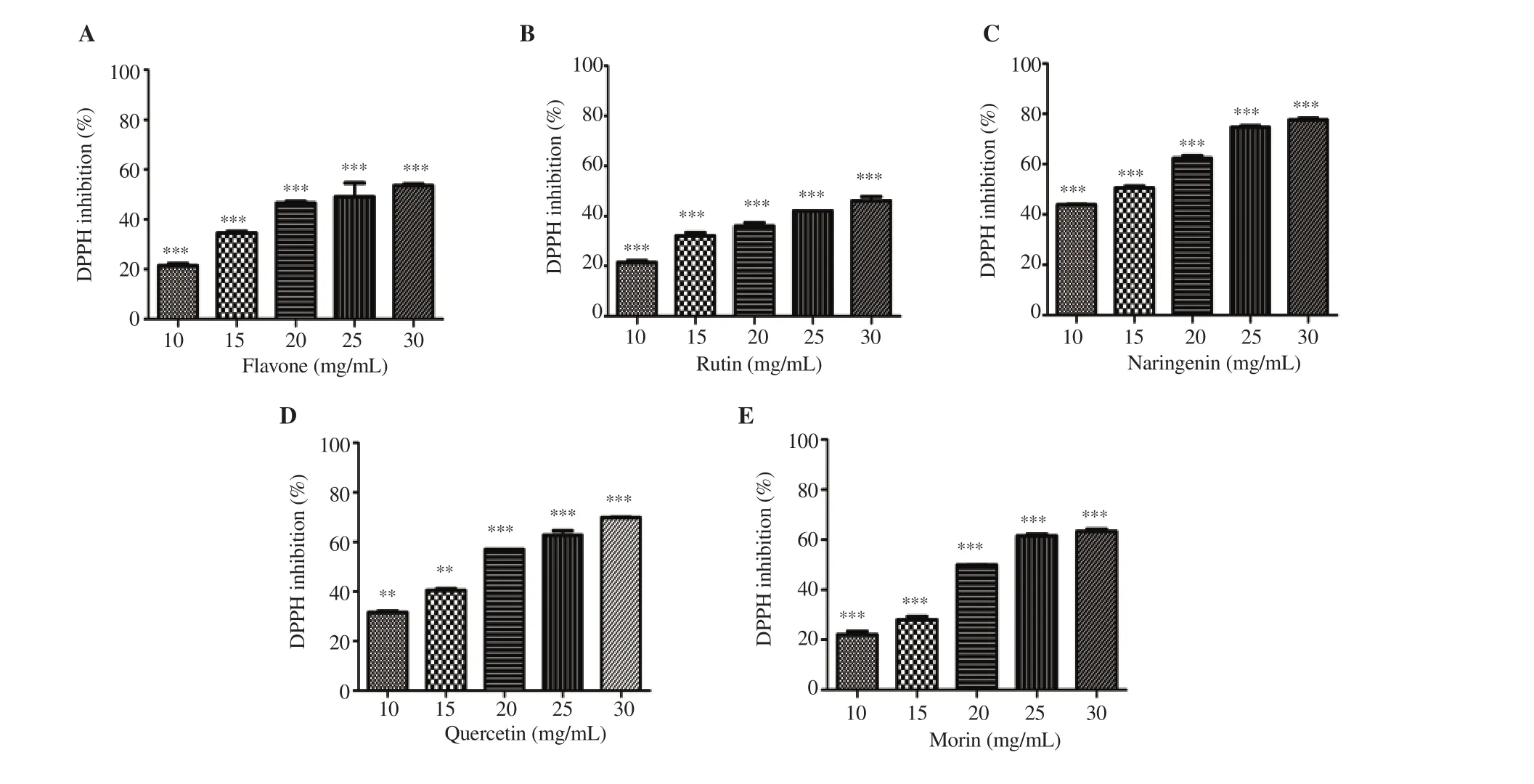
Figure 3. Antioxidant activity of the flavonoids using DPPH scavenging method. (A) flavone, (B) rutin, (C) naringenin, (D) quercetin and (E) morin. Values are expressed as mean ± SD, and ***P<0.001 and **P<0.01 represent significant difference.
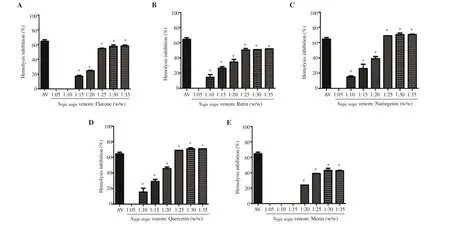
Figure 4. Anti-hemolytic activity of (A) flavone, (B) rutin, (C) naringenin, (D) quercetin and (E) morin. Values are expressed as mean ± SD, and *P<0.05 represents significant difference. Inhibition by antivenom (35 mg/mL; AV) is given for reference.
3.6. Venom neutralizing activity of flavonoids
Changes in the survival time were inferred after administrating synthetic flavonoids pre-incubated with 2×LDof the venom. Mice injected intraperitoneally with N. naja venom survived only for 6.5 h, whereas mice injected with venom pre-incubated with naringenin(200 mg/g) were alive even after 48 h (Table 1). Mice injected with the highest concentration (200 mg/g) of naringenin (without pre-incubation with venom) did not show any toxic symptoms or behavioural changes.
3.7. Histopathological results
Histopathological study of the organs including heart, liver,and kidney injected only with venom revealed haemorrhage in heart, denaturation of hepatocytes and necrosis (Figure 5.1 A-C).Significant decrease in toxicity was observed in the organs treated with venom pre-incubated with naringenin (Figure 5.3 A-C).However, no major histopathological abnormalities were observed in tissue samples of heart, liver, and kidney of mice injected with only naringenin and only saline (Figure 5.2 A-C) and (Figure 5.4 A-C).
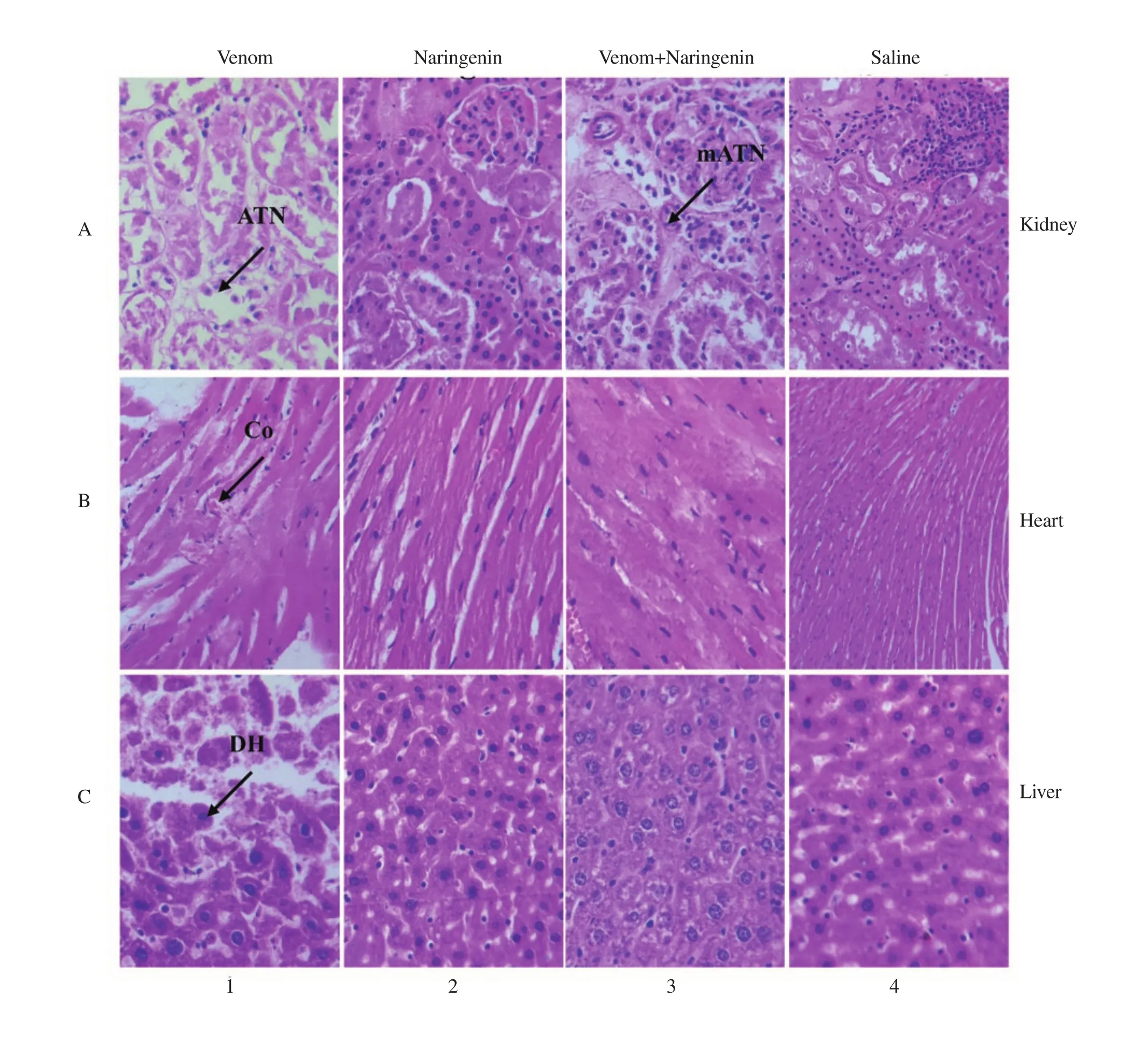
Figure 5. Histopathological changes at 340× magnification (A) kidney, (B) heart and (C) liver; 1(A-C): the organs of mice injected with the only venom at 0.9 mg/kg b.w show pathological changes [acute tubular necrosis (ATN) in the kidney, congestion in the heart (CO), denaturation of hepatocytes (DH)]; 2(A-C):the organs of mice injected with only naringenin show no major abnormalities; 3(A-C): the organs of mice injected with a pre-incubated sample of venom and naringenin (1:200 w/w) show mild acute tubular necrosis (mATN) and a significant reduction in the venom toxicity; 4(A-C): the organs injected with only saline (control) show no abnormalities.
3.8. Combinational inhibition studies
Initial studies with combination of flavonoids indicated that these molecules might act either additively or synergistically. This behaviour is dependent on the flavonoid type. However, inhibition studies with combinations of AV and flavonoids indicated mostly synergistic behaviour (Table 2). For all these experiments, 1.12 µg/µL of AV (half the ED) was used so that minimum concentration of AV can be utilised. The highest synergistic effect was observed when AV was used along with the flavonoids, except for rutin (Table 2). From the inhibition observed, AVE for different flavonoids was calculated. The value indicates the amount of AV that is required to produce the effect and therefore, lower value refers that the potential of the flavonoid is less compared to the AV. Equivalence was highest for quercetin (0.90 µg/µg), followed by naringenin (0.65 µg/µg)and lowest for rutin (0.55 µg/µg). The synergistic effect was also observed when combinations of flavonoids were used.
4. Discussion
Snakebite envenomation has become an emergency medical threat and the frequencies of bites in developing countries are on the rise.Even though AV therapy is practised worldwide, the post-treatment side effects are commonly seen[27]. For instance, administration of polyvalent AV was found to have strong adverse effects in approximately 43% in India (Sri Lanka)[28]. These adverse effects vary and include IgE mediated anaphylactic reaction (immediate reaction), anaphylactoid reaction, pyrogenic, and serum sickness(late reaction). Predominantly, adrenaline glucocorticoids[8] and antihistamines are used as pre-AV treatment protocols[8]. However,observations from clinical practices reflect a lack of efficacy in administration of these premedication drugs (antihistamines and hydrocortisone).
One such technique that is used to develop promising antibodies for venom toxins is through phage display[29], and this technique uses human-sourced monoclonal antibodies to avoid antagonistic reactions and also the loss of efficacy in the individuals receiving the antibody. This approach in AV study bypasses the immunization and screening standard protocols and it also overcomes the toxicity and lowers the immunogenicity of the target protein/peptide. In addition to this technique, single-chain variable fragment/antigenbinding fragment format to the full human immunoglobulin G format was found to express in mammalian cells to ensure correct protein folding and also modification(s) that occurred after protein translation[30].
Flavonoids are polyphenolic compounds with a wide range of therapeutic applications, including their ability to inhibit snake venom enzymes. The flavonoids used in this study and several others have been found in plants. Use of flavonoids for the treatment of snakebite dates back to 1985 when the effects of hypolaetin-8-glycoside were studied on PLA. In addition, other flavonoids such as rutin, quercetin, isoscutellarein, and kaempferol also have the potential to inhibit venom PLAenzyme, with values from 20% to 60%[31]. The use of myricetin for the inhibition of proteolytic and hemorrhagic activity indicates that myricetin can also be an appropriate candidate to reduce tissue damage due to envenomations[32]. A recent study has compiled the novel therapeutic strategy for snakebites in combination with small molecules[33].Identification and screening of new techniques and natural compounds that inhibit snake venom enzymes are essential so that the molecules thus identified can be used as an alternate, additive and effective agents to enhance the treatment[34]. Snake venom phospholipase and metalloproteases are the major key enzymes that cause severe tissue necrosis and affect the degradation of the extracellular matrix[35].
Among the other constituents, PLAis a multifunctional enzyme and contributes significantly to the lethality of snake venom[36].PLAcan be considered as the black box in snake venom, as it is a multifunctional enzyme that elicits both site-specific and systemic effects in humans (mitochondria, red blood cells, nerve endings,and also skeletal system)[37]. PLAalso interrupts the release of prostaglandins, platelet-activating factor, and leukotrienes, which has inflammation and pain-inducing effects. Therefore, inhibition of PLAcan block most of the pathways, in a single attempt and will be an effective measure in the management of snake bites[38].
According to experimental trials[39], natural and synthetic small molecules have been found to be effective against various diseases and hence reports suggest that they can be used in combination with commercial AV for snake venom enzyme inhibition. In the present study, selected flavonoids were evaluated for their inhibitory PLApotential. The results demonstrate concentration dependency by all the flavonoids used, even though the percentage of inhibition is dependent on the flavonoid. Significant inhibition was observed in the case of quercetin, naringenin, and flavone. However, complete inhibition was not observed with any of these flavonoids at the maximum concentration used. The maximum PLAinhibition (56%)was observed with quercetin and it was less than 50% inhibition for other flavonoids. However, the inhibition potential was found to increase when combinations of the flavonoids were used. Also, these flavonoids when used in combination with AV produced pronounced inhibition effects. Results clearly indicate that these flavonoids can be used as additives, along with AV, during the management of snake venom bites. Gil et al.[40] reported a maximum of 30% PLAinhibition by a group of flavonoids.
PLAactivity is neutralized, possibly, by inactivating the toxic nature of the enzyme or by adjuvant mode of actions or even by competitive chelation of metal ions that are responsible for the activity of these enzymes. Snake venom proteases are considered to involve in the destruction of hemostasis that in turn results in bleeding. Results of the SDS-PAGE in the presence of quercetin were reported in our earlier study[14], which clearly depicted the inhibitory activity. The current study authenticates that flavonoids inhibit the proteolytic activity of snake venom. Of the flavonoids used, quercetin and naringenin are very effective in inhibiting the snake venom proteases. In-silico studies on citrus flavanone hesperetin were reported to bind significantly to snake venom serine protease, thereby inhibiting the proteolytic activity[41]. Myricetin, a flavonoid, inhibits the proteolytic activity of Bothrops atrox venom by more than 50%[34].
The antioxidant activity using DPPH assay revealed that DPPH inhibition increased by flavonoids in a concentration-dependent manner. Naringenin exhibited higher hydroxyl scavenging property compared to quercetin and other tested flavonoids. Flavonoids such as quercetin, kaempferol, and myricetin exhibit both antioxidant activity and PLAinhibitory activity, and therefore can be promising anti-inflammatory compounds[42]. Rupturing of red blood cells is another vital action of snake venom hemotoxins.These hemotoxins are classified, as direct and indirect, based on their mode of action. The current study also reveals substantial inhibition of snake venom hemolytic activity by these flavonoids. It can be noted that the percentage of hemolytic inhibition, generally,is greater than the inhibition of other proteins in the snake venom.Quercetin and naringenin display considerable inhibition compared to other flavonoids such as rutin, flavone, and morin. The result may be due to the possibility of the protective effect produced by flavonoids under stress conditions, and it is strongly believed that the antioxidant property can assist in preventing the lysis of red blood cells by controlling the free-radicals[42].
Asp48 is important for the catalytic activity of PLA. This amino acid residue is conserved in D-type PLAof Bothrops sp. and N. naja. Previous studies on the PLA-metabolites interactions revealed changes in the conformation of PLAupon binding to these ligands[43]. The inhibitory effect of flavonoids may be either by chelating to calcium ions or by direct binding with the substrate.The flavonoids exhibit different binding affinity and intermolecular hydrogen bonding patterns (flavone, morin, naringenin, quercetin,and rutin). Structural variation of the flavonoid is reflected in the binding affinity and also in the number of possible hydrogen bonds between the flavonoid and the PLAenzyme. All the tested flavonoids exhibit a higher binding constant than the natural substrate, glycerophospholipid. Therefore, these molecules can competitively inhibit the action of PLAin a concentrationdependent manner. The results obtained in our analysis are in line with the previously published reports related to examining the effects of different compounds on snake venom toxicity[14,16,30]. The intermolecular interactions were observed to involve the catalytic site residues such as Cys44 and Tyr63. Other amino acids that were consistently found to be involved in binding the flavonoids include Asp48, Gly29, and Gly31. These three residues are involved in calcium ion binding of PLAand therefore, binding of flavonoids to these amino acid residues will definitely lower the enzyme activity.Further, hydrophobic interaction of flavonoids with the enzyme(PLA) supports the enzyme inhibition activity. Similar interaction of PLAwith biflavonoids (ginkgetin and bilobetin) was reported by Herowati and Widodo[44].
Venom neutralization effects of quercetin-3-O-rhamnoside against crude N. naja venom were previously reported from our laboratory[25]. In present work, the naringenin was used to study the in vivo neutralization of crude snake venom. Venom toxicity gradually decreased with increasing concentration of naringenin(pre-incubated with venom). Animals that were treated only with naringenin and only with saline did not show any abnormalities,thus strongly indicating non-toxicity of the flavonoid. The histopathological study showed that abnormalities were improved in the heart, kidney, and liver of mice injected with venom and flavonoids.
In addition, a combination of AV and flavonoids was found to be effective in inhibiting the venom enzyme with synergetic effect.Derivatives of flavonoids such as pinostrobin and quercetin were also reported to significantly neutralize the snake venom PLAactivity[45].
In conclusion, the present study highlights the inhibition effects of flavonoids on selected snake venom activities involving PLA,proteolysis, and haemolysis. Antivenom combined with flavonoids can be effective in the management of snake bites, primarily by reducing post-therapeutic effects caused by overuse of AV. However,an in-depth study is required to understand the synergistic effects caused by such combinatorial methods.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Authors’ contributions
GJ and SR conceptualized and formatted the study design. SR performed, analyzed, interpreted and wrote the first draft. GJ supervised, revised and reviewed the manuscript. All authors read and approved the final manuscript.

