Modification of nanoparticles for the strength enhancing of cementstabilized dredged sludge
Lei Lang,Bing Chen,*,Haijuan Duan
a State Key Laboratory of Ocean Engineering,Shanghai Jiao Tong University,Shanghai,China
b Shanghai Key Laboratory for Digital Maintenance of Buildings and Infrastructure,Department of Civil Engineering,Shanghai Jiao Tong University,Shanghai,China
Keywords:Cement-stabilized dredged sludge (CDS)Nano-modification Strength development pH level Microstructure
ABSTRACT This paper investigates the effectiveness of nano-modification on the strength enhancement of cementstabilized dredged sludge(CDS).Three types of nanoparticles including nano-SiO2(NS),nano-Al2O3(NA)and nano-MgO (NM) were used as cement admixtures for dredged sludge stabilization.Effects of single nanoparticle content,mass ratio of composite nanoparticles and curing time on the strength development of CDS were evaluated via a series of unconfined compressive strength (UCS) tests.The pH evolutions of CDS caused by nanoparticles were also examined by a range of pH tests.Furthermore,micromechanisms reflecting the strength evolutions were analyzed by performing scanning electron microscopy (SEM) and X-ray diffraction (XRD) tests.The results indicated that adding nanoparticles can significantly improve the UCS of CDS.For single nano-modification,the optimum contents of NS,NA and NM were 4%-6%,6% and 8%,which can increase the 7-and 28-d UCSs of CDS by 38% and 50%,17% and 35%,65%and 67%,respectively.Compared with single nano-modification,composite nano-modifications were more effective in improving the strength gain of CDS.The optimum mass ratios of composite nanoparticles,namely NS/NA,NS/NM and NA/NM,were 9/1,3/7 and 3/7,respectively.Based on the strength growth rate,the composite nanoparticles with NS/NM of 3/7 were highly recommended.The addition of nanoparticles obviously affected the pH evolution of CDS,which was mainly determined by the difference of OH- production and consumption inside nano-modified CDS.The microstructural analysis revealed that C-S-H and C-A-H gels are the main cementitious products,and the addition of nanoparticles can obviously contribute to a denser and more homogenous microstructure of CDS.
1.Introduction
Dredging operations on rivers,lakes and ports produce huge volumes of dredged sludge (DS),which is usually characterized by fine particles,high moisture content,high compressibility,low strength and rich organic matter(He et al.,2020).Due to these poor engineering properties,the rational disposal of DS challenges many cities in China (Wang et al.,2020).Traditional approaches mainly focus on land disposal and offshore dumping,which inevitably occupy land resources together with a series of environmental pollution (Lang et al.,2020a,b).Chemical stabilization is a wellestablished soil improvement technology,and cement is the most commonly adopted binder because of its low cost,high strength gain and extensive sources (Chian et al.,2016;Yao et al.,2019a,2020a).However,the manufacture and usage of cement have raised serious environmental issues such as energy and non-renewable resource consumptions as well as CO2emissions (Arulrajah et al.,2018;Wang et al.,2019a;Yaghoubi et al.,2019;Rafiean et al.,2020;Lang et al.,2020c;Gu et al.,2020).The production of cement results in 5%-8% of artificial CO2emissions,leading to increasing greenhouse effect(Wang et al.,2019b;Lang et al.,2020d;Li and Yi,2020;Yu et al.,2020).In addition,the high alkaline environment caused by cement may have side effect on the plant growth performance and pollutant stability of reclaimed soils (Li and Yi,2019).Therefore,looking for admixtures that can reduce cement usage seems promising.Many previous studies have investigated the stabilization of soft soils using different alternative materials including industry by-products,fibers and other chemical additives (Yi et al.,2014;Changizi and Haddad,2015;Jafer et al.,2018;Lang et al.,2020d).
With the rapid development of nanotechnology in recent years,nano-based binder has attracted great attention.This stems from the fact that the hydration product C-S-H gel of cement is also a natural nano-structured material (Zyganitidis et al.,2011;Ghasabkolaei et al.,2017;Yao et al.,2019a).Nanoparticles can be used either to partly replace cement or as the cement admixtures,and both of these two cases can effectively improve the performance of cement(Stefanidou and Papayianni,2012).Furthermore,nanoparticles can be acted as the nuclei to accelerate cement hydration (Bahmani et al.,2014).It is interesting to observe from previous studies that the nanoparticle dosage was commonly very low (less than 10% by dry cement weight) (Ghasabkolaei et al.,2017;Lo et al.,2017;Choobbasti et al.,2019;Lang et al.,2020a;Mahmoudian et al.,2020).Thus,the use of nanoparticles as cement modifier can achieve a better performance compared with other materials.The most widely used nanoparticles in cement-based binders are SiO2,MgO,Al2O3,TiO2and carbon nanotubes (Jo et al.,2007;Bahmani et al.,2014;Ma et al.,2016;Gowda et al.,2017;Lo et al.,2017).Additionally,other nanoparticles such as nano-Fe2O3(NF)and nano-CaCO3(NC)have also been investigated for the performance improvement of cement products (Sato and Beaudoin,2011;Choobbasti et al.,2019).It can be speculated from above studies that using nanoparticles as cement admixtures can effectively improve the strength of cement-stabilized soils.
The contribution of nanoparticles to the strength development of stabilized soft soils has been evaluated previously by several studies.Bahmani et al.(2014) conducted a series of tests to investigate the effect of nano-SiO2(NS)on the geotechnical properties of cemented residual soil,and the results showed that the addition of 0.4%NS can increase the compressive strength of cemented soil up to 80%.Changizi and Haddad (2015) reported that the shear and compressive strengths of soil can be significantly improved by incorporating NS.Both Meng et al.(2017) and Choobbasti et al.(2019) investigated the effects of NC on the strength development of cement-stabilized marine soil,and found that the addition of NC effectively increases its compressive strength.Choobbasti and Kutanaei (2017) confirmed that the addition of optimum NS can obviously enhance the strength and associated microstructural properties of cemented sand.Yao et al.(2020b)pointed out that the shear strength of cement-stabilized silty clay can be effectively improved by adding 1% nano-MgO (NM).Furthermore,Yao et al.(2019a) also observed that NS can effectively reduce the deterioration of cemented soil strength caused by sulfuric acid attack.Wang et al.(2019c)studied the effectiveness of NM on the strength properties of cement-stabilized seashore soft soil,and observed that the inclusion of NM increases its shear strength.Lang et al.(2020a) revealed that adding 1% NS can obviously improve the strength of cement-stabilized DS containing humic acid.Thomas and Rangaswamy (2020) investigated the strengthening of NS on cement-treated soft clay via a range of strength and microstructural tests,and they proved that the presence of NS in cemented clay leads to a positive influence on the strength gain.Review of these researches related to the positive effects of nanoparticles on the strength development of cemented soils shows that the previous studies mainly focus on the contribution of single nanoparticle to the strength enhancement of cemented soils.However,little attention has been paid to the effect of composite nanoparticles,especially the mass ratio of different nanoparticles on the strength development and associated micro-mechanisms of cementstabilized DS (CDS).
This study aims to quantify the effects of various dosages of single and composite nanoparticles on the strength of CDS.A series of unconfined compressive strength (UCS) tests was conducted to achieve these purposes.Furthermore,the impacts of these variables on the pH evolutions were also evaluated via a range of pH tests.Finally,the microstructure characteristics revealing the macro-strength development were analyzed by scanning electron microscopy (SEM) and X-ray diffraction (XRD) tests.The results obtained in this study can encourage the utilization of nanoparticles as cement admixtures for DS stabilization.
2.Materials and methods
2.1.Raw materials
The DS used in this study was obtained from a river training works,which was located in Minhang District,Shanghai,China.The basic physical properties of used DS were measured in the laboratory based on the following specifications.The DS has an initial water content in the range of 80%-120% (ASTM D2216-10,2010).The liquid and plastic limits are 64.3% and 26.9%,respectively,as per ASTM D4318-10 (2010).Therefore,its plasticity index is 37.4%,indicating the high compressibility.The specific gravity of DS is 2.69(ASTM D854-14,2014),and the void ratio is about 2.12(GB/T50123-2019,2019).The optimum water content and maximum dry density are 22% and 1.73 g/cm3,respectively,determined by the modified Proctor compaction tests (ASTM D1557-12,2012).These values show that this DS has notable characteristics of high water content and high compressibility,which require stabilization treatment before reusing in practical engineering.The water content of DS used in stabilization tests was fixed at 70% by dry weight,which conformed to the actual water content after a period of natural dehydration and air drying.According to X-ray fluorescence (XRF)tests,the chemical composition of DS was determined and is listed in Table 1.By conducting SEM and XRD tests,the microstructure and crystalline phases of DS are given in Figs.1 and 2,respectively.It could be observed that the DS particles exhibit irregular shaped,and are mainly composed of quartz,illite and kaolinite.Fig.3 shows the particle size distribution curve of DS determined by a laser particle size analyzer.The cement adopted in this study was 42.5 Portland cement (PC),and its chemical composition is given in Table 1.The nanoparticles used in this study were NS,nano-Al2O3(NA) and NM in the state of white powder.The basic physical properties of the three nanoparticles are provided in Table 2.Moreover,the micro-images of the three nanoparticles obtained by SEM tests are shown in Fig.4.
2.2.Sample preparation
The mix design and testing scheme are provided in Table 3.A total of six series including CDS-S,CDS-A,CDS-M,CDS-SA,CDS-SM and CDS-AM were prepared.In CDS-S,CDS-A and CDS-M series,single nanoparticle was used as cement admixture,with contents of 0%,2%,4%,6%,8%and 10%by dry mass of cement.As previously introduced,the nanoparticle dosage is usually controlled within 10% (by cement weight),thus in CDS-SA,CDS-SM and CDS-AM series,the total content of composite nanoparticles was selected as 10%.Based on the prior studies (Chian et al.,2016;Yao et al.,2019b,2020c),the cement dosage used for soft soil stabilization is commonly more than 20%or even 30%.To achieve the purpose of reducing cement dosage,this study fixed the cement content at 15%,which is characterized by the mass ratio of cement or water to dry DS.Note that the CDS without nanoparticles was also used as the benchmark for comparison.The DS was first dried in an oven at a temperature of 70°C,and then was ground into powder and passed through the 2-mm sieves.The mass of the raw materials including DS,cement and nanoparticles was calculated and weighed according to the mix design.The dry DS and binders (i.e.cement and nanoparticles)were firstly mixed in a bench-top mixer for 5 min until the mixture became uniform.Then,the weighedwater was gradually added and mixing was continued for another 5 min.The homogenized DS-binder mixture was slightly poured into a cylindrical mold of 50 mm in diameter and 50 mm in height.During the preparation,vibration compaction was performed to remove air bubbles inside the mixture.Because the water content adopted was slightly higher than the liquid limit,the DS-binder mixture was close to the slurry form,and their dry density was considered to be almost the same.The well-prepared samples together with molds were sealed with fresh-keeping films,and placed in a curing room where the temperature and relative humidity were 25°C±3°C and 90%±5%,respectively.After cured for 24 h,the samples were re-molded and cured continuously to 7 d and 28 d for subsequent tests.For each test,three samples were prepared and a total of 180 samples were used for UCS testing.

Table 1Chemical composition (%) of DS and PC.

Fig.1.The microstructure of DS with 5000 × magnification.
2.3.Testing methods
The UCS tests were conducted as per ASTM D4219-08 (2008).The sample was loaded vertically at a constant displacement rate of 1 mm/min until failure,and then the UCS was calculated.For each mix at the same curing age,three parallel samples were measured,and their average and standard deviation (error) values were determined as the final UCS results.The pH measurements were conducted on the samples cured for 28 d in accordance with ASTM D4972-13 (2013).After UCS tests,for each mix,the broken sample was crushed to powder with a grinding rod and passed through a 2-mm sieve.Afterwards,10 g powder sample was weighed and placed in a 100 mL breaker,and then 50 mL distilled water was added.The mix was stirred well in the beaker with a stirring rod for 5 min.After standing for 6 h,a pH-100 tester was used to directly measure the pH value of supernatant.Typical 28-d samples (at optimum mixes) were selected for microstructural analyses by conducting SEM and XRD tests.These samples were taken from the failure samples after UCS tests.Before SEM testing,the samples were frozen drying by liquid nitrogen after 7 d of soaking in ethanol.Then,the samples were sublimated for 2 d in a vacuum.The dried sample pieces of about 1 cm3were prepared.It should be noted that a layer of gold was needed to be coated on the surface of samples so as to induce conductivity and avoid charging effect.For XRD tests,the milled samples were scanned for 2θ in the range of 5°-75°,with step length of 0.02°and scanning rate of 8°per minute.The XRD results revealing crystalline phases were analyzed by the MDI Jade 6.0 material analysis software.

Fig.2.The crystalline phase of DS obtained from XRD test.

Fig.3.Particle size distribution curve of DS.

Table 2Basic physical properties of nanoparticles.
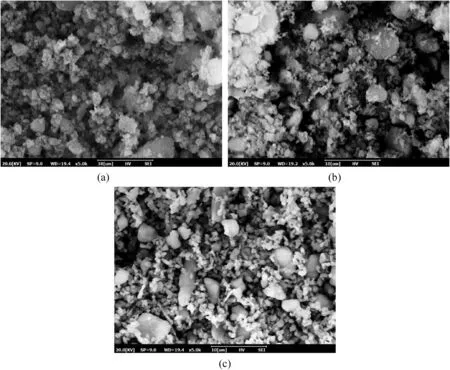
Fig.4.The micro-images of (a) NS,(b) NA and (c) NM.
3.Results and discussion
3.1.Unconfined compressive strength (UCS)
3.1.1.Effect of single nanoparticle
The effect of single nanoparticle content on the UCS of CDS is shown in Fig.5.For CDS-S as plotted in Fig.5a,the UCS achieves the peak for the samples with NS contents of 4% and 6% cured for 7 d and 28 d,respectively.Thus,the optimum NS content is determined to be 4% and 6%,which can increase the 7-and 28-d UCSs by 38%and 50% compared to that of CDS (without nanoparticle),respectively.It should be noted that the optimum NS content varies with the curing time,which may be related to the hydration degree of cement.The NS was mainly consumed by Ca(OH)2,which was produced by the cement hydration,and then additional C-S-H gel was produced.The amount of Ca(OH)2produced in 7 d was lower than that in 28 d.This led to the required optimum NS content in 7-d CDS,which was less than that in 28-d CDS.This indicates that the addition of 4%-6%NS is reasonable to improve the strength of CDS.The UCS of CDS-A versus NA content is illustrated in Fig.5b,indicating that the UCS enhancing with NA content has a tendency of increasing at first and then decreasing.The maximum 7-and 28-d UCSs occur at 6% NA content.By adding 6% NA,the 7-and 28-d UCSs of CDS increase by 17% and 35%,respectively.This confirms that the optimum NA content is 6%.Similar tendency is also observed for CDS-M,as shown in Fig.5c,i.e.both 7-and 28-d UCSs of CDS-M increase to the peak with increasing NM content to 8%and then decrease slightly.The addition of 8% NM to CDS can increase 7-and 28-d UCSs by 65%and 67%,respectively,compared to that of CDS without nanoparticle.
The above results indicate that regardless of nanoparticle type added,the UCS of CDS can be effectively improved.This further confirms that nanoparticle is suitable for improving the strength of CDS.It is well known that nanoparticles have high specific surface area together with high surface energy,which can accelerate the cement hydration (Zyganitidis et al.,2011;Taha and Taha,2012;Bahmani et al.,2014).The mechanisms for this improvement in strength can be attributed to the following three aspects.First,the high surface energy of nanoparticles makes the hydration products of cement rapidly deposited on its surface,and then bonded with Ca(OH)2to form C-S-H gel under the nano-induced effect.Second,the addition of nanoparticles can also alter the soil properties by interactions between nanoparticles and soil matrix particles,causing a large number of soil particles to contact together(Changizi and Haddad,2015;Mahmoudian et al.,2020).Third,nano-filling is another main reason for improving the strength of stabilized soils(Lo et al.,2017;Meng et al.,2017).A great number of nano-scale pores and capillary pores exist inside CDS,which provide room for nanoparticles to fill the pores and then improve the compactness.Additionally,the average diameter of most hydration product,i.e.C-S-H gel,is about 10 nm,thus the nanoparticles can also fill the pores inside C-S-H gel matrix (Bahmani et al.,2016).However,it can be observed from Fig.5 that adding excessive nanoparticles has an adverse influence on the strength development of CDS.Adding excessive nanoparticles leads to the decreaseof the distance between nanoparticles,which prevents the suitable Ca(OH)2crystal growth and then weakens the formation of C-S-H gel (Choobbasti and Kutanaei,2017).The incomplete Ca(OH)2crystal growth may cause shrinkage and associated microcracks,and then decrease the strength gain.Similar observations were also found by previous researchers(Bahmani et al.,2014;Ye et al.,2015;Choobbasti and Kutanaei,2017;Yao et al.,2019a).Furthermore,the dispersion problems of excessive nanoparticles may also responsible for the strength drop(Bahmani et al.,2014).It should be noted that the strength enhancement varies with the type of nanoparticles.Further analysis of this observation will be conducted in the later stage.
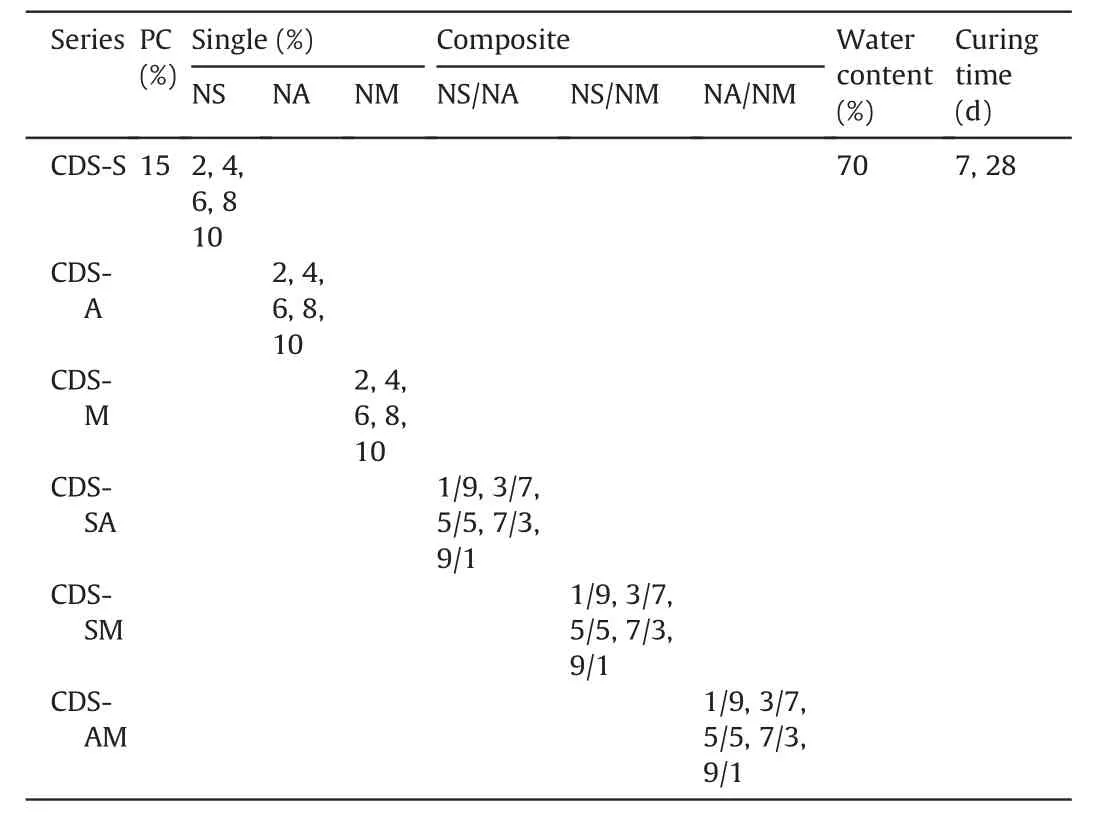
Table 3The mix design and testing scheme.
The productiveness evaluation of nanoparticles with curing time on the strength of CDS is important to determine the optimal cost-effective nanoparticle content.As Kang et al.(2016)elaborated previously,the curing time was essential to the strength enhancement of cement-treated marine dredged clay.Because only 7-and 28-d UCSs of CDS were adopted in this study,it is impossible to fully consider the efficiency of nanoparticle modification on the strength gain with curing time.Therefore,based on the 7-and 28-d UCS results,the strength increment (ΔUCS) and corresponding growth rate(UCSgr)of CDS were quantitatively evaluated,which are respectively calculated by the following equations:

where UCS7and UCS28represent the 7-and 28-d UCSs,respectively.
Fig.6 presents the ΔUCS and UCSgrof CDS against three single nanoparticle contents.For CDS-S given in Fig.6a,both ΔUCS and UCSgrachieve the maximum at the NS content of 6%,and the corresponding UCSgris 0.77.This indicates that the NS content of 6%is the most effective to improve the strength of CDS.In combination with the optimum NS content of 4%-6%obtained from UCS results,it is suggested to use 6% NS as the cement admixture for DS stabilization.Fig.6b shows the ΔUCS and UCSgrversus NA content of CDS-A,indicating that they all achieve the maximum at the NA content of 6%.This agrees well with the optimum NA content(6%)from UCS tests.Fig.6c shows the ΔUCS and UCSgrversus NM content of CDS-M,and it is clearly observed that both ΔUCS (170 kPa)and UCSgr(0.68) reach the maximum at the NM content of 4%.Different from what is expected,the most effective NM content is lower than the optimum value(8%).From Fig.5c,the UCS of CDS-M containing 4% NM is 12.6% lower than that of CDS-M with 8% NM.Therefore,it is suggested to determine the reasonable nanoparticle dosage based on the considerations of strength growth efficiency,maximum strength and economic cost.
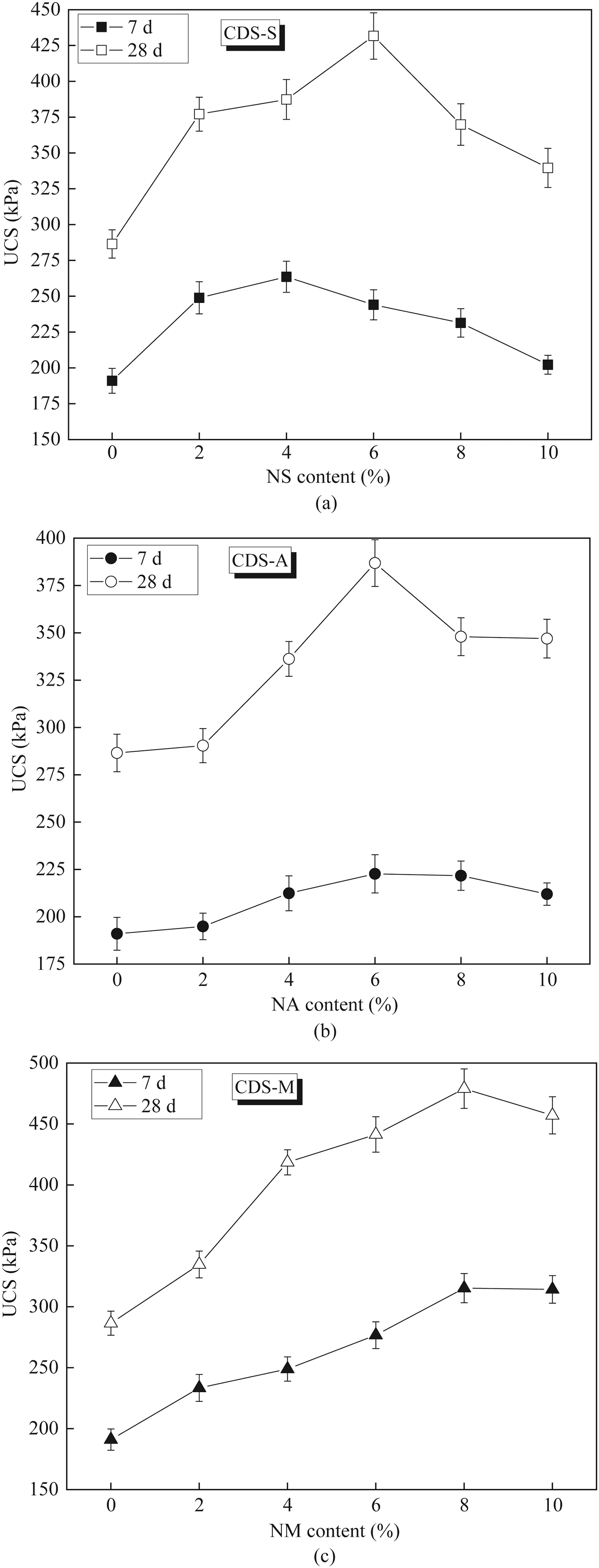
Fig.5.Effect of single nanoparticle on the strength development of CDS:(a)NS,(b)NA,and (c) NM.

Fig.6.The ΔUCS and UCSgr of CDS versus (a) NS,(b) NA,and (c) NM contents.
3.1.2.Effect of composite nanoparticles
Fig.7 presents the effect of mass ratio of composite nanoparticles on the strength of CDS.For comparison purposes,the 7-and 28-d UCSs of CDS with equal amount of single nanoparticle are also marked in the form of dotted line.For CDS-SA presented in Fig.7a,the UCS of CDS-SA decreases with the mass ratio of NS/NA,indicating that the optimum NS/NA ratio is 9/1.Compared to CDS with NS or NA alone,the CDS containing composite nanoparticles at NA/NA ratio of 9/1 has higher UCS.The maximum 7-d UCS of CDSSA is even higher than the 28-d UCS of CDS containing 10%NA.The maximum 28-d UCS of CDS-SA is 44.5%and 41.1%higher than that of CDS with 10% NS and NA,respectively.For CDS-SM as shown in Fig.7b,the UCS of CDS-SM increases with the mass ratio of NS/NM up to 3/7 and then decreases.Therefore,the optimum NS/NM ratio for CDS-SM is determined to be 3/7.Taking the UCS of CDS-M as the benchmark,the maximum 7-and 28-d UCSs of CDS-SM increase by 31.8% and 25.6%,respectively.Fig.7c presents the effect of NA/NM mass ratio on the UCS of CDS-AM.It can be seen that the UCS of CDS-AM slightly increases with the NA/NM ratio and reaches the peak at the NA/NM mass ratio of 3/7,implying that the optimum NA/NM mass ratio is 3/7.The 7-and 28-d UCSs of CDS-AM with optimum NA/NM mass ratio increase by 20.5% and 19.9%,respectively,when compared to those of the CDS-M with 10% NM.The above results confirm that the coupling effect of two nanoparticles exists in CDS,which further improve the strength of CDS compared to that of single nano-modification.Fig.8 shows the optimum UCS comparison of CDS containing various single and composite nanoparticles,together with the UCS of CDS without nanomodification as a benchmark.Obviously,the UCS of CDS can be significantly improved by incorporating nanoparticles,regardless of single or composite form.For the single nano-modification,NM was the most effective to improve the UCS of CDS,followed by the NS and NA,respectively.For instance,the optimum 28-d UCSs of NM-,NS-and NA-modified CDS increase by 67%,50% and 35%,respectively,when compared to that of CDS without nanomodification.For composite nano-modification,the CDS-SM has the highest UCS,followed by CDS-AM and CDS-SA,respectively.By comprehensive comparison,the composite nanoparticles were more efficient than single nanoparticle in improving the UCS of CDS.Specifically,the optimum 7-and 28-d UCSs of CDS-SM are 117% and 91% higher than that of CDS without nanoparticles.It should be noted that the optimum contents of NS,NA and NM are 4%-6%,6% and 8%,respectively,while the composite nanoparticle content is 10%.However,this does not affect the above conclusion,because the UCS of CDS containing 10%single nanoparticle is even smaller (see Fig.5).Therefore,it is highly suggested to use composite nanoparticles,especially NS/NM of 3/7,to improve the strength of CDS to the greatest extent.
Fig.9 shows the ΔUCS and UCSgragainst the mass ratio of composite nanoparticles.For CDS-SA provided in Fig.9a,both ΔUCS and UCSgrdecrease with NS/NA mass ratio from 9/1 to 7/3,and then increase until the NS/NA mass ratio reaches 1/9.Combined with the results in Fig.7a,the lower 7-d UCS of CDS-SA is mainly responsible for the observation that the highest UCSgris achieved at NS/NA ratio of 1/9.Therefore,as per the evolution of ΔUCS and UCSgr,the NS/NA mass ratio of 9/1 is also recommended for the CDS-SA,which is highly in agreement with the strength results.The effect of NS/NM on ΔUCS and UCSgrof CDS-SM is given in Fig.9b,indicating that the evolutions of ΔUCS and UCSgrwith NS/NM mass ratio can be divided into three stages:increase rapidly,decrease slowly and increase rapidly again.The number of cementitious products in CDS-SM may be responsible for this observation.The highest UCSgrof 0.53 is achieved at the NS/NM ratio of 1/9.Fig.9c shows the ΔUCS and UCSgrversus NA/NM mass ratio for the CDS-AM.It is clear that the maximum ΔUCS and UCSgroccur at the NA/NM ratio of 5/5.Due to the fact that UCSs of CDS-AM at NA/NM ratios of 5/5 and 3/7 are close to each other,as shown in Fig.7c,the NA/NM mass ratio of 5/5 may be more suitable for the long-term strength of CDS-AM.It is suggested that the maximum UCS and UCSgrshould be noted simultaneously to determine the reasonable composite nanoparticle dosage.
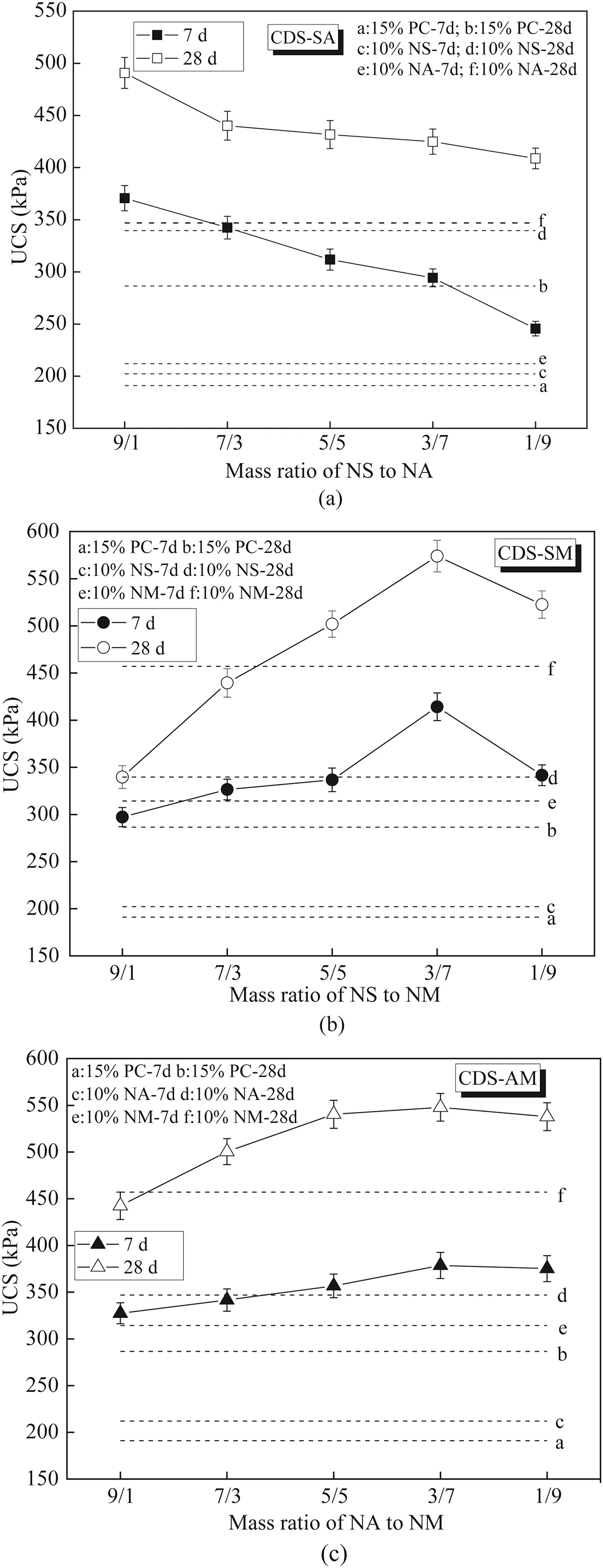
Fig.7.Effect of mass ratio of composite nanoparticles:(a) NS/NA,(b) NS/NM,and (c)NA/NM on the strength development of CDS.
3.2.pH level
The pH level of soils is one of the important indices which can significantly affect its strength(Sargent et al.,2013;Zainuddin et al.,2019).The variations of pH level of CDS versus single nanoparticle content are given in Fig.10.As a reference,the pH of CDS without nano-modification of 11.6 is also plotted by a dotted line,which exhibits obvious alkalinity.The tricalcium silicate(3CaO·SiO2,C3S)and dicalcium silicate (2CaO·SiO2,C2S) are the main ingredients,the sum of which is about 65%-80%of PC(Xing et al.,2009).When PC meets water,hydration reaction immediately occurs and produces C-S-H gel and Ca(OH)2(see Eqs.(3) and (4)).Meanwhile,some Ca(OH)2ionizes to form OH-(see Eq.(5)),which is responsible for the alkalinity of CDS.

Fig.8.Comparison of optimum UCS of CDS modified by different nanoparticles.

For CDS-S,the pH level decreases from 10.25 to 9.55 with increasing NS content up to 6%,and then keeps a moderate evolution as the NS content continues to increase.This observation is in line with the previous findings by Bahmani et al.(2014).On one hand,Ca(OH)2is consumed by NS via pozzolanic reaction (see Eq.(6)),which directly causes an reduction in pH level.On the other hand,the dispersion problems caused by excessive NS content may inhibit further pozzolanic reactions between NS and Ca(OH)2and thus delay the decrease of pH level.It is known that alkaline environment is necessary to elevate strength gain of stabilized soils.Unconventionally,the optimum 28-d UCS of CDS is achieved at the lowest pH level.This is attributed to the reactions between NS and Ca(OH)2which not only produce C-S-H gel,but also decrease the pH level to some extent.Thus,using NS as PC admixture is beneficial to control the pH level of CDS.

For CDS-A,the pH level decreases from 11.26 to 10.01 with increasing NA content from 2% to 10%.The formation of Ca(OH)2produced by PC hydration provides an alkaline environment,which contributes to the dissolution of Si-O and Al-O,and then leads to the formation of cementitious products (Xu and Yi,2019).The reaction between Ca(OH)2and Al2O3is responsible for the reduction in pH level (see Eq.(7)).Furthermore,the rates of Ca(OH)2production and consumption significantly determine the variations of pH level (Xue et al.,2017;Sun and Yi,2020).

For CDS-M,it is clearly observed that the pH level increases firstly with increasing NM content up to 4%and then decreases and becomes stable as the NM content reaches 6%.The reason for this observation can be simplified below:
(1) MgO meets with water and directly produces OH-and Mg2+ions(Yi et al.,2016).This process leads to the increase in pH level(see Eq.(8)).

Fig.9.ΔUCS and UCSgr of CDS versus mass ratios of (a)NS/NA,(b)NS/NM and (c)NA/NM.
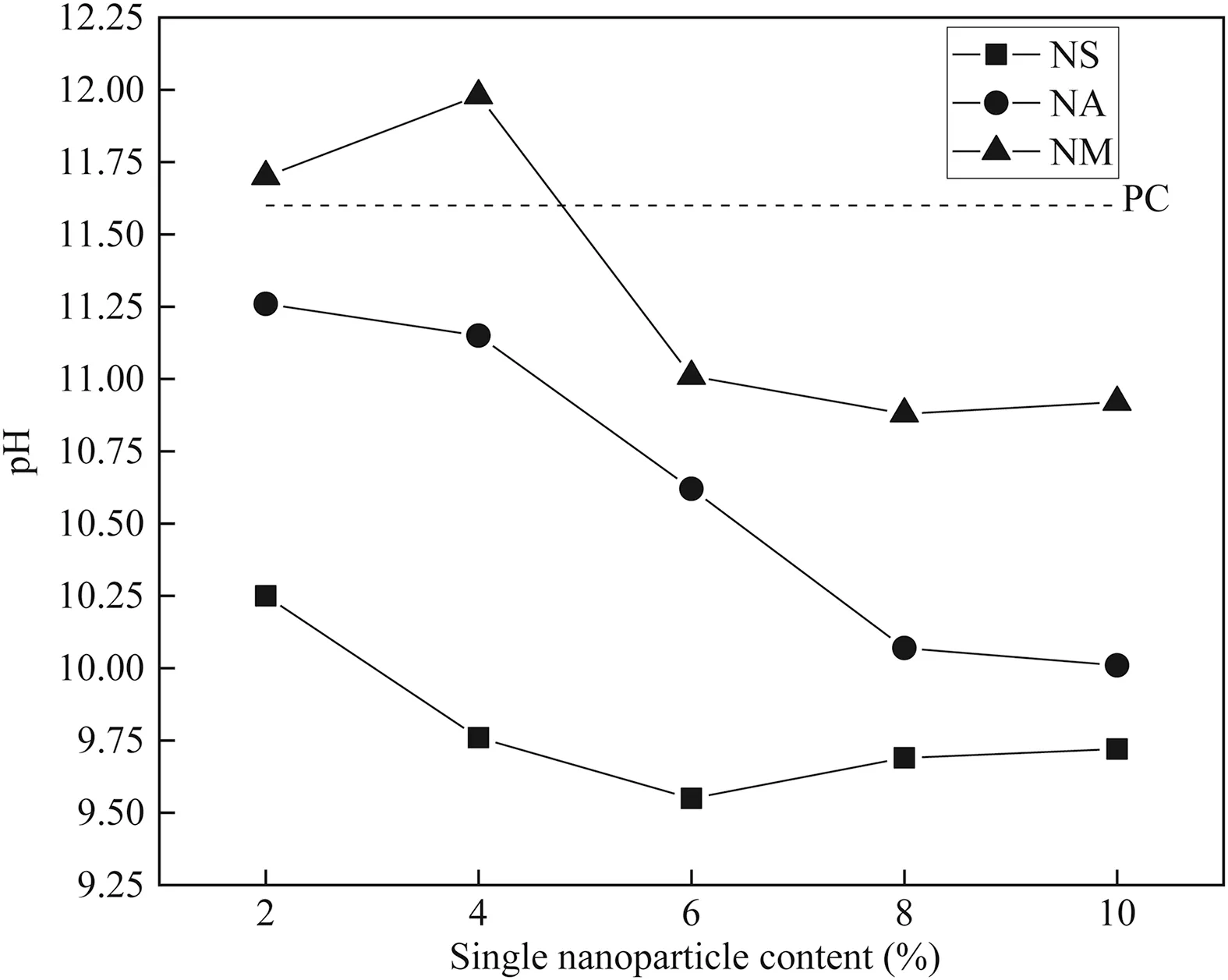
Fig.10.The pH variations of CDS with single nanoparticle content.
(2) The OH-ion is consumed in the formation of M-S-H(MgO·SiO2·H2O) gel (see Eq.(9)).This reaction not only improves the bonding between soil particles,but also decreases the pH level.As the OH-consumption approaches its production,the variation of pH keeps moderate.In general,the pH level of CDS-M is the largest,followed by CDS-A and CDS-S,respectively.
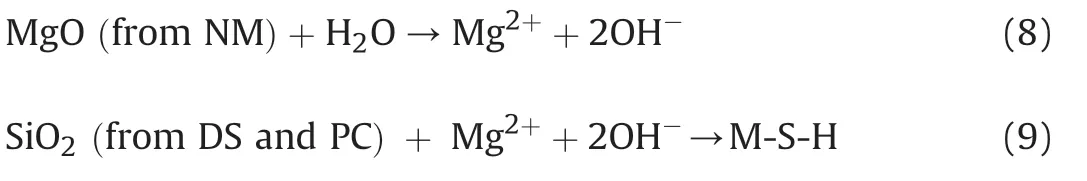
Fig.11 shows the pH evolution of CDS with mass ratio of composite nanoparticles.For CDS-SA,the pH value increases marginally with the NS/NA mass ratio.Fig.10 displays that the pH level decreases with increasing NS (0%-4%) and NA contents.Thus,the slight increase of pH level with NS/NA mass ratio indicates that the OH-consumption capacity of NA is weaker than that of NS.For CDS-SM,the pH level increases first and then decreases with the NS/NM mass ratio,and achieves the highest at NS/NM ratio of 5/5.The addition of NM yields OH-and causes an increase in pH level with the NS/NM ratio from 9/1 to 5/5(see Eq.(8)).Meanwhile,with the improvement in alkaline environment caused by incorporating NM,pozzolanic reactions become more active and then consume more OH-conversely(see Eq.(9)).This may be responsible for the decrease of pH level with the NS/NM ratio from 5/5 to 1/9.For CDSAM,the pH value increases with the NA/NM mass ratio from 9/1 to 7/3,and then decreases with the NA/NM ratio from 7/3 to 1/9.This may be attributed to that the production of OH-is not sufficient to buffer its consumption.By comparison,the pH level of CDS-AM is the highest,followed by CDS-SM and CDS-SA,respectively.The above observations confirm that for CDS with composite nanoparticles,the pH evolution is mainly determined by the difference between OH-production and consumption.
3.3.X-ray diffraction (XRD)

Fig.11.The pH evolution of CDS with mass ratio of composite nanoparticles.
Combined with the UCS results,the crystalline phases,represented by XRD patterns for typical 28-d CDS samples at optimum mixes,are shown in Fig.12.The XRD patterns of CDS without nanoparticles were selected as a comparison benchmark.The clay minerals,i.e.quartz,kaolinite and illite,were detected in all samples,reflecting the nature of DS used.Furthermore,the main hydration products of PC,C-S-H and C-A-H gels were also detected in all the samples.This indicates that the addition of nanoparticles to CDS does not change its mineral compositions,and even no new hydration products are produced.Compared to CDS without nanoparticles (a),Ca(OH)2is obviously absent in CDS-6S (i.e.CDS containing 6% NS,similarly hereafter) (b),the pozzolanic reaction between NS and Ca(OH)2from PC hydration is responsible for this observation,and then additional C-S-H gels are produced.For CDS-6A (c),it is interesting to find that no additional C-A-H gels are obviously detected compared to CDS (a),together with Ca(OH)2,which is also detected.This confirms that the pozzolanic reaction between Ca(OH)2and NA is insufficient,which causes Ca(OH)2not to be completely consumed in producing additional C-A-H gels.For CDS-8M (d),it can be observed that more Ca(OH)2is detected.When NM is mixed into CDS mixture,the Mg2+and OH-ions are immediately yielded.Meanwhile,the chemical reactions between SiO2(from PC and DS),Mg2+and OH-occur and then produce M-SH gel.This process accelerates the further hydration of PC,and thus additional Ca(OH)2is detected.Furthermore,the high specific surface energy of NM also promotes the hydration of PC.However,the M-S-H gel is not detected from XRD pattern of CDS-8M.This result may be caused by the miscibility of C-S-H gel,and the similar observation was also reported by Brew and Glasser(2005).For CDSSA (e),it can be seen that compared to CDS with single nanomodification,more C-S-H and C-A-H gels are detected,confirming the advantage of composite nano-modification in promoting the production of hydration products inside CDS.Similarly,the XRD patterns of CDS-SM (f) and CDS-AM (g) also exhibit an improvement in producing C-S-H and C-A-H gels,compared to CDS with single nano-modification.The above analysis further confirms the high efficiency of nano-modification,especially composite nanomodification,in improving the strength of CDS.
3.4.Scanning electron microscopy (SEM)

Fig.12.XRD patterns for CDS containing optimum nanoparticle mixes at 28 d:(a)15%PC,(b)15%PC+6%NS,(c)15%PC+6%NA,(d)15%PC+8%NM,(e)15%+9%NS+1%NA(1/9),(f)15%PC+3%NS+7%NM(3/7),and(g)15%PC+3%NA+7%NM(3/7).1-quartz;2 -kaolinite;3 -illite;4 -Ca(OH)2;5 -C-S-H;6 -C-A-H.
Fig.13 presents the typical SEM micrographs,taken at 5000× magnification,for 28-d CDS with optimum mixes.For CDS without nanoparticle,as shown in Fig.13a,large soil particles are randomly displayed along with obvious pores.The main hydration products of PC,gel-like C-S-H and cubic-shaped C-A-H gels fill some pores and cement soil particles.Furthermore,the plate-shaped Ca(OH)2is also seen adhering to the surface of soil particle.By adding 6%NS to CDS,as shown in Fig.13b,the soil particles tend to agglomerate and the whole structure becomes denser and more homogenous since the pores are significantly reduced.The additional hydration product C-S-H gel caused by NS contributes to this good integrity in the microstructure.Furthermore,the addition of NS can also narrow the pore size distribution by filling the micro-pores(Lo et al.,2017).Note that the plate-shaped Ca(OH)2was not clearly imaged,which was caused by the pozzolanic reaction between NS and CH,and this has been confirmed by XRD result.For CDS containing 6%NA shown in Fig.13c,more cubic-shaped C-A-H gels are clearly seen,distributing among soil particles,implying the positive effect of adding NA on producing C-A-H gel among soil particles.Comparison of Fig.13a and c indicates that a denser matrix is achieved and smaller pores exist inside CDS-A to a great extent.However,the space of the pores between soil particles in CDS-A is larger than that in CDS-S,which helps to explain the result that the optimum UCS of CDS-A is lower than that of CDS-S.For CDS with 8%NM,as shown in Fig.13d,the microstructure of CDS-M is obviously denser,more uniform and flatter,and exhibits stronger binding ability between soil particles.Except that the nano-filling effect can improve the packing density,the micro-expansion caused by the formation of Mg(OH)2is probably responsible for this uniform and solid microstructure,which was also found by Yao et al.(2019a).Furthermore,the honeycomb-like hydration product M-S-H gel is observed,which adheres to the surface of soil particles and leads to a denser matrix.It is remarkable that some microcracks emerge among the soil particles,and this may weaken the strength gain to some extent.Fig.13e-g shows the microstructure changes of CDS modified by three types of composite nanoparticles with optimum mass ratio.From the perspective of microstructure integrity,the CDS with composite nanoparticles exhibits a strengthened integrity together with the close-knit structure,when compared to CDS with single nanoparticle.From Fig.13e and f,it can be clearly found that large cemented soil particles are formed,which lead to the formation of close-knit structure and improved the compactness.These observations confirm that composite nano-modification has the advantage over single nano-modification in improving the strength of CDS.By comparison,the microstructure of CDS-SM exhibits the best overall cementation,followed by CDS-AM and CDS-SA,respectively.This goes well with the optimum UCS comparison result presented in Fig.8.It should be noted that microcracks are observed once again in CDS-SM(Fig.13f),which further confirms the expansion caused by NM added.More details about this observation need to be systematically investigated in the future.
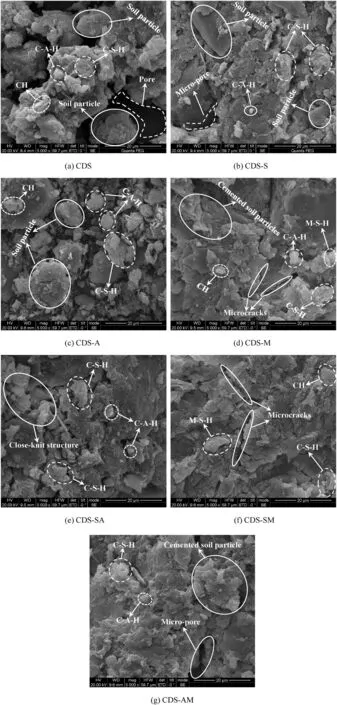
Fig.13.SEM images of CDS containing optimum nanoparticle mixes.CH -Ca(OH)2.
4.Conclusions
This study investigated the effectiveness of nano-modification on the strength and microstructural properties of CDS.Three types of nanoparticles including NS,NA and NM were used.The effects of single nanoparticle content,mass ratio of composite nanoparticles and curing time on the UCS and associated microstructure changes of nano-modified CDS were evaluated via a series of UCS,SEM and XRD tests.The key findings from test results can be drawn as follows:
(1) The UCS of CDS can be significantly improved by the nano
modification.The 7-and 28-d UCSs of CDS can be increased by 38%and 50%,17%and 35%,65%and 67%by adding 4%-6%NS,6%NA and 8% NM,respectively.The high surface energy,nano-induced and nano-filling effects of nanoparticles were responsible for this observation.Excessive nanoparticle has side effect on the strength of CDS,which may be related to the dispersion issues of nanoparticle.Based on the strength growth rate (UCSgr),the cost-effective addition of 6% NS,6%NA or 4%NM was recommended,respectively.
(2) The optimum mass ratio of composite nanoparticles,i.e.NS/NA,NS/NM and NA/NM,was 9/1,3/7 and 3/7,respectively.With regard to UCSgr,the most effective NS/NA,NS/NM and NA/NM mass ratios of 9/1,1/9 and 5/5 were suggested.For single nano-modification,NM was the most effective to improve the UCS of CDS,followed by NS and NA,respectively.For composite nano-modification,the CDS-SM exhibited the highest strength enhancement,followed by CDS-AM and CDS-SA,respectively.The composite nano-modification was obviously more effective than single nano-modification in improving the strength of CDS.The composite nanoparticles with NS/NM mass ratio of 3/7 were highly recommended.
(3) The addition of nanoparticles obviously affected the pH evolution of CDS.For single nano-modification,the pH level of CDS decreased to a certain value and then kept stable with increasing single nanoparticle content.For composite nanomodification,the pH values of CDS-SM and CDS-AM increased at first and then decreased with the mass ratios of NS/NM and NA/NM,respectively,while that of CDS-SA increased slightly with the NS/NA mass ratio.The OH-(hydroxy ion) production and consumption inside nanomodified CDS were responsible for the pH evolutions.
(4) The XRD analysis indicated that the C-S-H and C-A-H gels were the main cementitious products determining the strength of CDS.Furthermore,no new cementitious products were clearly detected after incorporating nanoparticles.The SEM results showed that adding nanoparticles to CDS can lead to a denser and more homogenous microstructure.The nano-filling effect and the formation of additional cementitious products caused by nanoparticles were mainly responsible for the microstructure evolutions.The expansion caused by excessive NM may create microcracks inside CDS.
Declaration of competing interest
The authors wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant No.51972209).
 Journal of Rock Mechanics and Geotechnical Engineering2021年3期
Journal of Rock Mechanics and Geotechnical Engineering2021年3期
- Journal of Rock Mechanics and Geotechnical Engineering的其它文章
- Effects of oil contamination and bioremediation on geotechnical properties of highly plastic clayey soil
- Bearing capacity of surface circular footings on granular material under low gravity fields
- Effect of natural and synthetic fibers reinforcement on California bearing ratio and tensile strength of clay
- Engineering and microstructure properties of contaminated marine sediments solidified by high content of incinerated sewage sludge ash
- An improved specimen preparation method for marine shallow gasbearing sand sediments and its validations
- Rock-like behavior of biocemented sand treated under non-sterile environment and various treatment conditions
