An improved specimen preparation method for marine shallow gasbearing sand sediments and its validations
Yong Wang,Lingwei Kong*,Min Wang,Yanli Wang,Peng Cheng
a State Key Laboratory of Geomechanics and Geotechnical Engineering,Institute of Rock and Soil Mechanics,Chinese Academy of Sciences,Wuhan,430071,China
b Fluid Dynamics and Solid Mechanics,Theoretical Division,Los Alamos National Laboratory,Los Alamos,87545,USA
c Key Laboratory of Geotechnical Mechanics and Engineering of the Ministry of Water Resources,Yangtze River Scientific Research Institute,Wuhan,430010,China
d Hunan University Design Institute Co.,Ltd.,Changsha,410082,China
Keywords:Shallow gas Gas-bearing sediments Bubbles Mechanical behaviour Bender element
ABSTRACT Instabilities of shallow gas-charged seabed are potential geological hazards in ocean engineering.In practice,the conventional field sampling techniques failed to obtain undisturbed gas-bearing sediments from the seabed for laboratory mechanical testing because of sensitive gas exsolution and escape from sediments.However,preparation of representative remoulded gas-charged specimens is a challenging issue,because it is rather difficult to quantitatively control the gas content and obtain uniform distribution of gas bubbles within the specimen.Given the above problems,this work proposes a reliable approach to reconstitute the high-saturation specimen of gas-charged sediments in the laboratory by an improved multifunction integrated triaxial apparatus (MITA).This apparatus is developed based on an advanced stress path triaxial system by introducing a temperature-controlled system and a wavemonitoring system.The temperature-controlled system is used to accurately mimic the in situ environments of sediments in the seabed.The wave-monitoring system is utilized to identify exsolution point of free gas and examine the disturbance of gas to specimens during gas exsolution.The detailed procedure of gassy specimen preparation is introduced.Then,the quality of prepared specimens using our improved apparatus is validated by the high-resolution micro-X-ray computed tomography (μCT)scanning test,from which bubble occurrence and size distribution within the gassy sand specimen can be obtained;and preliminary mechanical tests on gassy sand specimens with various initial saturation degrees are performed.The proposed specimen preparation procedure succeeds in proving the postulated occurrence state of gas bubbles in coarse-grained sediments and accurately controlling the gas content.
1.Introduction
Gas-charged sediments are widely distributed in marine,deltaic,lacustrine,and interactive marine and terrestrial sedimentary environments(Fleischer et al.,2001).In most cases,gas in sediments is a by-product of metabolism by methanogenic bacteria and the upwardly migrated gas of deep thermogenic or hydrate dissociation,namely shallow gas (Lee and Chough,2002;Oung et al.,2006).Usually,gas occurs in sediments in three ways:gas dissolved in pore water,free gas in a form of gas-filled voids,and gas hydrates(Sills and Wheeler,1992).Except for solid gas hydrates that are often formed in deep marine environment,free gas found in most sediments in the shallow sea(depth<300 m)is in a form of discrete and insular gas bubbles (Sills et al.,1991;Christian et al.,1997;Anderson et al.,1998;Boudreau et al.,2005).Fredlund and Rahardjo (1993) argued that when the water content of soil is higher than 85%,the gas phase occurs as insular bubbles within voids.
The shallow gas-bearing sediment is a balanced product of the overlying seal clay,pore water,pressure gas,and temperature,which coexist in a metastable equilibrium in nature.The balance is so vulnerable that any natural or artificial disturbance,such as deposition,earthquake,drilling and excavation,could cause catastrophic failure of the structures built on the sediments (Wang et al.,2018).In recent years,shallow gas-charged seabed has been frequently reported to be an adverse geological unit,posing the safety risk for the offshore infrastructure,ferry terminals,ports,tunnels,pile foundations,pipelines,cables,etc (Wheeler et al.,1991;van Kessel and van Kesteren,2002;Tang et al.,2003;Tjelta et al.,2007;Mabrouk and Rowe,2011).The generation of free gas weakens the strength of sediments and reduces the bearing capacity of foundation,which has been regarded as a conceivable trigger mechanism for failures of submarine slopes (Elger et al.,2018).The shallow gas-bearing sediments are attracting more and more attentions in the research community because of their global distribution and profound scientific,engineering and environmental significance (Wang et al.,2020).Since extraction of in situ undisturbed specimens is challenging due to sensitive gas exsolution and subsequent escape from sediments,preparation of uniform reconstituted gas-bearing soil specimens in the laboratory becomes an alternative way for investigating engineering properties of marine shallow gas-charged sediments (Sobkowicz,1982;Thomas,1987;Vanoudheusden et al.,2004;Hong et al.,2017).
Over the past few decades,great efforts have been made to develop several reconstituted methods for gas-bearing specimens in the laboratory,including chemical,biological,physical and unsaturated soil test methods.A review on the previous methods of gassy specimen preparation is provided in Table 1.Comparatively,the physical method seems to be the promising way to reconstitute gassy specimens for laboratory investigations of marine shallow gas-charged sediments,because it is safe to operate,easy to control,and free of any pollution problems.Sobkowicz and Morgenstern(1984) firstly proposed a method of carbon dioxide (CO2) solution replacement,where the pore water in a saturated specimen will be replaced by CO2-dissolved water under a certain hydraulic gradient.Grozic et al.(2000)further developed the method and investigated the mechanical behaviour of marine loose gassy sand sediments.However,the used method was conducted at the room temperature without strict temperature-controlled condition,and it cannot mimic different in situ temperature conditions on the seafloor(e.g.0°C-5°C in deep sea).Moreover,1% fluctuation of room temperature could cause the saturation degree in gassy specimens to increase or decrease by 4%-5%,which is not allowed to mimic the original highly saturated marine gas-charged sediments(saturation is above 85% in general).In addition,to the best of authors’knowledge,the real-time monitoring on disturbances of gas extrusion and expansion to soil skeletons has not been reported.Free gas in marine gas-charged sediments has different forms.Wheeler (1988) argued that gas forms relatively small occluded bubbles existing within the pore spaces in coarse-grained sediments,while in fine-grained sediments,gas forms relatively large bubbles surrounded by a saturated matrix.Anderson et al.(1998)postulated that there were three types of bubbles in sediments,which are interstitial gas bubbles,airbags and sediment displacing bubbles.Unfortunately,due to the limited technology in the past,the gas occurrence type in the specimen of different reconstituted methods for marine gas-charged sediments was not examined carefully.The recent advancement of micro-X-ray computed tomography (μCT) imaging technologies,which can enhance the spatial resolution in the order of 1 μm or even higher,provides a powerful and nondestructive testing approach to study the free gas bubble in sediments.For instance,Johnson et al.(2017)investigated the evolution of bubble populations generated in consolidated soft sediments by a high-resolution CT.Based on the time-lapse μCT scanning tests,Liu et al.(2016,2018) tracked the dynamic process of methane bubble formation and migration,and estimated the gas content in aquatic sediments.But few of studies were conducted toquantitatively examine the representative volume element(RVE)of a prepared gassy specimen and assess its reconstituted quality through measuring the interior bubble occurrence state,size distribution,and homogeneity.To overcome aforementioned problems,this work first develops a multifunction integrated triaxial apparatus (MITA) by introducing a temperature-controlled system and a wave-monitoring system based on an advanced stress path triaxial system in Section 2,where the function of temperaturecontrolled system and wave-monitoring system is addressed in detail.Then,the improved preparation procedure for high-quality gassy specimens and determination of their state parameters are given in Sections 3 and 4,respectively.It is followed by quantitative validations of the prepared specimens using the high-resolution μCT scanning test and basic mechanical response of gassy specimens with various saturation degrees in triaxial tests.Then,discussion on the proposed method and the importance of our improvement for obtaining high-quality gassy specimens is given before conclusions are drawn.

Table 1Comparison of previous gassy specimen preparation methods.

Fig.1.Image of the customized MITA.BES:bender-extender element system.
2.The multifunction integrated triaxial apparatus
In situ gassy sediments are usually found in the seabed in different ambient temperatures and pressure conditions.It is well known that the occurrence state of gas is sensitive to pressure and temperature.To mimic and control precisely the in situ temperature condition of marine sediments,a temperature-controlled system is introduced into the process of gassy specimen preparation.Additionally,how to accurately measure the gas content in a prepared gassy specimen is extremely difficult.One effective way is to obtain the value of gas content from back analysis based on the ideal gas equation of state,which strictly depends on a stable constant temperature condition.That is another important reason why the temperature-controlled system is used.
Fig.1 shows the self-developed MITA for the preparation of gasbearing specimens.It is mainly composed by four parts,i.e.an advanced stress path triaxial system,a temperature-controlled system,a wave-monitoring system,and a circulation system of gas-dissolved water.
2.1.Advanced stress path triaxial system
The advanced stress path triaxial system is the basic equipment,which should be equipped with a double-walled cell,in which specimens are prepared(Fig.2).Between the inner and outer cells,there is an electronic volume change device (VCD).It is used to accurately measure the volume change of specimens during the preparation process (Ng et al.,2002).
2.2.Temperature-controlled system
To provide a temperature-controlled environment for the triaxial device,a seamless copper pipe (φ6 mm) is installed inside and outside the inner cell,as shown in Fig.2b.The copper pipe installed on the inner cell is connected to an external circulating water bath device(2 kW,0°C-100°C,accuracy of 0.01°C).Outside wrapping wires are connected in parallel to guarantee the synchronous temperature-control inside and outside the doublewalled cell (difference ≤0.5°C).Fig.3 shows the performance of the triaxial system equipped with the temperature-controlled apparatus,from which constant temperature ranging from low to high values can be achieved quickly and maintained over a long time.
2.3.Wave-monitoring system
A bender-extender element system(BES)is introduced into the MITA as the wave-monitoring system for the gassy specimen preparation (Fig.2a).The BES has twofold functions,which can generate compressive(P)and shear(S)waves(Lings and Greening,2001;Cheng et al.,2020).Since P-wave is sensitive to gas content,it can be used to identify the exsolution point of gas bubbles,while Swave is insensitive to gas but soil skeleton and suitable to examine the skeleton disturbance of specimens caused by gas exsolution and expansion.The BES consists of a function generator,a power amplifier,and a mixed oscilloscope(Fig.1).Both velocities of P-and S-wave are measured in real time.S-wave transmitter and P-wave receiver are mounted on the top cap,while S-wave receiver and the P-wave transmitter are mounted on the pedestal.Both sinusoidal and square input waveforms at different frequencies can be stimulated,with a maximum voltage of±10 V.The sampling frequency is 10 MHz,corresponding to a sampling interval of 0.1 μs.The delay of BES is 5 μs,which is determined by the calibration test with the element tips in direct contact (Viggiani and Atkinson,1995;Wang et al.,2007).During the BES test,the propagation time of wave is determined by the difference between the starting point of transmitted wave and the corresponding point of received wave (startto-start,S-S method).Fig.4 illustrates the S-S method used to interpret BES test results(Leong et al.,2009;Gu et al.,2015).

Fig.2.The inner cell of the triaxial system:(a) Schematic diagram of the inner cell;and (b) The improved temperature-controlled inner cell.

Fig.3.The performance of temperature-controlled system.

Fig.4.Wave velocity determination method in BES tests (S-S method).
2.4.Temperature-controlled circulation system
Another important unit of the MITA is the circulation system of gas-dissolved water,which is independent of the triaxial system and used to generate gas-dissolved water.Referring to the principle of a circulation system proposed by Sobkowicz and Morgenstern(1984),the customized circulation system of gas-dissolved water enhanced by temperature-controlled device is shown in Fig.5.It is comprised of two high-pressure reactors (capacity of 5 MPa and accuracy of 5 kPa),which are wrapped by foam-insulating materials(thermal insulation)to avoid influences of the variation of ambient temperature.The reactors are made of stainless steel materials,and each of them is equipped with the concentric wire wrapping pipe,which is connected to the water bath (see Fig.5b).Through a continuous circulation of water driven by the water bath in the concentric wire wrapping pipe,an accurate control of temperature condition inside the reactors is achieved.In Fig.5a,one of the reactors is an inlet reactor(volume of 1.5 L),which is located no more than 1 m above the pedestal of the triaxial cell and connected to pipeline joint B.The other is an outlet reactor with the same volume,which is parallel to the level of the triaxial cell and connected to pipeline joint A.Each reactor is equipped with an electromagnetic stirrer (10-1000 revolutions per minute (rpm)).Before preparing specimens,the two reactors are filled with half-tank of deaired distilled water.The remaining volume of the reactors is charged with gas (in general CO2or CH4) with a target pressure(usually over 300 kPa),which depends on the in situ hydrostatic pressure of marine gas-bearing sediments.Stirrers are opened to accelerate the dissolution of gas into the de-aired distilled water.The preparation process of water saturated by dissolved gas in the reactors should be completed before the gassy specimen preparation.
3.Gassy specimen preparation procedure
3.1.Tested materials
Sand specimens were collected from a typical area of shallow gas-charged sediments in Hangzhou Bay,China.Sand particles have fine psephicity,and the primary minerals are quartz and feldspar.First,the specimens are air-dried,and the impurities with grain size greater than 1 mm are removed.Then,the sand is mixed evenly.The specify gravity of sand,Gs,is 2.68;and the maximum(emax)and minimum (emin) void ratios are 1.239 and 0.739,respectively.Through the standard test of sieve analysis (ASTM D422-63(2007)e2,2007),Fig.6 shows the particle size distribution curve of the sand.The particle sizes are mainly distributed in the range of 0.1-0.6 mm,containing a small number of silt particles.It belongs to silty sand,with coefficient of uniformity(Cu)of 19.7 and coefficient of curvature(Cc) of 7.5 (ASTM D2487-17e1,2017).
3.2.Saturated specimen preparation
Sand specimens are initially oven-dried,and then the cooling specimens are prepared for triaxial specimens.According to the original occurrence conditions(in situ temperature and hydrostatic pressure level) of marine sediments,the fully saturated sand specimen with ultra-pure water should be prepared by the airpluviation,water-sedimentation,or moist tamping methods in the inner cell of triaxial system (Ladd,1977;Vaid et al.,1999;Yamamuro and Wood,2004).In this work,sand specimens are prepared on the pedestal with the air-pluviation method(φ50 mm × 100 mm,ρd=1.283 g/cm3).Referring to the methodology of conventional triaxial test on saturated soil,the sand specimen is saturated with a constant effective stress of 20 kPa.In this process,the backpressure is ramped up to the same pressure level of reactors of the circulation system (e.g.450 kPa),and the temperature is controlled at 20°C.When the sand specimen is fully saturated (the Skempton’s parameter B ≥0.99),P-wave velocities greater than 1700 m/s could be observed by the BES tests.By increasing the cell pressure(up to 500 kPa)while maintaining the backpressure constant (450 kPa),the saturated sand specimen is isotropically consolidated (non-isotropic consolidation and different consolidation ratios can also be permitted).The axial deformation of the specimen is recorded using an external linear variable displacement transducer(LVDT),while the volume change is monitored by the VCD.The consolidation compression process continues for 1 h until the target mean effective stress(e.g.50 kPa)is reached.
3.3.Pore water replacement
As shown in Fig.5a,the pipeline joint B of the temperaturecontrolled circulation system is connected to the drainage port mounted on the pedestal of triaxial cell,while the joint A is connected to the specimen cap.The prepared water with dissolved gas in the inlet reactor permeates into the pre-prepared saturated specimen in the triaxial cell from the bottom to the top and flows into the outlet reactor due to the hydraulic head difference between the inlet and outlet reactors (H ≤1 m).During this process,the water with dissolved gas displaces the pore water of the saturated specimen,and it would take approximately 2-3 h until the volume of the displaced water exceeds the specimen at least 5 times.The flow rate is controlled by adjusting the hydraulic head difference between the inlet and outlet reactors to minimise the specimen disturbance as possible,according to the permeability of soil.The whole procedure mentioned above is called the pore water replacement,which should be conducted at a constant temperature condition (e.g.20°C).
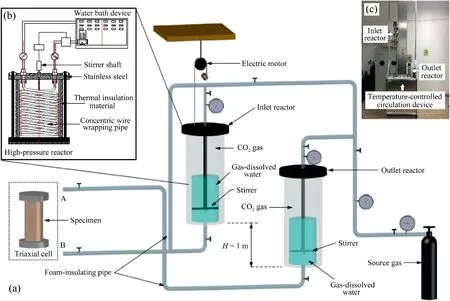
Fig.5.The customized temperature-controlled circulation system:(a) The system schematics;(b) The profile of temperature-controlled reactor;and (c) The image of circulation system of gas-dissolved water.

Fig.6.The grain size distribution of sand specimens in Hangzhou Bay.
3.4.Gassy specimen formation and monitoring
After the procedure of pore water replacement is completed,the circulation system is disconnected.At this point,the backpressure valve is closed and the specimen is ready for undrained unloading.Then,the cell pressure is reduced in a stepwise manner.At each step,the cell pressure is unloaded to no more than 50 kPa (an empirical reduction value is estimated by prior wave velocity monitoring tests of BES).Keeping the cell pressure unchanged,the pore pressure response of the specimen is observed and recorded.Meanwhile,the height and volume variations of specimens should be recorded.The BES alternatively stimulates P-and S-wave to pass through the specimen at a certain interval,and the wave forms and average wave velocities are recorded and used to monitor the status of sand specimen.

Fig.7.Variation of pore pressure response during the unloading steps.
As shown in Fig.7,at the beginning of each unloading step,the same reduction (50 kPa) in pore water pressure as the decrease in cell pressure is observed.For instance,when the cell pressure is unloaded from 500 kPa to 450 kPa (unloading step I),the pore pressure decreases from the initial pressure of 450 kPa to 400 kPa instantaneously.Then,the pore pressure starts to recover and stabilise at 419 kPa.The recovery of pore pressure is caused by gas exsolution due to the unloading (Wong and Maini,2007).Because the P-wave velocity is very sensitive to gas bubbles,it is used to monitor the gas exsolution in the specimen(Kokusho,2000;Yang,2002).Next,the backpressure valve is opened and the backpressure is controlled at the same level as the current pore pressure(419 kPa),while the cell pressure is increased to 469 kPa (i.e.the current mean effective stress recovers to the initial stress of 50 kPa).Subsequently,the backpressure valve is closed,and the pressure is maintained for a few minutes to stabilise.Then,the S-wave is used to test the skeleton disturbance of specimen under the constant effective stress (50 kPa).Following the same routine(cell pressure is progressively unloaded from 500 kPa to 250 kPa),gassy specimens with different initial gas contents are obtained through the stepwise unloading.The changes in the height and volume of specimens are recorded during each unloading process to calculate the state parameters at each step.
It should be noted that,in the process of gassy specimen preparation,P-waves are used to monitor the bubble nucleation because of gas exsolution,and S-waves are used to monitor skeleton disturbance in the specimen,which helps to select a suitable pressure reduction at each unloading step(a larger unloading value could cause unrecoverable disturbance of specimens).It is the main reason why the wave-monitoring system should be equipped in MITA.The measured P-wave velocity of a sand specimen during unloading is shown in Fig.8 (input frequency of 33.3 kHz).The obvious change in the P-wave velocity is an important signal for gas exsolution.For example,when the cell pressure is reduced from 450 kPa to 400 kPa,the measured P-wave velocity shows a significant decrease(unloading step II).This phenomenon indicates that a large number of free gas bubbles form in the specimen.The initiation of gas exsolution corresponds to the liquid/gas saturation pressure.It is irrelevant to the mean effective stress and initial density,but very sensitive to the ambient temperature.Therefore,strict temperature-controlled conditions are important during the gassy specimen preparations.Moreover,the P-wave velocity is also influenced by the temperature(Zou et al.,2017).
Theoretically,gas and liquid cannot resist shearing,thus the variation of saturation degree almost has no influence on the Swave velocity of sand at a high saturation.Therefore,the change in S-wave velocity can reflect the skeleton disturbance of specimen(Pham et al.,2017).Only if the measured S-wave velocity does not show obvious variation(difference ≤5%),the specimen preparation is considered to be successful.Fig.8 shows the measured S-wave velocity of the specimen during unloading (input frequency of 20 kHz).The S-wave velocity at each unloading step is almost equal to the initial value,implying that there is no significant disturbance of the specimen skeleton during the unloading process.
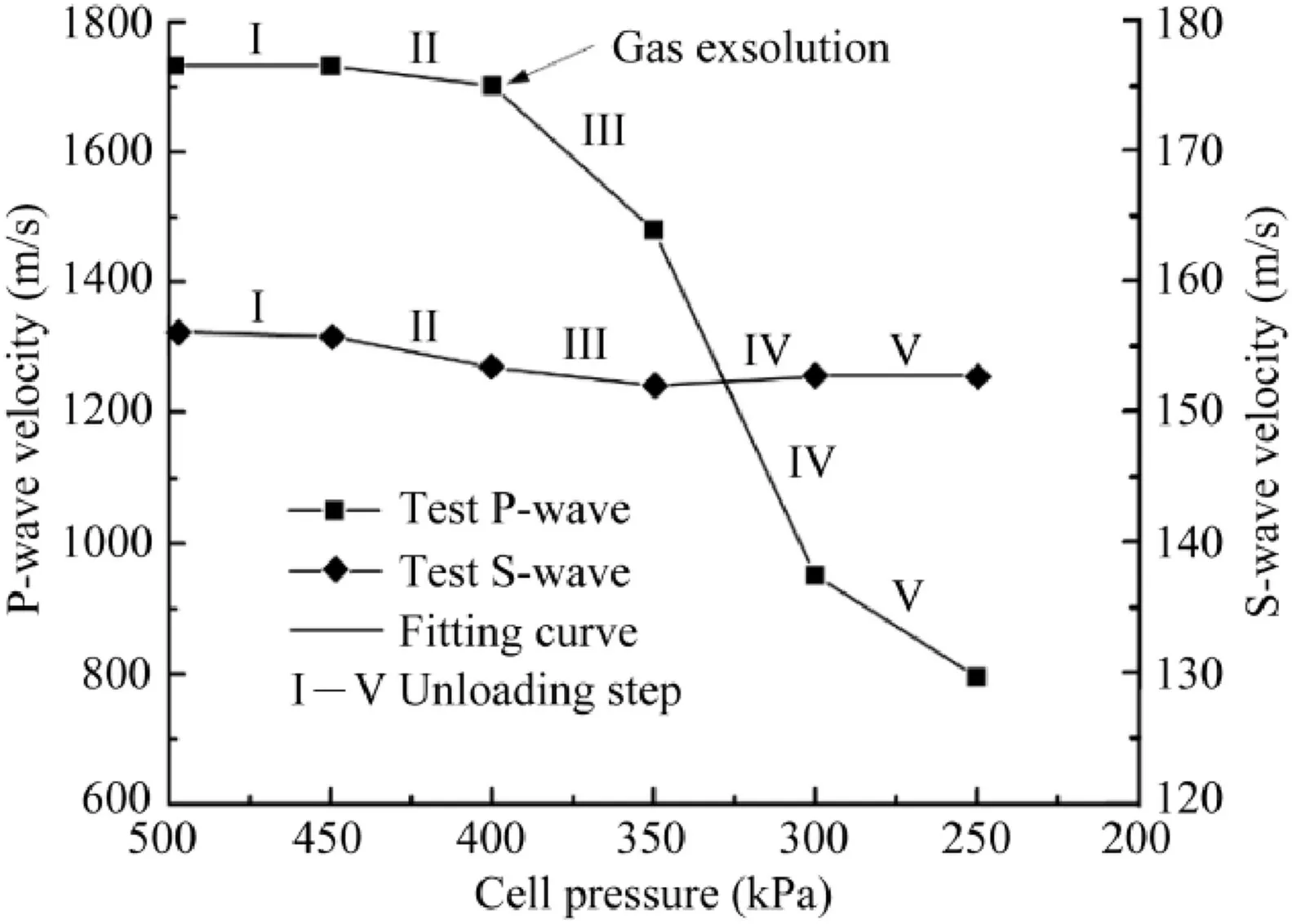
Fig.8.Variations of P-and S-wave velocities of specimen during unloading steps.
To facilitate illustration,the specimen preparation procedure for marine gas-bearing sandy sediments based on the MITA in the laboratory is summarised in Fig.9.
4.Determination of state parameters for the specimen
Volume change is an important indicator and a measurable index to obtain the state parameters of gassy specimens,such as saturation degree,void ratio and porosity.It is well known that the initial saturation of typical marine gassy sediments is usually high(generally above 85%).Due to the limitations of the present testing techniques,including the time domain reflectometry (TDR),it is difficult to directly detect a tiny saturation variation in the specimen because of gas exsolution.Therefore,an indirect method based on the volume change of specimen is adopted.If the volume change is known at each unloading step during the specimen preparation,state parameters of the specimen can be calculated and updated before the next decrement of total stress.However,it must be emphasised that the indirect method is reasonable only at a constant temperature.
The pore fluid of the gassy specimen consists of miscible fluids,i.e.pore water,dissolved gas,and free gas.Fredlund and Rahardjo(1993) demonstrated that Boyle’s law for free gas and Henry’s law for dissolved gas can be combined by applying Boyle’s law to the total volume of both free and dissolved gases in the system.Ignoring the volume compressibility of water,the total volume of water is constant,and the volume of dissolved gas in water can be assumed as a constant,irrespective of the pressure (≤2 MPa) at which the gas is dissolved (Anderson,2002).Hence,based on the Boyle’s law and Henry’s law,Amaratunga and Grozic(2009)derived the following equation to describe the relationship between the saturation of gassy soil and volume change resulting from stress reduction:

where Viand ΔVijcorrespond to the initial total volume and change in the total volume of the current unloading step,respectively;niis the porosity of specimen;Siis the saturation degree before the current unloading step;ΔPijis the change in the gauge pressure(absolute pressure);Pjis the final pore water pressure after the unloading step;and H′is the Henry’s volumetric coefficient of solubility,which varies with temperatures and gas/liquid combinations,e.g.at 20°C,for air/water combination,H=0.02 L/L;for CO2/water,H=0.86 L/L;and for CH4/water,H=0.034 L/L(Rad et al.,1994).
Eq.(1)is an alternative form of Hilf(1948)’s equation.After each stress decrement,saturation degree,porosity,and void ratio values are updated:

Based on the volume changes of specimen and the indirect method,saturations of the gassy specimen at different unloading steps were obtained (the initial saturation is 100% by default),as shown in Fig.10.

Fig.9.The specimen preparation procedure for marine gas-bearing sandy sediments with MITA.
5.Validations of prepared specimens
5.1.X-ray tomography characteristics
The recently developed μCT scanning technique can be used to examine the quality of gassy specimen prepared by the proposed method.Firstly,a saturated sand specimen is prepared in auxiliary devices(a miniature chamber specially made for CT scanning),with the inner diameter of 10 mm.Because the maximum particle size of the sand specimen is only 0.6 mm,the size effect of device can be negligible.Meanwhile,in order to enhance the gas-water contrast of X-ray imaging,20% (by weight) KI solution is prepared in the reactors to replace the air-free water (van Loo et al.,2014).Then,based on the above-mentioned specimen preparation method by MITA,a gassy sand specimen with the initial gas content of 7.9% is obtained after five unloading steps.
The μCT scanning test is carried out on a 300 kV/500 W industrial scanning system,which is mainly constituted with a microfocus X-ray tube and an X-ray sensitive imager.The X-ray tube focus allows geometrical magnification of the image according to different applications,and the maximum detail detectability can reach 1 μm.The cone-beam scanner uses the three-dimensional(3D) filtered back-projection algorithm (FBP) for tomographic reconstruction from two-dimensional (2D) projection data(Feldkamp et al.,1984;Kak and Slaney,1987),based on its selfcarried datos|x reconstruction software (phoenix,GE Measurement &Control Solutions,Germany).Before the image reconstruction step,correction of beam-hardening(suppression of the cupping effect)should be made by preprocessing the projection images (Pease et al.,2012).The band-pass filter,acting in the Fourier domain,is applied on the images of slices (Elaqra et al.,2007).Then,a total of 720 transmission images reconstructed in 2048 × 2048 pixels of 1536 slices (containing the greyscale information of each phase of the specimen) are imported into the commercial software Avizo©Fire 8.0(Visualisation Science Group,2013).The 3D structure of specimen is reconstructed by stacking the slices.Then,representative segment is selected to investigate and eliminate the influence of external shadows in images.According to suitable greyscale threshold values,different phase segmentation is performed and digital RVE is reconstructed.Next,Avizo is used to extract the relevant information of RVE,such as porosity,water content,and pore and bubble distributions.The main procedure of CT image reconstruction and RVE analysis is shown in Fig.11.

Fig.10.Variation of saturation of the gassy specimen under the unloading steps.

Fig.11.The procedure of CT image reconstruction and volume analysis.
As the contrast medium (KI) is added into the pore water of specimen before the scanning test,the phases of gas,water,and sand grain can be quantitatively distinguished in process of the phase segmentation.The greyscale histogram and reconstructed images of the RVE of specimen are shown in Fig.12.From Fig.12a,the peaks of three phases (gas bubble,water,and sand grain) are clear,equal to 94,118 and 172,respectively.Thus,the water phase is easily separated from the gas and sand grain,according to the greyscale threshold values(109 and 139).A watershed algorithm is used in Avizo to perform different phase segmentation (Vincent and Soille,1991).
Since the shape of gas bubbles is very irregular,the equivalent sphere algorithm (Hall et al.,2010) is used to statistically analyse the bubbles in the RVE.The extracted gravimetric water content(by the number of pixels)varies from 37.6%to 39.8%alongside the RVE height direction.The mean value is a bit larger than the water content(37.4%)measured by oven-drying(S0=92.1%).The possible reason for such deviation probably results from the effect of adding KI and inevitable CT reconstruction errors(Li and Tang,2019).Using the shortest square Euclidean distance (SED) method to simulate fluid intrusion (Shih and Wu,2004),Avizo can perform the digital mercury injection test to obtain the pore size distribution characteristics of RVE.The capillary force corresponding to mercury saturation is calculated as (Ku et al.,1968):

where pcis the capillary force (mN),γ is the surface tension of mercury (mN/m),θ is the contact angle between mercury and air(°),and r is the pore throat radius (μm).Through simulating the mercury injection test,the mercury injection curve and pore diameter distribution curve can be obtained.
Fig.13 shows the extracted characteristics of pores and gas bubbles in the RVE.From Fig.13b,it can be found that bubbles are uniformly distributed throughout the RVE,which demonstrates the prepared gassy sand specimen with a good homogeneity.As shown in Fig.13c,the sizes of pores and bubbles approximately obey the lognormal distribution,which is consistent with the study of gas bubble transport in granular materials(Geistlinger et al.,2014).The peak equivalent diameter of pore sizes is about 275 μm;and the maximum pore size is close to 500 μm,less than the maximum grain size.The peak bubble sizes are mainly distributed in 20-30 μm,which are far smaller than pore size of grain skeleton.This result reveals that the occurrence state of free gas in the gassy sand specimen is insular bubbles entrained in the pore water.
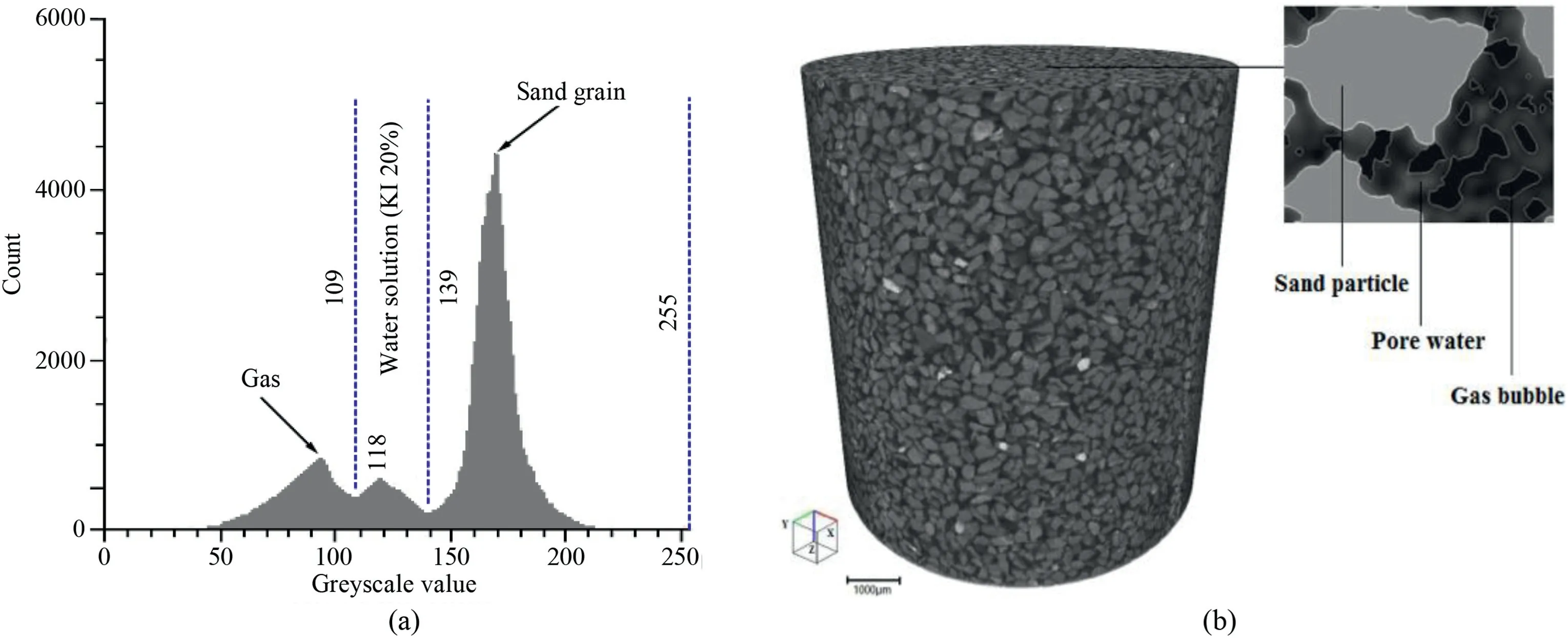
Fig.12.Greyscale histogram and reconstructed image of a RVE of the gassy sand specimen:(a) Greyscale histogram of the specimen mixed with 20% KI solution;and (b) 3D reconstructed image of a RVE and the three-phase partitioning.

Fig.13.The size distribution of pores and gas bubbles of the RVE:(a) Pores,(b) bubbles,and (c) the equivalent diameter distribution.
Existing studies show that bubble nucleation results from dissolution of gas from a supersaturated liquid (Hemmingsen,1977;Lubetkin,2003).Gas molecules occupy cavities between water molecules until fluid reaches the supersaturation threshold that prompts bubble nucleation (Ronen et al.,1989).The tiny bubbles or“embryos”that form at bubble nucleation sites are unstable until a critical size is reached (Finkelstein and Tamir,1985).The critical radius Rcriis defined as

where Tsis the surface tension(0.072 N/m for water at 20°C),Ssupis the supersaturation,and u is the pressure at which bubbles nucleate.Bubbles with size smaller than Rcritend to re-dissolve into pore water.While stable bubbles(R>Rcri)can migrate or coalesce to form larger bubbles that can eventually become trapped at pore throats.Based on Henry’s law,Ssupcan be defined as

where qgenis the gas concentration in fluid,and qeqis the gas concentration soluble in liquid under experimental conditions.Lubetkin (2003) provided the measured values of supersaturation needed to cause bubble nucleation for different gases,including CO2(Ssup≈4.62-20).Given the pore pressure of the prepared gassy specimen,the estimated minimum diameter of stable bubble nuclei is about 1.25 μm (from Eq.(6)).
Although the μCT scanner failed to identify the fine bubbles,whose size is smaller than a few microns,due to the limited resolution,it is capable of examining the occurrence state of gas in the prepared specimen.From Fig.13,we can see that the average bubble size is at least one-order smaller than the minimum pore size.It reveals that shallow gas occurs as the type of insular bubbles entrained in pore water of the highly saturated marine gas-charged coarse-grained sediments.
5.2.Undrained mechanical behaviour
To further validate the effectiveness of the developed MITA and the specimen preparation method,basic mechanical tests are carried out.It is known that tidal drawdown,excavation or erosion can produce undrained unloading,resulting in detrimental effects of gas-charged sediments in the marine environment.Therefore,firstly,isotropic undrained unloading tests on saturated and unsaturated specimens with the same initial dry density(ρd=1.283 g/cm3,and σ′=200 kPa) are conducted by the MITA system.Fig.14 presents the pore pressure response of sand specimens under different undrained unloading paths.For the saturated sand,unloading the radial pressure results in synchronous reduction of pore pressure,keeping the Skempton’s parameter B constant(Fig.14a).However,for the gassy sand,the pore pressure response is different from that of the saturated sand (Fig.14b).At the beginning of unloading,the behaviour of pore pressure(450 kPa)is similar to that of the normal saturated soil,and it decreases commensurately with the reduction of radial stress.However,when the radial pressure decreases from 600 kPa to 550 kPa,the pore pressure shows a rebound phenomenon (from 350 kPa to 379 kPa) due to a large amount of gas exsolution.The same behaviour can be observed in subsequent unloading steps.The mean effective stress (σ′=200 kPa) of gassy sand decreases with time,while the saturated sand remains almost constant throughout the test.When the radial pressure further decreases (e.g.to 300 kPa),the pore pressure(e.g.296 kPa)of the gassy sand almost equals the total stress,resulting in the failure of liquefaction.That is one of the key features of gassy sand,which differs from the general saturated sand.Moreover,it is an important reason why the marine gas-bearing sediments could collapse during tidal drawdown,excavations or erosion.Meanwhile,the test phenomenon also indicates a limitation of the proposed specimen preparation method,which is not suitable for preparing gassy specimens with gas content less than 85%.In this case,more unloading steps should be used during the preparation,which could cause liquefaction effect and significant skeleton disturbance of the specimens.

Fig.14.The pore pressure response of sand specimens under isotropic undrained unloading path:(a) Saturated and (b) gassy sands.
5.3.Temperature effects
To investigate the influences of ambient temperature and free gas content on mechanical behaviour of marine gassy sandy sediments,drained shear tests on sand(e0=1.067)with different initial saturation degrees (S0=100% and 92.9%) and different temperatures(i.e.5°C and 20°C) are carried out under the same effective confining pressure (σ′=200 kPa).As shown in Fig.15,both saturated and gassy sands show a slight strain-softening behaviour after peak strengths.Moreover,with the increases of the ambient temperature and initial gas content,there is an ascending trend in the drained shear strength of gassy sand.Under the same temperature,the strength of gassy sand is slightly larger than that of the saturated sand.During the process of shearing,free gas bubbles in pore water of gassy sand are prompted to migrate around,and they are trapped at pore throats due to the capillary resistance.The accumulated bubbles could coalesce and form larger bubbles around the contact locations in particles.Suction generated by the meniscus in gas-liquid-solid interfaces provides additional shear resistance for gassy sand,resulting in its relatively higher shear strength.Meanwhile,it can also be found that the behaviour of gassy sand is sensitive to the ambient temperature.Marine shallow gas-bearing sediments can occur at various water depths,where the ambient temperature is different.With the increase of temperature,the solubility of gas in water decreases and the amount of free gas bubbles goes up.Furthermore,pore water becomes less viscous and soil permeability increases,resulting in relatively larger pore ratio reduction under the same effective stress.Therefore,the strength of gassy sand slightly increases with the growing ambient temperature.Test results are consistent with the known laws of related previous studies (Morin and Silva,1984;Burghignoli et al.,2000).

Fig.15.The stress-strain curves of gassy sand specimens at different temperatures.
In summary,the preliminary tests on gassy sand demonstrate that the developed MITA and specimen preparation method are feasible and controllable,which can meet the requirements for experimental study of marine gas-bearing sediments.
6.Discussion
In this work,an improved preparation method to reconstitute the specimen of marine shallow gas-bearing sediments is proposed based on the customised MITA.By improving the inner cell and connecting it to an external water bath device,the temperaturecontrolled function is accomplished for the triaxial system;and a circulation system of gas-dissolved water enhanced by the temperature-controlled device is also developed into the MITA.These improvements enable the MITA to provide efficient and accurate temperature-controlled conditions in the preparation of gassy specimen.It is well known that the sediments at the sea floor are in different temperature and pressure environments at different depths.The occurrence state of free gas in sediments is very sensitive to the in situ temperature and pressure conditions,which determine the size of gas bubbles.Consequently,acoustic and mechanical properties of gas-bearing sediments are significantly affected by temperature and pressure.Secondly,the amount of shallow gas dissolved in water is determined by the temperature and pressure conditions.Only in a constant temperature and limited pressure (≤2 MPa) condition,the assumption that Henry’s coefficient H can be considered as a constant is reasonable,because the Poynting correction term of Krichevsky-Kasarnovskey equation is very small (Anderson,2002).If Henry’s constant H' varies with the temperature,there is no reliable and effective way to determine state parameters for gassy specimens during specimen preparation.Therefore,the previous specimen preparation methods carried out at room temperature should be improved to mimic the actual in situ temperature and pressure environments of marine gas-bearing sediments.
Previous methods almost cannot monitor the real-time status of gassy specimens during preparation.To overcome this issue,the BES is introduced into the MITA.P-wave excited by the BES is utilised to identify the exsolution point of a large number of gas bubbles in the specimen,and S-wave is used to examine the disturbance of soil skeleton due to the gas exsolution or expansion.Thus,the effective feedback information can be obtained by the wave-monitoring system to control the gassy specimen preparation.Once the wave velocity is abnormal,the preparation procedure can be modified or terminated in a timely manner.
Additionally,it is known that the free gas in marine gas-charged sediments has different forms according to the soil types of sediments.The form of free gas occurring in sediments determines their mechanical properties.Most of previous studies on gassy specimen preparation were mainly focused on how to entrain gas into the soil matrix,but few of them examined the RVE quality of preparation.Undoubtedly,there is no unified method developed to mimic all forms of gas occurrence in marine gas-charged sediments at present.Examining the RVE becomes a promising way to evaluate the effectiveness and reliability of a specimen preparation method developed.As the μCT scanning technique is a powerful nondestructive testing method,it is very suitable to quantitatively examine the RVE similarity between the gas occurrence state of gassy specimen and that of in situ gas-charged sediments.With the μCT scanning tests,the proposed specimen preparation method in this study succeeds in proving the postulated occurrence state of gas bubbles in marine coarse-grained sediments.
Although methane (CH4) is the predominant gas found in marine seabed sediments,it is flammable and explosive in nature,and requires a very high pressure to dissolve in water.In the laboratory,CO2is selected as the dissolved gas in the circulation system because of the safety consideration.Compared to methane gas,CO2is readily available,noncorrosive and nonflammable.To use CO2instead of CH4should lead to relatively conservative results from the viewpoint of engineering safety,without influencing the recognition of fundamental laws in marine gas-charged sediments(Grozic et al.,2005).
7.Conclusions
Shallow gas is encountered frequently in ocean engineering,where gas-charged sediments have been considered as a problematic soil.Gassy specimen is the premise to experimentally study the geomechanical properties of marine shallow gas-bearing sediments in the laboratory.However,it is extremely hard to collect in situ undisturbed gassy specimens due to sensitive gas exsolution and subsequent escape from sediments.The previous studies mainly focused on in situ acoustic characteristics of marine gasbearing sediments,but few investigated their mechanical properties.One important reason is that a quantitatively controllable and repeatable specimen preparation method for marine gas-charged sediments is rather difficult in the laboratory.
In this work,an MITA system is developed for the reconstituted specimen preparation of marine coarse-grained gassy sediments.In this method,water with dissolved gas is used to displace pore water of saturated specimens,and the controllable gas bubble exsolution is achieved through the unloading process.During the preparation,constant temperature conditions are easily obtained by the temperature-controlled system.Meanwhile,S-wave generated by the BES is used to examine the disturbance of gas exsolution to soil skeletons,and real-time volume change is utilised to quantitatively determine instant gas content in the specimen.
Moreover,the high-resolution μCT scanning test is adopted to assess the quality of prepared gassy specimen and examine the occurrence state of free gas in sand sediments.Preliminary tests on the mechanical behaviour of gassy sand validates that the developed specimen preparation method based on MITA is a feasible,effective and controllable way for investigations of marine gasbearing sediments in the laboratory.
Declaration of competing interest
The authors wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos.51979269,51579237 and 51779017).
List of symbols
GsSpecific gravity
e0Initial void ratio
emaxMaximum void ratio
eminMinimum void ratio
CuCoefficient of uniformity
CcCoefficient of curvature
n Porosity
ρdDry density
σ′Effective confining stress
S Saturation degree
SsupSupersaturation
H′Henry’s volumetric coefficient of solubility
pcCapillary pressure
TsSurface tension
u Pressure at bubbles nucleation
V Total volume of specimen
P Gauge pressure
ΔP Change of pressure
RcriCritical radius of a bubble
R Radius of a stable bubble
 Journal of Rock Mechanics and Geotechnical Engineering2021年3期
Journal of Rock Mechanics and Geotechnical Engineering2021年3期
- Journal of Rock Mechanics and Geotechnical Engineering的其它文章
- Effects of oil contamination and bioremediation on geotechnical properties of highly plastic clayey soil
- Bearing capacity of surface circular footings on granular material under low gravity fields
- Effect of natural and synthetic fibers reinforcement on California bearing ratio and tensile strength of clay
- Engineering and microstructure properties of contaminated marine sediments solidified by high content of incinerated sewage sludge ash
- Modification of nanoparticles for the strength enhancing of cementstabilized dredged sludge
- Rock-like behavior of biocemented sand treated under non-sterile environment and various treatment conditions
