Chemically induced changes in the geotechnical response of cementing paste backfill in shaking table test
Imad Alainachi,Mamadou Fall
Department of Civil Engineering,University of Ottawa,Ottawa,Ontario,K1N 6N5,Canada
Keywords:Cemented paste backfill (CPB)Liquefaction Shaking table Tailings Mine Sulphate
ABSTRACT Cemented paste backfill (CPB) is extensively used for underground mine support and/or tailings management.However,CPB behavior under cyclic loadings might be affected by the chemistry of its porewater,which often contains sulphate ions.Till today,no studies have addressed the effect of sulphate on the response of CPB to cyclic loadings by using shaking table technique.This study presents new findings of assessing the effect of the sulphate in the pore water of CPB on its geotechnical response to cyclic loading by using shaking table.CPB mixtures were prepared (with and without sulphate),poured into a flexible laminar shear box,cured to 4 h,and then exposed to cyclic loading using one-dimensional(1D) shaking table.Several parameters (e.g.pore water pressure,settlement,lateral deformation,acceleration,electrical conductivity,effective stress,and liquefaction susceptibility) were monitored or determined before,during,and after shaking.Obtained results indicate that the sulphate-bearing CPB cured to 4 h can be prone to liquefaction under the studied conditions.However,sulphate-free CPB samples are resistant to liquefaction.These results are expected to contribute to a better understanding of the effect of water chemistry on the cyclic behavior of CPB,consequently enhancing the cost-effective design of CPB structures.
1.Introduction
Mining is one of the industries that have been significantly influencing the world economy.For instance,in 2017,mining activities produced total revenue of around 20 billion USD in Canada,and around 600 billion USD around the world (Statista,2019).However,these activities may involve several environmental,economic,and safety challenges,such as the production of huge quantities of mining solid wastes like tailings,that become a potential source of environmentally harmful substances or processes(e.g.acid mine drainage,heavy metals) if they are improperly deposed.Also,mining extraction creates large underground openings (stopes) that expose the surrounding areas to several geotechnical hazards,such as ground subsidence.Moreover,the safety of people in the mine workplace and the surrounding area can be endangered owing to the instability of these underground openings beside the economic damages to the failed mine ground(Ghirian and Fall,2015;Aldhafeeri et al.,2016).
One of the solutions to deal with these challenges was developed by reusing these mine wastes(tailings)and mixing them with certain percentage of water and binder materials(e.g.cement).This mixture is called cemented paste backfill(CPB).This novel method of waste management allows large amount of mining wastes(tailings)to be returned to the mine stope.It can also improve the short-and long-term stability of the stopes besides ensuring the safety of the workers in the mine and the neighboring areas(Landriault et al.,1997;Abdul-Hussain and Fall 2012;Thompson et al.,2012;El Mkadmi et al.,2014).CPB is considered as a finegrained (silt) soil undergoing cementation from a geotechnical point of view.
Typically,CPB mixture consists of 70%-85% of tailings,fresh or mine processed water,and often 3%-7%(by total weight of solid)of hydraulic binder(usually cement).These components are generally mixed and prepared in paste backfill plant usually located on the mine surface.Afterwards,the prepared paste is delivered into the mine stope by gravity and/or pumping (Ercikdi et al.,2009a;Aldhafeeri and Fall,2016;Jiang et al.,2016;Li and Fall,2016;Walske et al.,2016;Xue et al.,2020).CPB is extensively applied in mine backfilling as it can be produced and delivered to the mine stope in a short period,leading to complete the stope backfilling process in a matter of days,whereas it might take weeks or months with older backfilling methods.This allows additional revenue to be generated(Ercikdi et al.,2009b;Thompson et al.,2009;Yilmaz et al.,2009).
There are several occasions at which the underground mines can be exposed to cyclic or seismic loadings.The origins of these cyclic loadings are either natural earthquakes or mining-induced seismic events.Mining-induced seismic events include many sources:fault slip (earthquake),rockburst (violent ejection of rock into mine opening),pillar burst,bump (sudden slip of a quasiviscous seam,such as coal at great depth or a tremor of unknown origin),and outburst (sudden violent ejection of coal into mine opening).They also include pillar punching,disturbance of geological structures due to active longwall mining,failure of overburden strata,and events of coal bursts(Hasegawa et al.,1989;Ahn et al.,2017).The high rate of volume extraction and the depth of mining increase the frequency and severity of mine-induced seismic events (Hasegawa et al.,2009).Due to the low availability of ores in shallow depths and the increase in the rates of the greater volume extraction,it is noted that underground mining operations around the world have significantly increased with greater volume and at greater depths.Thus,more severe and/or frequent seismic events are expected,and the risk of seismic-induced liquefaction of CPB structures at the early ages could increase.Liquefactioninduced failure of CPB structures can cause injuries and/or fatalities in the mine workers and the surrounding public besides having negative environmental impacts and economic damages(Poulos et al.,1985;Fall and Samb 2008;Abdelaal,2011;Becker et al.,2014).Therefore,understanding liquefaction potential of CPB under cyclic loading conditions at the early ages is critically important for a cost-effective and safe design of CPB structures.
Nevertheless,only a limited number of experimental studies(e.g.Saebimoghaddam,2010;Abdelaal,2011)have been conducted to assess the behavior and liquefaction potential of early-aged CPBs under cyclic loading conditions.Moreover,all these previous studies examined the cyclic behavior of CPB by applying cyclic loading on small-size CPB samples using common small cyclic triaxial test apparatus (Saebimoghaddam,2010;Abdelaal,2011).None of these previous studies have evaluated the effect of sulphate(present in the pore water of CPB)on the response of CPB to cyclic loading.
Previous studies have concluded that they are several sources of sulphate in CPB systems.These sources include(i)the oxidation of sulphide minerals (such as pyrite) that are commonly present in hard rock tailing materials;(ii) the mine processing water used as the mixing water in CPB preparation;(iii) sulphur dioxide and air method used in the elimination of cyanide in gold mines;and (iv)chemical additives (such as gypsum and anhydrite) that are often added to the clinker to control the cement setting (Ercikdi et al.,2009a,2017;Pokharel and Fall,2013;Aldhafeeri,2018).Several previous studies (e.g.Fall and Pokharel,2010;Li and Fall,2016)were undertaking on the sulphate-induced changes in the mechanical or geotechnical behavior of CPB under static loading conditions.These studies have revealed that the sulphate ions in the pore water of CPB can significantly deteriorate its static mechanical properties (strength),and thus jeopardize its mechanical stability.However,the fundamental question whether the sulphate ions in the pore water of CPB can deteriorate its liquefaction resistance when subjected to cyclic loadings still remains unanswered.There is a need to address this knowledge gap,since CPB often contains sulphate ions,which are commonly originated from the mine processed water (used as mixing water) and/or the tailings as noted above.
In addition to the facts mentioned above,no study has been conducted to assess the behavior and liquefaction potential of sulphate-bearing CPB by using shaking table testing techniques.Shaking table has become a valuable tool to assess and comprehend the cyclic response of geomaterials and engineering structures since the mid of the past century despite its high cost and the difficulty in reproducing or simulating the in situ stress (Moncarz and Krawinkler,1981;Bairro and Vaz,2000;Ngadimon,2006).Nowadays,shaking tables are being used in numerous researches.For instant,numerous studies (e.g.Bairro and Vaz,2000;Prasad et al.,2004;Dungca et al.,2006;Ueng et al.,2006;Chen et al.,2013,2015;Mohamed,2014;Komak Panah et al.,2015) have evaluated the behavior and liquefaction potential of natural soils subjected to cyclic loadings,where only few researches(e.g.Poulos et al.,1985;James et al.,2003;Pépin et al.,2009,2012a,b;Özgen et al.,2011) have used shaking table to assess the cyclic behavior of tailings (without cement).Nevertheless,there are no studies to date that have been conducted to assess the effect of sulphate on behavior and liquefaction potential of early-aged tailings undergoing cementation under cyclic loadings by using shaking table.
Accordingly,this study aims at using shaking table technique to understand the effect of the chemistry of the pore water(sulphate content)of hydrating CPB material on its geotechnical behavior and response,particularly liquefaction potential,at the early ages during cyclic loadings.
2.Materials and equipment used in the experiments
2.1.Materials
Silica tailings (ST),which is synthetic tailings material (manufactured by the U.S.Silica Co.) made of ground silica,was used in this study as the key component of CPB mixtures.The grain size distribution of ST is similar to the average grain size distribution of nine mine tailings (9MT) extracted from nine different mines in eastern Canada(Fig.1).The minerals of the ST are essentially made of quartz,which is the predominant mineral in Canadian hard rock mine tailings.ST was selected in this study thanks to the high percentage of silica (99.8% SiO2) within ST,which makes ST a chemically inert material and reduces the uncertainties related to the use of natural tailings to a minimum level by minimizing/controlling the potential chemical interactions of the tailings with other ingredients in the CPB mixture(Carraro et al.,2009;Fall et al.,2010;Aldhafeeri and Fall,2016;Jiang et al.,2016).Physical properties of the tailings used in this study are illustrated in Fig.1 and Table 1.

Fig.1.Grain size distribution of the silica tailing(ST)vs.average grain size distribution of tailings extracted from nine Canadian mines (9MT).
To improve the mechanical properties of CPB,sufficient amounts of binder,such as Portland cement(PC),are usually added to a mixture of tailings and water.In this study,the binder agent used in the preparation of CPB mixtures is Portland cement type I(PCI) (Table 2),which is the most frequent binder used in the preparation of CPB worldwide.Tap water was used as mixing water.
2.2.Preparation of paste backfill mixture
In this study,two CPB specimens were prepared.In both specimens,ST was mixed with PCI (4.5 wt%) and water,with watercement ratio (w/c) of 7.6.CPB specimens were mixed for 10 min in order to obtain homogenous mixture.The degree of water saturation (S) of the prepared CPB was determined to be equal to 100%.Also,the slump of the prepared backfill mixture was tested according to ASTM C143/C143M-15a(2015),and it was found to be equal to 18 cm,which is one of the most frequent slump values being used in paste backfill operations in Canadian mines.In order to determine the effect of the chemistry of mixing water,sulphate salt (FeSO4·7H2O) was added to one of the CPB specimens.The initial content of the chemical additive was determined to be 5000 ppm.The other CPB specimen was prepared without adding sulphate.
Afterwards,CPB mixtures were poured into the developed laminar shear box (described below).To avoid changes in water content due to evaporation,laminar shear box with CPB mixtures was sealed and kept in a room with stable temperature(~20°C)for curing until reaching the testing age of 4 h.It should be underlined that,after its placement,the CPB specimen is expected to undergo some self-weight consolidation.This is because the self-weight of the CPB material can induce consolidation of the pore voids generated due to self-desiccation,as observed in previous studies(e.g.Ghirian and Fall,2014).Detailed testing program is described in Section 3.
2.3.Shaking table
Dynamic loading was simulated in this study by applying series of one-dimensional(1D)(longitudinal)sinusoidal cyclic motion on the CPB samples.In this regard,the shaking table of the University of Ottawa was used (Fig.2).
The platform of this shaking table is around 1200 mm(length)×1060(width)mm with a maximum base shear capacity and displacement limit of 27 kN and 120 mm,respectively.The shaking ranges from 1 Hz to 17 Hz and is driven by a digitally controlled hydraulic actuator (Mohamed,2014).
In order to evaluate the seismic behavior of CPB using the shaking table,a flexible laminar shear box(FLSB)(Fig.3)was used.The FLSB was designed and built at the Faculty of Engineering of the University of Ottawa for this research program.
The FLSB consists of 30 horizontal laminas,made of aluminum alloy box sections of 3.17 cm × 3.17 cm,with inner dimensions of each lamina of 75 cm×75 cm.In order to ensure the independent movement of each lamina,the FLSB was designed to have aclearance spacing of 0.2 cm between each lamina (2 mm gap between every 2 adjacent laminas).Accordingly,the total capacity of assembled FLSB is 75 cm (length) × 75 cm (width) × 1000 cm(height).

Table 1Primary physical properties of the tailings.
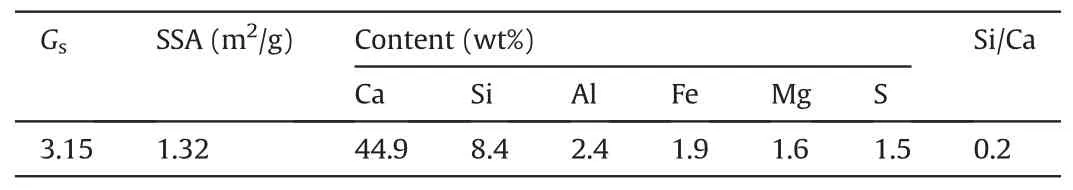
Table 2Primary physical and chemical properties of the Portland cement type I (PCI).
To contain/hold the CPB mixture,a flexible polyethylene membrane with a maximum thickness of 0.5 mm was placed inside the FLSB.Owing to its high flexibility,the membrane shows no (or negligible) effect on the movement of the FLSB (Mohamed,2014).The FLSB and the membrane were securely attached to the platform of the shaking table.The prepared CPB mixture was poured into the FLSB,with a final dimension of each tested CPB sample of 75 cm(length) × 75 cm (width) × 70 mm (height).
Numerous instruments or sensors were placed at different depths within the CPB model (FLSB and its content) as shown in Figs.4 and 5 and are described below:
(1) Pressure transducers(Item 1 in Fig.5)were used to monitor the change in pore-water pressure(PWP)before,during,and after applying the dynamic load.These transducers were placed at different depths (20 cm,40 cm and 60 cm) of the CPB samples inside the FLSB.The pressure transducers of PX309 series(-15 to+15 PSI(pound per square inch)range and ±0.25% static accuracy) were used in this regard.The dimension of each transducer was 7.6 cm (length) × 2.2 cm(diameter).
(2) Accelerometers (Item 2 in Fig.5) were used to measure the shaking acceleration.Endevco 7593A accelerometer with a full-scale range of ±2g and frequency response of 0-50 Hz were used and attached to the external side of the FLSB,and screwed with the lamina that is related to each testing depth of 20 cm,40 cm and 60 cm.In addition,another accelerometer was attached (screwed) to the shaking table to monitor its acceleration.The dimension of each accelerometer was 1.5 cm(length)×1.5 cm(width)×1.1 cm(height).
(3) Cable transducers (Celesco SP2-12 compact string with 317 mm range,and essentially infinite resolution (Item 3 in Fig.5)) were used to measure:
(a) The horizontal displacement of the CPB sample at different depths (20 cm,40 cm and 60 cm).These transducers were attached to the outside of the FLSB at the depth of related laminas.
(b) The vertical displacement of the CPB sample at different depths of 20 cm,40 cm and 60 cm.These transducers were attached to thin metal rods connected to 20 mm thick,20 mm × 20 mm,lightweight,perforated plastic plates that were installed at the three different depths within the sample.These plates were perforated to minimize its displacement due to seepage pressure.These metal rods were installed within cylindrical guidance towers (Fig.6) (made of thin metal mesh sheets) in order to avoid unwanted movement/tilting of these metal tubes during shaking process.Guidance towers were connected to the shaking table in order to avoid being a reinforcement factor and to allow the whole system to follow the same motion rhythm.
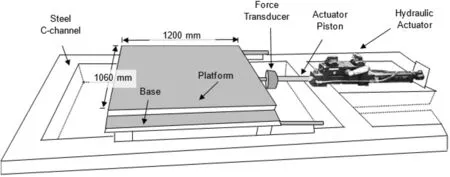
Fig.2.Shaking table used in this study.

Fig.3.Flexible laminar shear box (FLSB) used in this study.
(4) Linear variable differential transformer (LVDT) (Item 4 in Fig.5) was used to monitor the vertical displacement of the surface of the CPB sample.In this regard,HCD-1000 LVDTs of 2.5 cm range were used.
(5) Suction sensors (Item 5 in Fig.5) were used to monitor the suction development with time during CPB curing duration.Dielectric water potential sensors(ECH2-MPS6 sensors)that were designed to measure soil water potential were installed at different depths (20 cm,40 cm and 60 cm) within the sample.Measurement range of these sensors is -9 kPa to-100 MPa with a resolution of 0.1 kPa and its accuracy is±10% of reading +2 kPa,from -9 kPa to -100 kPa.The monitoring of suction also enables to assess the selfdesiccation of CPB.
(6) VWC/EC/Temperature sensors(Item 6 in Fig.5)were used to monitor the changes in electrical conductivity (EC),the volumetric water content (VWC),and temperature.The ECH2-5TE sensor was used.This sensor measures EC in the range of 0-23 dS/m with the accuracy of ±0.1 dS/m.It measures VWC in the range of 0-1 m3/m3with the typical accuracy of ±0.03 m3/m3,whereas the temperature measurement accuracy is ±1°C.These sensors were installed at different depths(0.2 m,0.4 m and 0.6 m)within the sample.Changes in EC reflect the rate of ion movement due to the chemical reactions between cement and water.Monitoring EC is an effective way to assess the cement hydration progress and the related structural changes(Li and Fall,2016).On the other hand,monitoring the VWC enables to assess the self-desiccation of CPB (capillary water consumed by the cement hydration) as well as the water flow within the CPB mass.The monitoring of the temperature provides valuable information about the progress of the cement hydration.

Fig.4.Schematic view of the FLSB and instrument locations.

Fig.5.Instrumented FLSB:(1) Pressure transducers,(2) Accelerometers,(3) Cable transducers,(4) LVDT,(5) MPS6 sensors,and (6) 5TE sensors.
The pressure transducers,multi-function sensors,5TE,and suction sensors,MPS-6,were connected to the guidance towers to avoid any changes in their positions.Transducers,accelerometers and LVDT were connected to signal conditioning and data acquisition systems(DAQS),while 5TE and MP6 sensors were connected to Decagon Em50 series data loggers.DAQS and Em50 were connected to computer in order to record/analyze the required data at almost 1 s interval during shaking and 10 min interval before and after shaking.Furthermore,digital camera was used to record each step of the testing program (mixing,installation,and shaking operation).
3.Testing program and procedure
The main goal of the testing program is to assess the influence of the chemistry of mixing water on the behavior of CPB material during shaking.It should be underscored that the objective is not to evaluate the cyclic behavior of CPB structure,but rather of the CPB material,with sulphate.Indeed,simulating the cyclic response of a CPB structure that can be up to 100-120 m high by using shaking table testing technique needs the use of an extremely large-scale shaking table testing facility to consider a reasonable height or the full size of the CPB structure and/or its complex in situ stress,which is an extremely or unrealistic costly approach,or to properly scale down the parameters of the model experiment based on the similitude laws.However,it is not possible to ensure that all or key factors meet the similitude laws in the 1g gravity field,if a full field scale prototype model is considered,due to complex field loadings conditions to which a CPB structure may be subjected.Though the CPB material with sulphate was assessed in this research,gaining insight into the response of sulphate-bearing CPB material to cyclic loading will enable to gain an understanding of the effect of sulphate on the cyclic behavior of CPB structure as well as develop future constitutive samples to describe the seismic behavior of hydrating CPB.Experimental testing program carried out in this study has been summarized in Fig.7 and Table 3 and is discussed below.
3.1.Shaking table tests
3.1.1.Cyclic parameters and test conditions
To better simulate a cyclic event,related cyclic parameters or test conditions have to be determined before conducting a shaking table test.These parameters include horizontal displacement amplitude(HDA),sinusoidal loading frequency(SLF),shaking peak horizontal acceleration (SPHA),and shaking duration (SD).Fig.7 shows the experimental program carried out and the testing conditions,whereas Table 3 summarizes the testing program.
Despite the fact that they might not all reflect the real situation of a seismic event,the values of the testing parameters were modified to accommodate some experimental limitations.These limitations include the maximum capacity of the shaking table and the sensitivity/limitation of the used monitoring instruments.Hitherto,this modification process is often used in liquefaction research(Ishihara,1996).Thus,shaking table tests were conducted in this study using a dynamic load that was applied in 1D signal,a uniform amplitude,and constant frequency.
The shaking table tests were performed in this study by using a sinusoidal loading frequency of 1 Hz.The selected applied frequency herein was based on previous liquefaction studies (e.g.James et al.,2003;Ueng et al.,2010;Pépin et al.,2012a,b;Geremew and Yanful,2013) and compatible with the capability of the used sensors in terms of the monitoring.Moreover,past studies have concluded(e.g.Sriskandakumar,2004;Srilatha et al.,2013)that the magnitude of loading frequency applied in laboratory seismic tests under undrained conditions has a negligible influence on the response of the tested material.The shaking table tests were conducted in the current study under undrained conditions.
The peak ground acceleration in this study was selected to be 0.13g.This acceleration is equal to the acceleration that was recorded in the Saguenay earthquake 1988 in Quebec (Canada)(Tuttle et al.,1990) (it should be stressed that only the peak acceleration corresponds to the peak of Saguenay Earthquake,not the whole time series).Despite the fact that the strongest mining induced ground motion in north eastern Ontario (Canada) has occurred in 2006 with a ground acceleration of 0.0027g (Atkinson et al.,2008;Natural Resources Canada,2019),the acceleration of 0.13g was selected based on the fact that tailings may liquefy when they are exposed to a ground motion with peak horizontal ground acceleration that exceeds 0.05g (Carter,1988;James et al.,2003).
As shown in Table 4,the cyclic loading(shaking)in this study is carried out for 30 min(1800 s).Although the recorded earthquakes or mine-induced seismic events do not last that much long(Natural Resources Canada,2019),the duration of cyclic loading in this study is not meant to be representative of the actual duration of earthquake.The duration of 30 min was selected to allow good observation of the dynamic behavior of the CPB sample and relative comparisons of their response.This will help better develop future constitutive models to describe the dynamic response of soils undergoing cementation and subjected to chemical attacks.Furthermore,similar duration has been used in several previous researches(e.g.James et al.,2003;Pépin et al.,2012b),and it was found that the cyclic peak of the liquefaction of tailings (without inclusions and/or cement) can be reached in shaking for 1000 s (James et al.,2003).As the material used in this study is cementing tailings(CPB mix),shaking duration was selected to range from 1 min to 30 min(60-1800 cycles)depending on material response,and the post loading monitoring duration (PLMD) to continue (depending on material response) for additional 24 h.
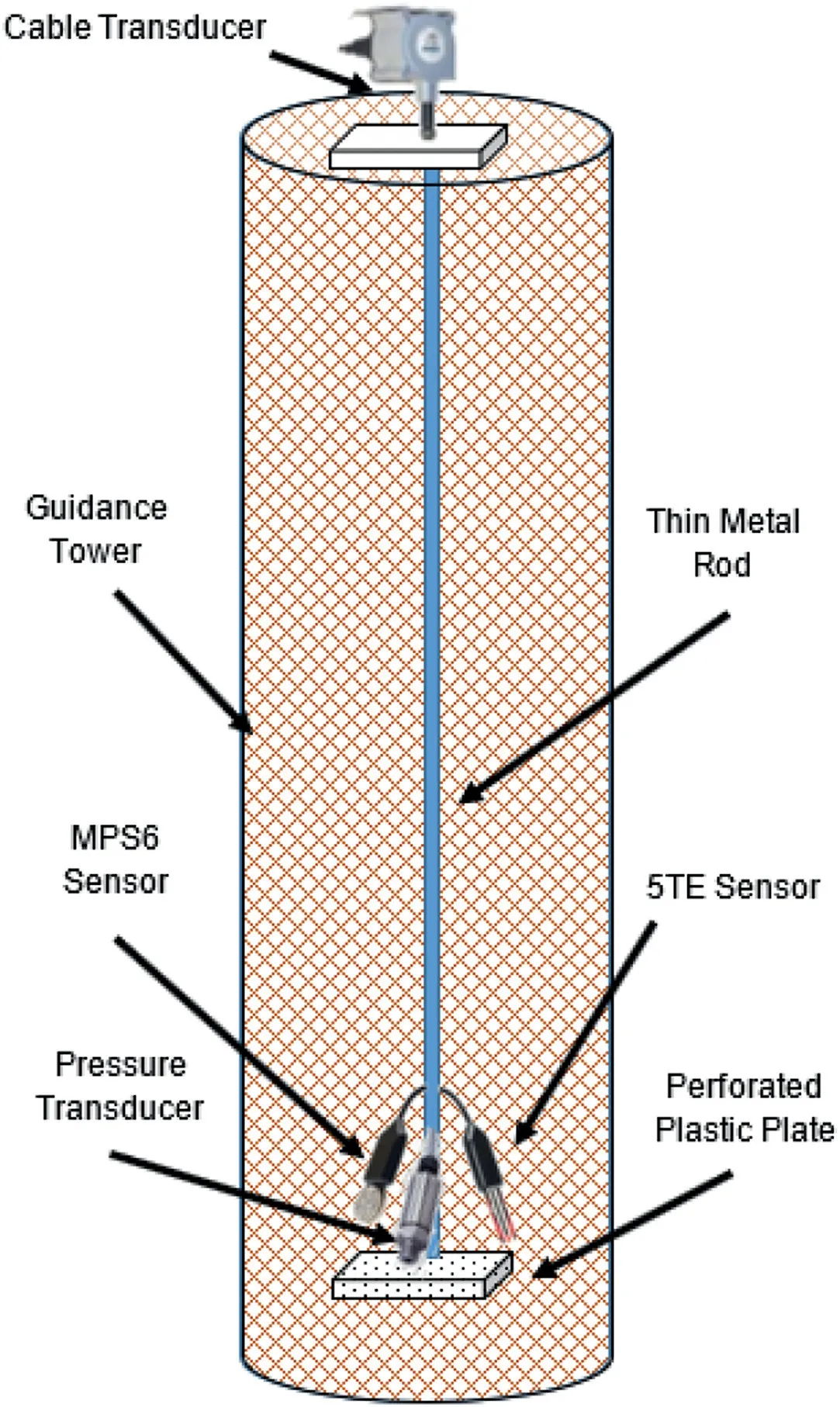
Fig.6.Schematic view of the guidance towers and related instrumentation setup.Figure is not to scale.

Fig.7.Flowchart of the experimental program and testing conditions.
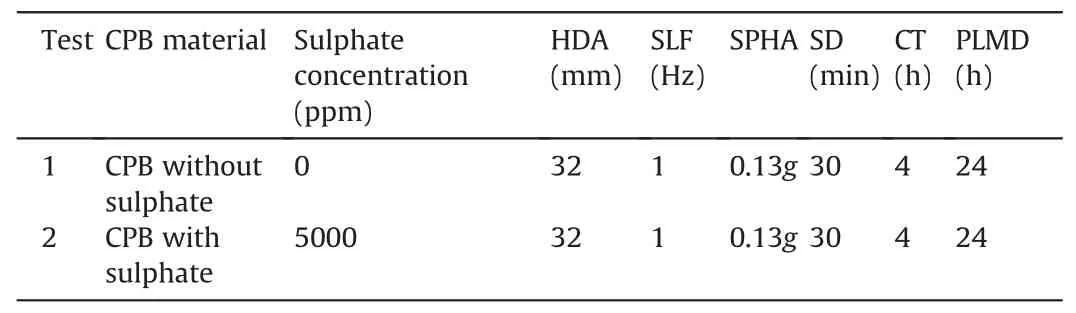
Table 3Summary of the testing program.
3.1.2.Effect of the chemistry of mixing water on the cyclic behavior of CPB
To evaluate the cyclic behavior of fresh CPB material under cyclic conditions besides assessing the effect of the presence of sulphateon this behavior,it was important to apply cyclic loadings(dynamic load) on CPB samples with different sulphate contents.Thus,two CPB mixtures were prepared;one mixture did not have sulphate salt added to the mixing water,and the other mixture was prepared with mixing water that contained 5000 ppm of sulphate.Both samples were then poured into FLSB,securely sealed and kept for curing (for the maturity age of 4 h) under stable temperature of 25°C.Afterwards,series of shaking table tests were performed on both CPB samples (see Fig.7).
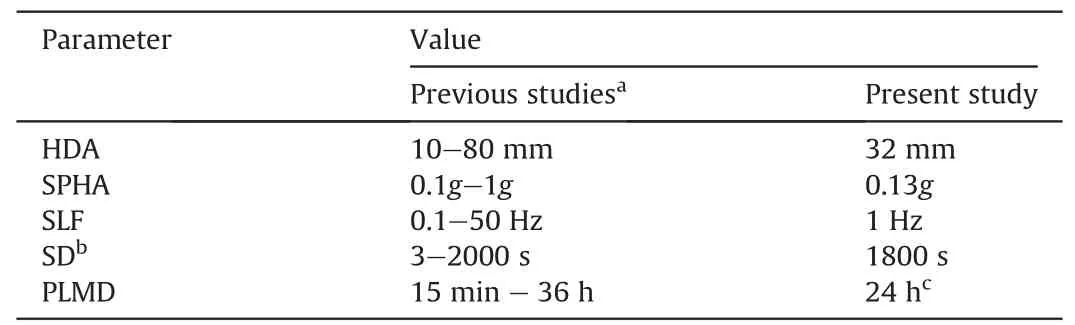
Table 4Selected cyclic parameters used in the present study and previous studies.
3.2.Microstructural analysis
Microstructural analysis was conducted on CPB samples that were prepared with and without sulphate to understand the effect of sulphate on its microstructural evolution.In this study,microstructural analysis included thermal analysis (differential thermogravimetry (DTG) and thermal gravimetry (TG)).The TGA Q 5000 IR from TA Instruments were used in this regard.Before conducting these tests,testing samples after curing of 4 h were first dried at 45°C in a vacuum oven up to mass stabilization.The various(dried)samples(about 20 mg each)were heated in an inert nitrogen atmosphere at the rate of 10°C per minute up to a temperature of 1000°C.
4.Results and discussion
4.1.Acceleration and lateral deformation
In this section,the results of acceleration and horizontal(lateral)displacement at different depths within each tested CPB model are presented with respect to initial content of sulphate(which affects the degree of cementation).The combined results of these two parameters can provide an indication about the shear resistance of the tested material at different depths(Takahashi et al.,2001;Nouri et al.,2006,2008).
4.1.1.Acceleration
Figs.8a,b and 9a,b show the peak acceleration histories at different depths during the shaking event for both CPB samples(prepared without and with sulphate) and cured for 4 h.These figures indicate that the presence of sulphate and the depth have impact on the measured acceleration values.It can be observed that CPB samples prepared without sulphate show higher peak acceleration values than CPB samples prepared with sulphate,which is more obvious at shallow depths.The recorded acceleration significantly varied with depth for the samples prepared with sulphate,while the variation of acceleration with depth was low for samples that do not contain sulphate.For instance,the peak acceleration of CPB sample prepared without sulphate (Fig.8a) was between 0.115g and 0.125g at 60 cm depth(10 cm above the shaking table),around 0.1g-0.11g at 40 cm depth(30 cm above the shaking table),and 0.09g-0.1g at 20 cm depth(50 cm above the shaking table)(no phase shift was observed in the comparison of the cycles).On the other hand,for the CPB that contains sulphate,the peak acceleration(Fig.8b)at 60 cm,40 cm and 20 cm depths was recorded to be around 0.1g-0.11g,0.08g-0.1g,and 0.06g-0.08g,respectively.Moreover,as it can be seen in Fig.9a and b,the shaking acceleration is significantly reduced within the sample with sulphate (Fig.9a)compared with the sulfate free sample (Fig.9b).

Fig.8.Measured peak acceleration histories at different depths vs.time for 4 h-CPB samples prepared (a) without and (b) with sulphate.
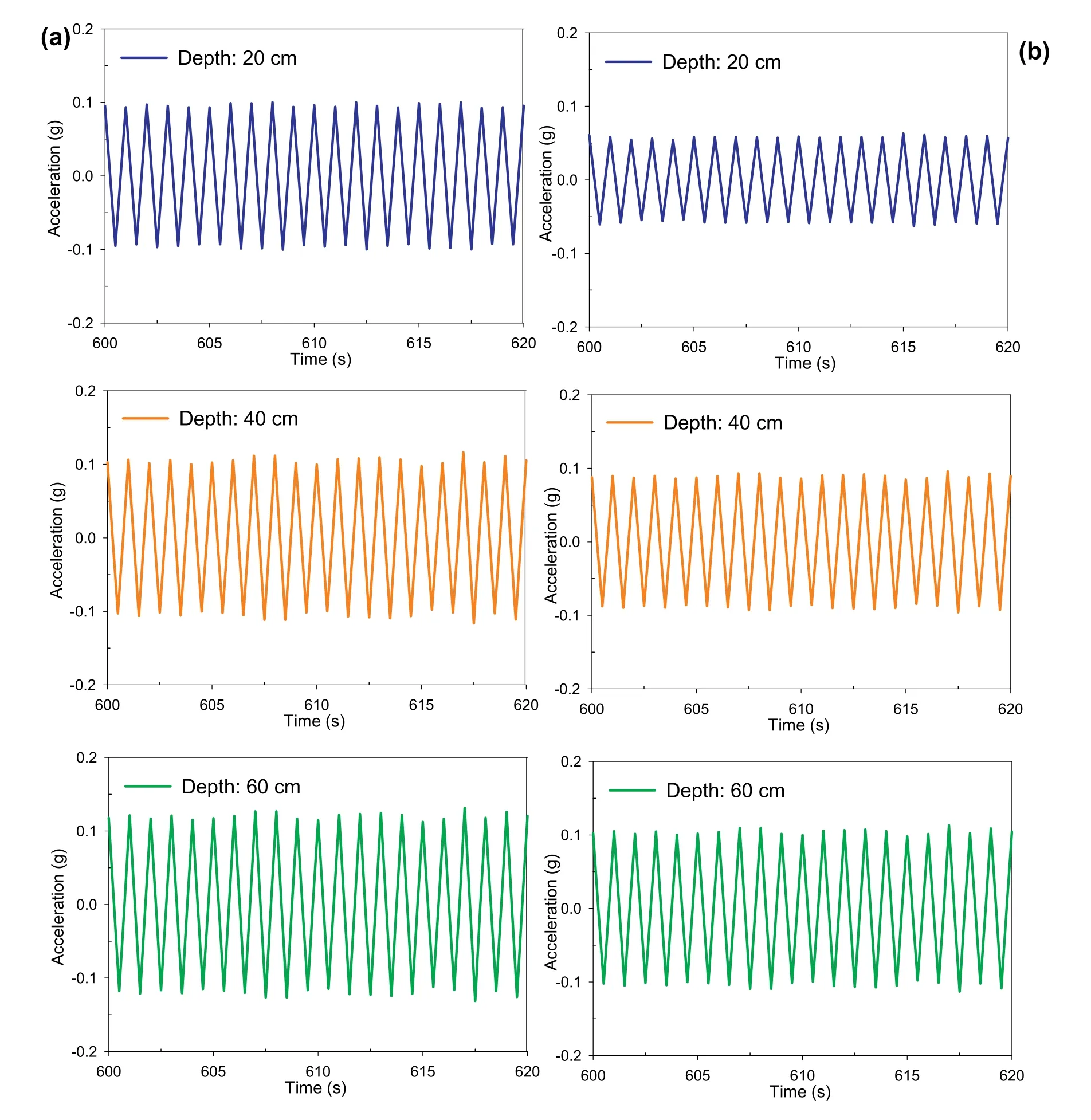
Fig.9.Variation in acceleration at different depths after 600 s of shaking 4 h-CPB samples prepared (a) without and (b) with sulphate.
This decrease in the peak acceleration as the sulphate content within CPB increases can be attributed to the effect of sulphate on the degree of cementation and consequently on the damping ratio.It was documented that the damping ratio increases when the degree of cementation is lower(Acar and El-Tahir,1986;Yang and Woods,2015).Furthermore,previous studies (e.g.Fall and Pokharel,2010;Li and Fall,2018) concluded that sulphate ions inhibit the cement hydration.This inhibition is attributed to the production of ettringite as a result of the reaction of sulphate ions in the pore-water with the C3A grains of the cement.The ettringite creates a thin coating of anhydrated cement grains,which will slow down the reaction between pore-water and C3A(Li and Fall,2016).Consequently,there will be a reduction in the degree of cementation within CPB that contains sulphate as compared to the sulphate-free CPB that is cured to the same age.It means that the CPBs that contain sulphate will have higher damping ratio,since the presence of sulphate is associated with lower degree of cementation (cement hydration degree).The progress of cement hydration increases the bonds between tailings(soil)particles and consequently reduces the deformation level of the material(Mamlouk and Zaniewski,2011);the less the cement hydration degree,the less the bonds between tailings particles.The observed significant reduction of acceleration with depth in the CPB prepared with sulphate can be attributed to nonlinearity,shear resistance and stiffness degradation of CPB caused by liquefaction as evidenced by the liquefaction analysis presented in Section 4.3.Liquefaction causes a large deformation in the CPB that contains sulphate,in which the cementation effect is still weak due to slow progress of cement hydration.This large deformation will cause more damage in the pore structure within CPB material.
4.1.2.Lateral (horizontal) displacement
The deformation of liquefiable soils due to shaking-induced liquefaction can be inferred from lateral (horizontal) displacement (Takahashi et al.,2001;Dungca et al.,2006).Indeed,ground shaking will result in a decrease in soil resistance against vibration because of the reduction in effective stress(Takahashi et al.,2001;Dungca et al.,2006).
Fig.10a and b illustrates the lateral displacement (measured at one side of the laminar shear box) at various depths during the cyclic loading durations for 4 h aged CPB prepared without and with sulphate,respectively.
From these figures,two key behaviors can be observed:
(1) In both CPBs,the lateral displacement decreased with the increase in depth.This behavior is consistent with the findings of several previous studies on liquefaction-induced lateral ground displacements in soils (e.g.Motamed et al.,2013;Srilatha et al.,2013) and is related to the increase in material density during shaking as a result of the dynamic densification(compaction) of the tailings particles(Zhu and Clark,1994;Anastasopoulos et al.,2010).However,the change in displacement with depth within the CPB that contains sulphate(Fig.10b)was higher than the variation in displacement with depth within the sulphate-free CPB(Fig.10a).This increase can be attributed to the effect of sulphate on slowing down the progress of cement hydration,and consequently reducing the CPB solidification.Slower cement hydration means a precipitation of a less amount of cement hydration products(e.g.the calcium silicate hydrate(C-S-H),calcium hydroxide (CH)),which plays the main role in increasing the cementation degree or cohesion of the CPB material (Fall et al.,2010;Xue et al.,2020).This argument regarding the decrease in the amount of cement hydration products with sulphate presence is experimentally supported by the results of TG/DTG on cement pastes of CPB,which will be discussed later.
(2) The magnitude of lateral displacement within sulphate-free CPB was lower in value as compared to the CPB that contains sulphate.This increase in the lateral displacement accompanied by the presence of sulphate is the consequence of the decrease in bonds between the tailings particles with the inhibition of cement hydration progress because of the sulphate ions (Mamlouk and Zaniewski,2011;Li and Fall,2018).
Comparative analysis of the results presented in Figs.8 and 10 suggests that the bonds between particles of 4 h-CPB that contains sulphate were low to resist the seismic induced shear stress,while those between the particles of the 4 h-CPB without sulphate were stronger and thus had higher shear resistance,which minimizes the acceleration and lateral displacement change.These facts strengthen the assumption that the presence of sulphate within CPB mixture might increase the susceptibility of seismic loadinginduced liquefaction,while the CPB material without sulphate is more resistant to liquefaction during ground movement.
4.2.Evolution of the pore water pressure,effective stress and settlement
4.2.1.Pore water pressure
Fig.11a and b illustrates the changes in PWP at various depths with time within the sulphate-free CPB (Fig.11a) and CPB that contains sulphate (Fig.11b),respectively,before,during,and after shaking (i.e.from the time of casting in the FLSB to about 24 h).
(1) Before shaking
During the first 1 h(3600 s),after depositing the fresh CPB in the FLSB,PWP rapidly increased in all depths of both 4 h-CPBs.The reason behind this increase is the reduction in the voids volume due to the self-weight settlement,which might reach more than 2%in 4 h after the end of filling process,and the rearrangement of the tailings particles at the very early age (Belem et al.,2016;Yilmaz,2018).Afterwards,PWP decreased in both samples as a result of the water consumption during cement hydration (Helinski et al.,2007;Ghirian and Fall,2014;Scrivener et al.,2015).However,the decrease in PWP within the CPB sample that contains sulphate was lower than the decrease in PWP within the sulphate-free CPB.For instant,after 4 h of curing,there was approximately 27%-45% of reduction in PWP within the sulphate-free CPB sample,while the reduction in PWP within the CPB sample that contains sulphate ions was about 10.7%-28.5%.This observation is consistent with the results of suction monitoring presented in Fig.12a and b (discussed later).In addition,the PWP near the surface of the CPB might also be subjected to water evaporation.
(2) During shaking
When shaking began,as expected,there was a rapid buildup of PWP at all depths within both 4 h-CPB materials until reaching peak values.Also,as expected,the greater depths showed greater buildup in PWP.This increase in PWP can be attributed to the contractive behavior of the saturated backfill material or particles causing rapid generation of excess pore water (Bouckovalas et al.,2009;Saebimoghaddam,2010;Jefferies and Been,2015;Porcino et al.,2015) (Fig.15).However,the increase in PWP within the CPB sample that contains sulphate was much higher than the increase in PWP within the CPB that does not contain sulphate.This can be attributed to the coupled effect of:(i)the positive impact of curing time on cement hydration,at which the cement hydration consumes more water (self-desiccation),and more cement hydration products will be precipitated as the curing time increases(This strengthens the cementing bonds between the tailings particles,thereby reducing the contraction of the CPB materials(Saebimoghaddam,2010;Ghirian and Fall,2013;Scrivener et al.,2015)),and (ii) the negative impact of sulphate ions on the cement hydration processes,which inhibits the progress of cement hydration and reduces the water consumption by the cement hydration(as mentioned above in Section 4.1.1)(Fall and Benzaazoua,2005;Fall and Pokharel,2010;Li and Fall,2016).
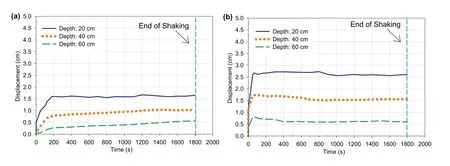
Fig.10.Lateral displacement histories at different depths vs.time for 4 h-CPBs prepared (a) without and (b) with sulphate.
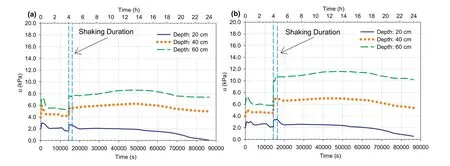
Fig.11.Pore water pressure (u) histories at different depths vs.time for 4 h-CPBs prepared (a) without and (b) with sulphate.
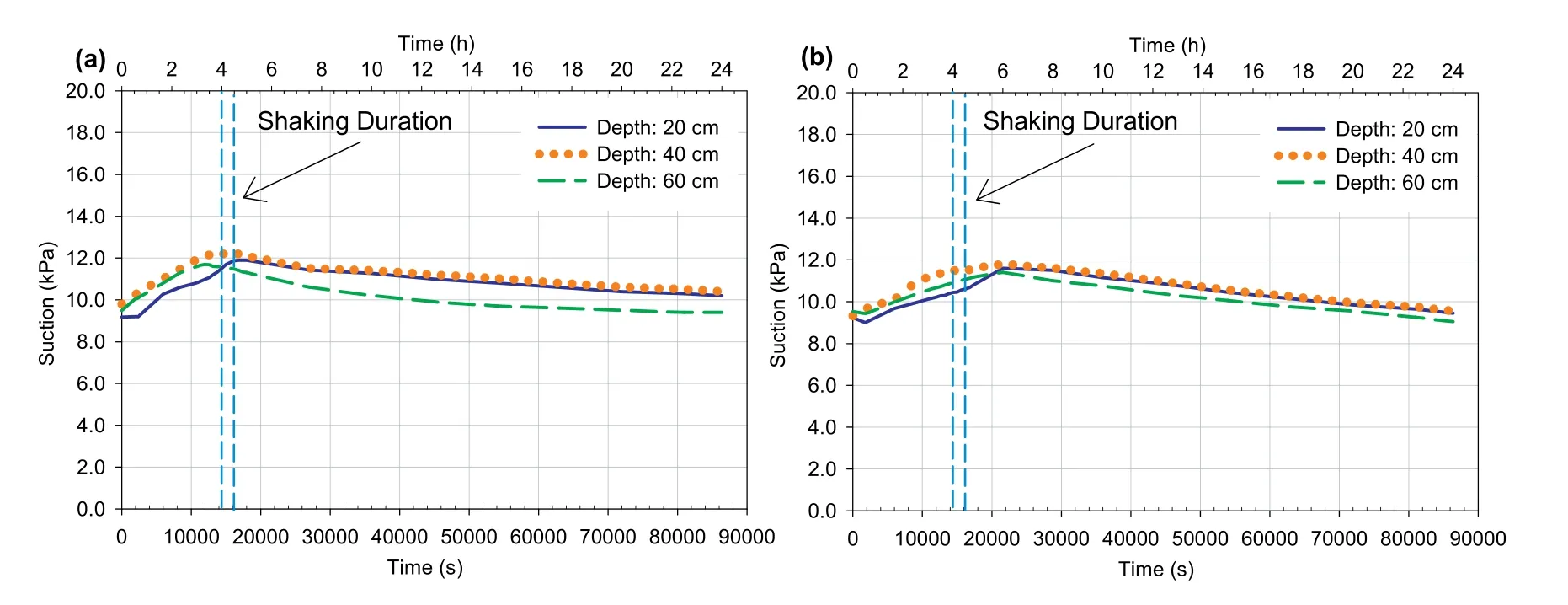
Fig.12.Suction evolution at different depths vs.time for 4 h-CPBs prepared (a) without and (b) with sulphate.
(3) After shaking
After the shaking,both 4 h-CPBs exhibited a slight increase in PWP with time at all depths(with different magnitudes)regardless of the progress of cement hydration.This observed slight increase in PWP at these depths may be caused by the settlement or contraction of some tailings particles,which might have been partly in suspension at the end of the cycling loading.This contraction generated additional PWP (Pépin et al.,2009).This slight increase in PWP at these depths was then followed by a gradually decrease in the PWP until the end of the monitoring period.The decrease in PWP observed at all depths is mainly due to the water consumption by cement hydration (i.e.self-desiccation)(Ghirian and Fall,2013).However,surface evaporation at shallow depths (close to CPB surface) of both samples caused additional dissipation of PWP to less than the hydrostatic PWP.This phenomena can be explained by the coupled effect of self-desiccation due to cement hydration and the near surface evaporation as a result of the difference of relative humidity (RH) between the ambient air and the surface of the CPB (Abdul-Hussain and Fall,2012;Ghirian and Fall,2013).To confirm this explanation,the evaporation-induced reduction in water content of a CPB material was experimentally determined in this study by exposing CPB samples(prepared with the same mix components and conditions as the CPB used in this study)to environmental conditions(RH and temperature) similar to those,in which the shaking tests were conducted.It was found that around 60% of the near surface reduction in CPB water content (water loss) was related to evaporation when the CPB sample is placed under the conditions of 20°C temperature and 24% of RH.Moreover,the contribution of the evaporation to the dissipation of the PWP decreases as the depth of the CPB increases.This is in agreement with the lower rate of dissipation of PWP observed at the depths of 40 cm and 60 cm compared to the depth of 20 cm.It should be also underlined,since the CPB is a low-permeability soil(k ≈10-7cm/s),the fluid(water)transport process is slow.Thus,long time will be required to withdraw water from deeper layers(i.e.40 cm and 60 cm)through evaporation.
4.2.2.Effective stress
Fig.13a and b presents the vertical effective stress evolution during shaking at different depths of 4 h-CPBs that were prepared with and without sulphate.During shaking,there was a reduction in these stresses from their initial values in both samples due to the contraction of CPB particles leading to development of excess pore pressure.However,the 4 h-CPB sample that contains sulphate experienced higher reduction in effective stress as compared to the sulphate-free 4 h-CPB sample due to the aforementioned effects of sulphate on cement hydration.
4.2.3.Vertical displacement (settlement)
The downward displacement(settlement)in soil due to ground shaking gives an indication of the contractive behavior of the particles of liquefiable soil and the increases in the soil density (Ueng et al.,2006;Pépin et al.,2012b).In this study,shaking-induced settlement was recorded in both 4 h-CPB samples,as shown in Fig.14a and b.However,this figure shows that the CPB that does not contain sulphate (Fig.14a) shows less significant settlement as compared to the CPB sample that contains sulphate(Fig.14b).This observation suggests that the presence of sulphate will reduce the degree of cement hydration,causing higher settlement or contraction (volume change) during shaking.This behavior,which is consistent with the results of PWP measurements and liquefaction analysis discussed in Sections 4.2.1 and 4.3,respectively,is due to the fact that less cement hydration degree (due to cement hydration inhibition by the sulphate ions)leads to the precipitation of less cement hydration products,thereby decreasing the strength of the CPB material (Fall et al.,2010;Scrivener et al.,2015).Consequently,the dynamic loading-induced contracting behavior or settlement of the CPB becomes higher (Saebimoghaddam,2010;Jafari,2020).From Fig.14,it can also be observed that the settlement of 4 h-CPB varied with the measurement depth.The settlement becomes higher in shallow depths.This could be explained by the fact that the CPB material may be slightly less dense at shallower depth due to tailings particles self-weight settlement.
4.3.Liquefaction analysis
Various liquefaction triggering criteria have been suggested and used to describe or determine soil liquefaction.These criteria comprise strength-based criteria,strain/deformation-based liquefaction criteria,energy-based liquefaction criteria,and (excess)pore ratio-based criteria.Each of these methods has advantages,but also limitations,as explained in various past studies (e.g.Wu et al.,2004).Moreover,the liquefaction assessment based on the evaluation of the stress-strain relationship,which can be interpreted from the accelerometer measurements,was conducted in previous shaking table tests (e.g.Turan et al.,2009).On the other hand,the excess PWP ratio criteria have been extensively used in assessing the liquefaction potential of soils or tailings,especially in shaking table tests (e.g.Adalier et al.,2003;Shahir and Pak,2010;Cetin et al.,2012;Pépin et al.,2012a;Wang et al.,2019;Bahadori et al.,2020).In this study,the excess PWP ratio was used as the evaluation factor of soil liquefaction susceptibility.The excess PWP ratio(Ru)represents the ratio between the excess PWP(Δu)and the initial effective stress(σ′0).Liquefaction is generally defined if Ru≥1.However,if Ru<1,there is no liquefaction (Wu et al.,2004).
Excess PWPs developed during shaking at different depths within 4 h-CPB samples prepared without and with sulphate are illustrated in Fig.15a and b,respectively.This figure depicts that the excess PWP developed within both samples varied with depth and shaking time.The PWP ratios determined during shaking of the sulphate-free 4 h-CPB sample and sulphate-rich 4 h-CPB have been depicted in Fig.16a and b,respectively.
It can be seen that 4 h-CPB sample that contains sulphate is susceptible to liquefaction (Ru≥1) when exposed to seismic loading,while the sulphate-free 4 h-CPB sample was resistant to shaking-induced liquefaction(Ru<1).In other words,the presence of sulphate in CPB mixing water increases the susceptibility of CPB liquefaction under seismic conditions.This reduction in liquefaction resistance of CPB prepared with sulphate can be attributed to the combined effects of the following two factors as discussed below:(i)the inhibition of cement hydration process because of the effect of sulphate leading to enfeeblement of self-desiccation of CPB;and (ii) production of less cement hydration products within the CPB pores due to the inhibition of the cement hydration,which diminishes the cementation or cohesion between the tailings particles,thus reducing the shear strength of the CPB material.These factors obviously result in the increase in the liquefaction susceptibility of the CPB.The aforementioned arguments are supported by the experimental evidences discussed below.
4.3.1.Cement hydration rate
The negative effect of sulphate ions on cement hydration rate is attributed to the reaction of the sulphate anions in the CPB poreliquid with the C3A grains of the cement to form ettringite.The ettringite forms a thin coating of anhydrated cement particles,thus preventing the quick C3A-water reaction (Li and Fall,2016).This will consequently reduce the intensity of the cement selfdesiccation within CPB particles,which plays a significant role in the strength of materials undergoing cementation (Persson,1997).
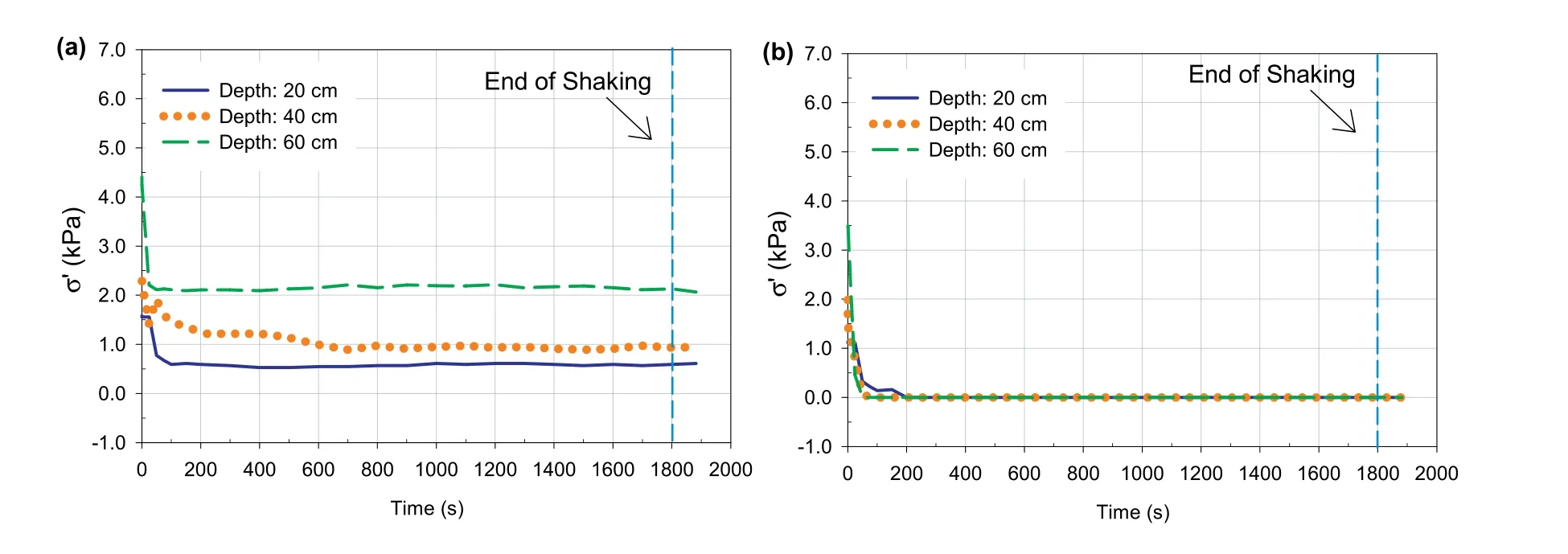
Fig.13.Effective stress at different depths vs.time for 4 h-CPBs prepared (a) without and (b) with sulphate.

Fig.14.Settlement at different depths vs.time for 4 h-CPBs prepared (a) without and (b) with sulphate.
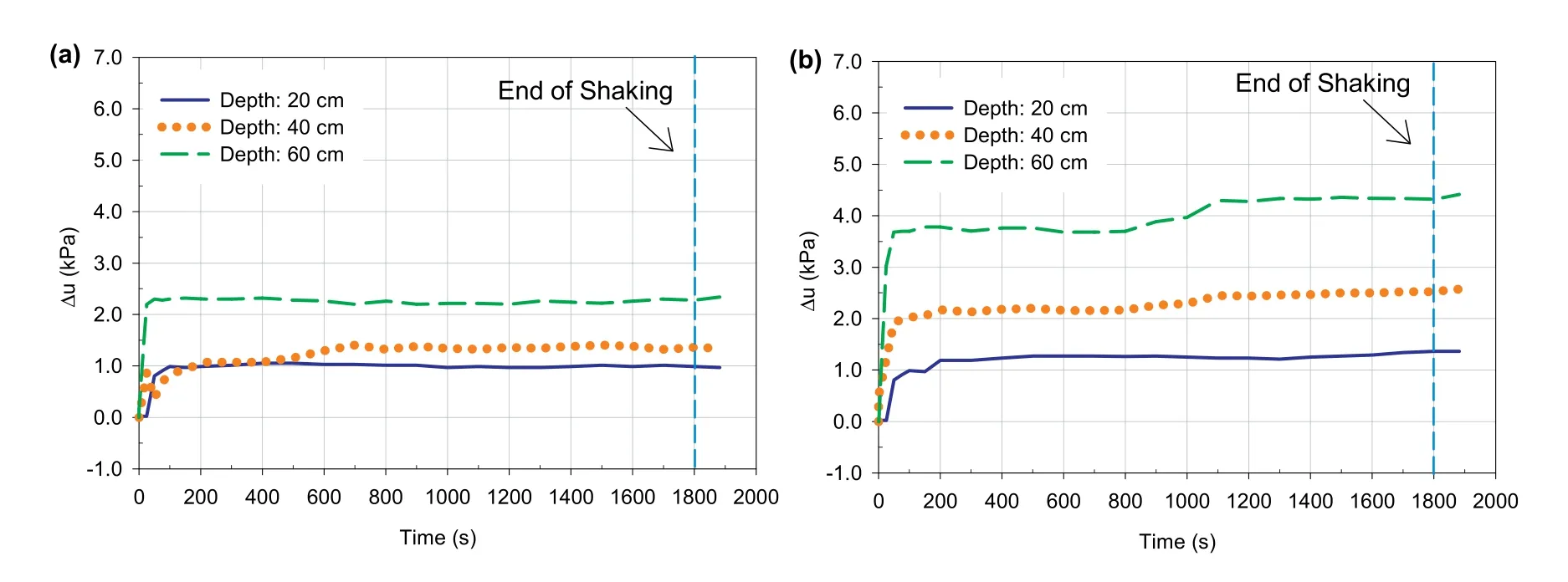
Fig.15.Excess pore water pressure (Δu) development at different depths vs.time for CPBs prepared (a) without and (b) with sulphate.

Fig.16.Pore-water pressure ratios determined at different depths vs.time for CPBs prepared (a) without and (b) with sulphate.
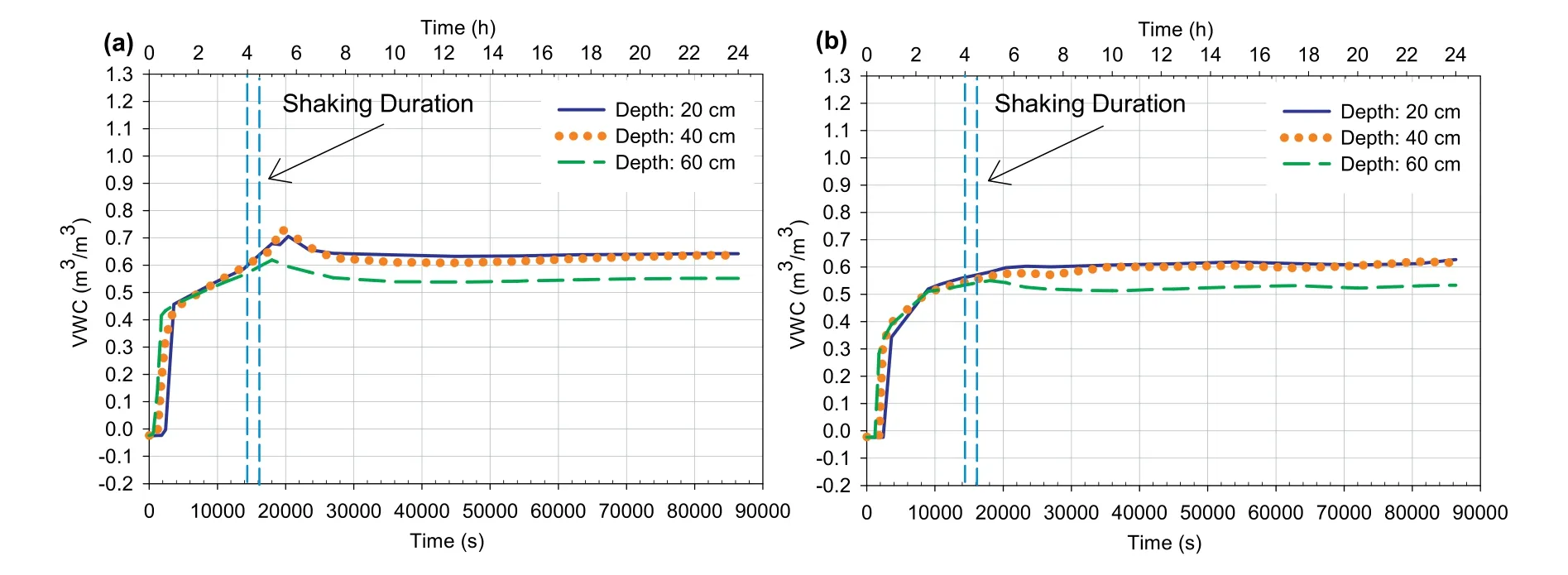
Fig.17.Change in volumetric water content at different depths vs.time for 4 h-CPBs prepared (a) without and (b) with sulphate.
Self-desiccation is the process of shrinkage in pores;consequently,the reduction in VWC of cemented-based materials,which are seal cured under saturated conditions (Bentz,2008).This will consequently decrease the PWP and/or generate negative PWP(suction)(Ghirian and Fall,2013).Hence,self-desiccation improves the effective stress and strength of these materials(Abdul-Hussain and Fall,2012;Fredlund et al.,2012;Ghirian and Fall,2014).Fig.17a,b and 12a,b,which show the evolution of the VWC and suction in the CPB at different depths,respectively,support the fact that selfdesiccation did take place in both 4 h-CPB samples.It was observed from Fig.17 that there was a gradual increase in the VWC in the first hours at all depths of both CPB materials,which reached its peak value after 6 h.However,it is evident that the sulphate-free CPB material experienced more increase in VWC as compared to CPB material that contains sulphate.For example,between 0.5 h (the end of filling process) and 6 h of curing (when VWC reached its peak),the VWC within the sulphate-free CPB sample increased from around 0.3 m3/m3to around 0.7 m3/m3,while the VWC within the sulphated CPB sample increased(in the same time period)from around 0.3 m3/m3to around 0.6 m3/m3.Accordingly,the less increase in VWC within the CPB material with sulphate indicates the effect of sulphate in inhibiting the self-desiccation process.Afterwards,the VWC slightly decreased and then remained relatively constant until the end of the monitoring period.This increase in VWC is attributed to the self-desiccation induced decrease in the total volume of the CPB since the VWC expresses the ratio of volume of water to the total volume of the soil.Also,the decrease in the amount of water or water content during this period was confirmed by the suction measurement results presented in Fig.12,at which a small suction has been recorded in both CPB samples shortly after its disposal into the laminar shear box.Afterwards,there was a gradual increase in suction until reaching its peak after 6 h.After that,the suction decreased slightly and then remained relatively constant until the end of the monitoring.The evolution of suction in both samples can be attributed to the chemical reactions of the cement hydration,which causes the consumption of water in the capillary pores of CPB(Wang et al.,2016)and reduces the water content in the hydrating backfill or any other cemented soils.This reduction in water content will allow air to enter the pores between tailings or soil particles,generating the air bubbles.As a result,the backfill or soil will turn from saturated condition to partially saturated conditions (Fredlund et al.,2012).The reduction in saturation leads to a decrease in the PWP,in other words to an increase in the effective stress,and thus to an increase in the liquefaction resistance of the CPB.Moreover,the air bubbles that enter inside the pores of a soil will reduce the pores volume,thus it will absorb the generated excess PWP and enhance liquefaction resistance (Okamura and Soga,2006).It was also noted that the suction values in the CPB material that contains sulphate were lower than those in CPB material that does not contain sulphate,which provides a strong indication of the negative effect of sulphate on the performance of cement hydration.In other words,this negative effect results in the higher water content and less air bubbles in the CPB that contains sulphate.Thus,the effective stress and effect of air bubbles in absorbing the excess PWP will be reduced in the CPB that contains sulphate,and liquefaction resistant will consequently decrease.It should be pointed out that,as mentioned in Section 2.3,the suction sensor(ECH2-MPS6)used has a measurement accuracy of±10%of reading+2 kPa,from 9 kPa to 100 kPa.Thus,considering the low values of suction obtained(Fig.12) and these suction sensor measurement limitations,the evolution of the suction presented in Fig.12 should be considered as qualitative.
4.3.2.Production of less cement hydration products
The production of less cement hydration products in the 4 h-CPB with sulphate as compared to the same sample without sulphate is experimentally supported by the results of the thermal analyses(TG/DTG),as shown in Fig.18.In this figure,a comparison of the TG/DTG diagrams between the CPB samples with and without sulphate is shown.The CPB with sulphate results in lower weight loss (TG)and the peaks (DTG) in the 400°C-500°C temperature,which means less amount of hydration products in CPB sample due to sulphate(Wang et al.,2016;Xu et al.,2020).The first peak(sudden change in weight) is due to the destruction of the C-S-H,ettringite,and gypsum (Taylor,1990;Fall et al.,2010),while the second peak is associated with the degradation of CH(Li and Fall,2016)and is much lower for the CPB sample that is prepared with sulphate compared to the sulphate free CPB sample.The third peak represents the decomposition of the calcite in the cement (Bian et al.,2019).
Moreover,Figs.19 and 20 present the results of the monitoring of the evolutions of EC and temperature with curing time progress at different depths of both CPBs,respectively.From these figures,it can be seen that the rate or progress of the cement hydration,in other words the amount of cement hydrations formed within the CPB,is relatively similar at all depths of each CPB sample.However,the presence of sulphate has affected these rates.

Fig.18.Effect of sulphate content on TG/DTG diagrams for 4 h-CPBs prepared without and with sulphate.

Fig.19.Electrical conductivity at different depths of the CPBs.

Fig.20.Evolution of hydration heat with curing time at different depths vs.time for 4 h-CPB samples prepared (a) without and (b) with sulphate.
For instance,the EC reaches the peak value in the CPB without sulphate (Fig.19a) faster than in the CPB with sulphate (Fig.19b).Moreover,it was noted that the EC peak value of CPB with sulphate is lower than the EC peak value of CPB without sulphate.According to the working principles of EC sensors,the initial increase in EC refers to the increase in the concentration of ions in the CPB pore water solution due to the dissolution of cement particles.Therefore,the delay in EC to reach the peak value with the presence of sulphate indicates the retardation of cement hydration process,and the lower EC values indicate a reduction in cement hydration intensity (Li and Fall,2016).
On the other hand,by comparing the generation of hydration heat of sulphate-free CPB sample (Fig.20a) and CPB sample that contains sulphate (Fig.20b),it can be seen that the presence of sulphate reduced the amount of generated hydration heat.It is agreed that the generated heat of hydration is a result of the exothermic reaction of aluminate (C3A),gypsum,and tricalcium silicate (C3S) with water to form ettringite and C-S-H (Bullard et al.,2011;Ghirian and Fall,2015).Thus,the reduction in the hydration heat generated within CPB samples that contain sulphate may confirm the above-stated assumption of the effect of sulphate on the production of less cement hydration products.
Thus,this production of less amount of hydration products due the sulphate ions,as demonstrated above,will reduce the cohesion between the tailings particles,thus decreasing the shear strength of the CPB material as well as increasing the CPB damping ratio.Indeed,the movement(due to shaking)of tailings particles,which did not react with cement (within the sulphate-bearing CPB),will cause high friction between particles and increase the material damping ratio.This assertion and the explanation are consistent with the above-discussed results of acceleration and displacement monitoring,such as the evident decrease in the acceleration values within sulphate-free sample.It should be underlined that the impact of the volume of cement hydration products generated or progress of cement hydration on the consolidation behavior of the CPB samples was not assessed in this study.Future study should address it.
5.Conclusions
This study has assessed and discussed the effect of the chemistry of CPB pore water (initial sulphate content) on the geotechnical response(e.g.deformation,excess of PWP,effective stress,and liquefaction potential)of paste backfill undergoing cementation to seismic loadings by using the shaking table testing technique.This assessment was conducted by applying seismic loading on paste backfills prepared with and without sulphate to the mixing water.Both samples were casted in an FLSB and securely cured to 4 h.The paste backfills were also instrumented with numerous sets of sensors and transducers to monitor the evolution of several parameters (PWP,suction,lateral and horizontal displacement,acceleration,temperature,and EC) before,during,and after shaking.The obtained results show that the presence of sulphate significantly affects the seismic response of the CPB in terms of acceleration,horizontal and vertical displacements,and excess PWP.Moreover,4 h old CPB material that contains sulphate can be susceptible to liquefaction,whereas the sulphate-free 4 h old CPB is resistant to liquefaction under the studied seismic conditions.This liquefaction susceptibility is due to the combined effect of two factors:(i) the sulphate induced decrease in cement hydration intensity that leads to a less self-desiccation within the CPB with sulphate.It results in lower effective stress in the sulphate-bearing CPB,and consequently increases the liquefaction susceptibility;and(ii) the sulphate-induced inhibition of cement hydration process that leads to the formation of less cement hydration products (CS-H and CH)within the CPB that contains sulphate.It reduces and weakens the cementation between the tailings particles,and thus increases the liquefaction susceptibility of CPB with sulphate.These 1g shaking table tests on CPB are time-consuming,however,they have allowed to acquire a better understanding of the effect of the pore water chemistry or chemistry of mixing water on the seismic behavior and liquefaction potential of CPB at early ages besides acquiring information and data that are useful for liquefaction assessment of CPB structures,and also for future development of constitutive samples to describe and predict the seismic behavior of hydrating paste backfill or soil undergoing cementation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank the National Natural Sciences and Engineering Research Council of Canada(NSERC)for financially supporting this project.Moreover,the authors would like to thank Dr.Mohammed Al-Umar,and Dr.Muslim Majeed for their help with the experimental tests.
 Journal of Rock Mechanics and Geotechnical Engineering2021年3期
Journal of Rock Mechanics and Geotechnical Engineering2021年3期
- Journal of Rock Mechanics and Geotechnical Engineering的其它文章
- Uncertainties of thermal boundaries and soil properties on permafrost table of frozen ground in Qinghai-Tibet Plateau
- Effect of natural and synthetic fibers reinforcement on California bearing ratio and tensile strength of clay
- Engineering and microstructure properties of contaminated marine sediments solidified by high content of incinerated sewage sludge ash
- Effects of oil contamination and bioremediation on geotechnical properties of highly plastic clayey soil
- Modification of nanoparticles for the strength enhancing of cementstabilized dredged sludge
- Rock-like behavior of biocemented sand treated under non-sterile environment and various treatment conditions
