Effect of fermented bee pollen on metabolic syndrome in high-fat diet-induced mice
Sh Yn, Ki Wng, Xioying Wng, Aiqun Ou, Feirn Wng, Liming Wu,b,*, Xiofeng Xue,*
a Institute of Apicultural Research, Chinese Academy of Agricultural Sciences, Beijing 100093, China
b Innovation Research Team of Risk Assessment for Bee Products Quality and Safety of the Ministry of Agriculture, Beijing 100093, China
c College of Food Science and Engineering, Shanxi Agricultural University, Taigu 030801, China
Keywords:
Bee pollen
Fermentation
Gut microbial community
Metabolic syndrome
ABSTRACT
Bee pollen has potential in preventing metabolic syndrome (MetS). The present study aimed to investigate the effect of yeast-fermented wall-broken bee pollen (YB) intervention on ICR mice with MetS induced with a high-fat (HF) diet. After YB intervention in mice for 16 weeks, the effect on alleviating MetS was evaluated based on MetS serum parameters, hepatic oxidant status markers and gut microbial populations. The results of animal experiment showed that YB intervention attenuated MetS. Based on multivariate statistical analysis results, YB treatment significantly increased glutathione S-transferase (GST) and catalase (CAT) activities and decreased the malondialdehyde (MDA) level in the liver. Further investigation showed that YB restored the Nrf-2-Keap-1 pathway to alleviate oxidative stress. Additionally, gut microbial community analysis revealed that YB restored the increase in the Firmicutes to Bacteroidetes (F/B) ratio (6.94 for the HF group and 3.74 for HF + YB group) and improved Lactobacillus and Lactococcus abundance induced by the HF diet. Overall, YB improved function and prevented MetS by modulating the gut microbiota and alleviating oxidative stress.
1. Introduction
Metabolic syndrome (MetS) is the constellation of obesity, blood pressure elevation, high serum triacylglycerol levels, hyperglycemia and low high-density lipoprotein cholesterol (HDL-C) levels.Generally, having 3 of the above 6 symptoms results in a diagnosis of MetS [1]. MetS ultimately leads to disorders in energy utilization and storage and is a factor for sudden death. Due to daily habits,low physical activity, and improper diet, the prevalence of MetS is growing at an alarming rate worldwide [2]. In particular, the high consumption of large amounts of saturated fat and carbohydrates promotes these metabolic changes. A long-term high-fat (HF) diet can destroy the balance between energy intake and expenditure, leading to the formation of free radicals [3]. Excessive increases in free radicals destroy redox homeostasis. Oxidative stress is easily induced when the body loses redox balance. High levels of reactive oxygen species impair mitochondrial function, and obesity and diabetes are associated with increased oxidative stress. Oxidative stress is closely related to the development of MetS [4].
Diet intervention is an effective strategy to control the development of MetS. Current evidence shows the protective effect of a Mediterranean diet on MetS, because this diet is rich in unsaturated fatty acids, phytochemicals, vitamins and antioxidants[5]. Due to a unique polyphenol profile and abundant dietary fiber,daily consumption of red raspberries can reduce the parameters of MetS in HF fed mice [4]. Ke fir, a typical fermented milk product, has potential to control MetS [6]. Bee pollen rich in active ingredients has been reported as a functional food for preventing MetS [7]. Bee pollen is an aggregate including flower pollen, nectar or honey and bee salivary substances [8]. The predominant constituents of bee pollen are proteins, carbohydrates (glucose, fructose and dietary fiber (DF)), lipids, and minor components including vitamins, amino acids, minerals and polyphenols [9]. Many studies have reported that the strong antioxidant activity of bee pollen depends mainly on bioactive components, such as vitamins, carotenoids and phenolic compounds [9]. Bee pollen also includes a wide range of prominent therapeutic properties, such as anti-inflammation, hepatoprotection,antiatherogenic, antimicrobial, antitumor activities and immunity enhancement [10]. For a long time, due to the lack of value-added products, bee pollen as a nutraceutical was directly consumed.However, due to an unpleasant smell, some people do not accept it.
Fermentation has been used to improve both organoleptic and nutritional properties of foods [11,12]. Our previous study indicated that yeast-fermented wall-breaking bee pollen (YB) not only significantly reduced sugar content but also increased some beneficial nutrients, such as oligopeptides and free phenolic and linolenic acids,which increased by 68.8%, 18.3% and 13.2%, respectively [8]. During the fermentation process, wall-breaking bee pollen (WB) is suitable for fermentation to transfer nutrients via microbes and enzymes [8].Previous results revealed that YB is characterized by less sugar,and more phenolic compounds, free amino acids, DF, vitamins, and monounsaturated fatty acids (MUFAs) compared to WB, meeting people’s expectations for healthy foods.
Bee pollen is effective in the prevention of MetS [7]. Whether the changes in bee pollen composition caused by different processes, such as yeast fermentation, would enhance the effect of bee pollen on MetS is unknown. In the present study, we provided bee pollen, WB and YB to mice fed an HF diet to evaluate whether an HF diet facilitates the appearance of MetS and to determine whether bee pollen, WB or YB intervention could prevent MetS and modulate the gut microbiota in HF diet-induced mice. The mice with MetS responding to bee pollen, WB or YB intervention were associated with alleviated oxidative stress, providing potential insights into the mechanisms of bee pollen products for attenuating MetS.
2. Materials and methods
2.1 Materials
Bee pollen (Brassica campestris L.) and WB samples were obtained from Sichuan Kuake Technology Development Co. Ltd. (Sichuan Province, China); the moisture content was below 7%. All the bee pollen and WB samples used in this study were commercially irradiated. All WB samples were examined by scanning electron microscopy (Hitachi,S-570, Tokyo, Japan) to ensure that pollen grains were broken into fragments and that the cytoplasm was released. Active dry yeast was purchased from Angel Yeast Co., Ltd. (Yichang, China).
2.2 Preparation of YB samples
According to our previous study [8], 500 g of WB was mixed well with 500 mL of distilled water, and the slurry was mixed with 3% (m/m)yeast and fermented for 48 h at 37 °C. After fermentation, the WB samples were treated to obtain a moisture content of approximately 7%by vacuum freeze drying (Boylkang, vacuum freeze dryer FD-1C-50,Shanghai, China) to produce YB. The YB pellet samples were stored at -20 °C for further analysis.
2.3 Analysis of the nutritional composition of different bee pollen samples
The proximate nutritional compositions of bee pollen, WB and YB, including the fat, protein, ash, DF and moisture levels, were analyzed according to the method described by Yang et al. [13]. The sterols of samples were extracted, derivatized and detected following the methods described by Xu et al. [14]. The contents of free amino acids, free fatty acids, fructose, glucose, phenolic compounds,thiamine, riboflavin, nicotinic acid and nicotinamide were obtained from our previous research [8].
2.4 Animals and experimental design
Thirty 6-week-old ICR mice (male) were obtained from the Laboratory Animal Research Center (Shanxi, China). The animal experiment was performed in the Animal Experimental Center of Shanxi Medical University (Taiyuan, China), following standard experimental protocols in strict accordance with the ARRIVE guidelines for reporting experiments involving animals. The experimental protocol was approved by the Animal Ethics Committee of the Institute of Apicultural Research, Chinese Academy of Agricultural Sciences (Beijing, China). Mice were kept 4 per cage under relative humidity of 35%-55% and constant temperature(22-26 °C) with a 12 h light/dark cycle. During acclimatization for one week, these mice were randomly divided into 5 different groups(n = 6): control diet (Ctrl), HF diet (HF), HF diet supplemented with bee pollen (HF + B), HF diet supplemented with WB (HF + WB) and HF diet supplemented with YB (HF + YB).
The Ctrl group was provided standard laboratory chow (Beijing Keao Xieli Feed Co., Ltd., China) containing 20% kcal protein, 70%kcal carbohydrate and 10% kcal fat. Other groups were provided a HF diet (Beijing Keao Xieli Feed Co., Ltd., China) containing 20%kcal protein, 35% kcal carbohydrate and 45% kcal fat. The HF + B,HF + WB and HF + YB groups received bee pollen, WB and YB solutions (0.1 g/mL, daily) by gavage at 1 g/kg, respectively. The Ctrl group and HF group received the same amount of water by gavage daily. During the experimental period, body weight was recorded once per week. After 16 weeks of gavage treatment, the mice were sacrificed. Blood was collected from the orbital vein and centrifuged at 3 000 r/min for 10 min at 4 °C to obtain serum. The internal organs,including the liver, kidney, heart, spleen and lung, were removed and weighed immediately. The organ index was calculated by the ratio of organ weight (mg) to body weight (g) [15]. Parts of liver tissues were weighed and placed in 4% formaldehyde for fixation. After 48 h, they were embedded in paraffin, then sectioned and stained.The pathological changes were observed and photographed with a microscope (Olympus Corporation, Japan). The internal organs and serum were stored at -80 °C for later analysis.
2.5 Fasting blood glucose, insulin and lipid parameter tests
Blood glucose was measured by collecting tail blood with a glucometer (Sinocare, Changsha, China). Serum insulin content was measured using insulin assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions. Total cholesterol (TC), triacylglycerol (TG), low-density lipoprotein cholesterol (LDL-C) and HDL-C contents in the serum were determined using assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following the corresponding instructions.The 96-well plates were read using an enzyme standard instrument(Molecular Devices, SpectraMax®i3, Shanghai, China).
2.6 Oxidant status parameters in the liver
Each liver sample (0.1 g) was homogenized in 900 μL of physiological saline buffer. The homogenate was centrifuged at 3 000 r/min for 10 min, and the supernatant was used for further analysis. Activities of superoxide dismutase (SOD), catalase (CAT),glutathioneS-transferase (GST), malondialdehyde (MDA) level and protein carbonyl content were measured using kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the corresponding instructions. The protein content in the liver was determined with a kit (Nanjing Jiancheng Bioengineering Institute,Nanjing, China) using the bicinchoninic acid (BCA) method.
2.7 Hepatic gene expression related to oxidative stress
For each mouse, RNA was extracted from a 50 mg piece of liver tissue. Total RNA was collected using a commercial kit(Carry Helix Biotechnologies Co., Ltd., Beijing, China). cDNA was reverse transcribed using a PrimerScript reverse transcriptionpolymerase chain reaction (RT-PCR) kit (TaKaRa, Dalian, China).The expression levels of specific genes were measured with SYBR premix EXTaq(PowerUpTMSYBRTMGreen Master Mix, Thermo Fisher Scientific, America) using a real-time qPCR instrument(QuantStudioTM1 real-time PCR, Thermo Fisher Scientific,America) following a standard two-step amplification procedure.The specific primer sequences used for oxidative stress genes are listed in Table 1.β-Actinwas used as the housekeeping gene, and the transcriptional levels of the target genes were calculated using the 2-ΔΔCtmethod (cycle threshold abbreviated as Ct) [15].

Table 1The specific primer sequences for oxidative stress genes.
2.8 Immunohistochemistry analysis
For the immunohistochemistry experiment, antibodies including oxidase quinone oxidoreductase 1 (NQO-1) (1:1 000) and hemeoxygenase-1 (HO-1) (1:1 000) were obtained from Proteintech Group (Germany). After adding primary antibodies, sections were incubated overnight at 4 °C, and then horseradish peroxidase labeling secondary antibodies were added. Diaminobenzidine was used as a chromogenic agent, and the stained tissues were imaged and scanned through the Pannoramic MIDI System (3D HISTECH, Hungary).Image viewing and analysis were performed by Pannoramic viewer(3D HISTECH, Hungary).
2.9 Gut microbial community analysis
Cecal digesta samples (100 mg) were collected from the cecal content of mice and stored at -80 °C for further analysis. A QIAamp DNA Stool Minikit (Qiagen, Hilden, Germany) was used to collect bacterial genomic DNA (gDNA). The purity of the gDNA was determined using a NanoDrop2000 spectrophotometer (Thermo Fisher Scientific, San Jose, CA, USA), based on a standardA260nm/A280nmratio between 1.8 and 2.0. The primers used for the 16S rRNA gene of the cecal microorganisms were 338F (ACTCCTACGGGAGGCAGCAG)and 806R (GGACTACHVGGGTWTCTAAT). The expression levels for specific genes were measured by PCR using TransGen AP221-02(TransStart FastPfuDNA Polymerase, 20 μL reaction system) in an ABI GeneAmp®9700 PCR system. PCR products were detected by 2% agarose gel electrophoresis. Samples with a bright main band of approximately 500 bp were chosen for further analysis. The purified PCR products were sequenced on an Illumina MiSeq platform.Through trimming, assembly (FLASH, V1.2.7) and filtering (QⅡME,V1.7.0), clean data were obtained [16]. Reads were clustered at 97%similarity, and operational taxonomic units (OTUs) were picked using UPARSE (V7.1) software [15]. Microbial abundance, diversity, and other parameters were analyzed based on OTU clustering and species classification [16].
2.10 Statistical analysis
Significant differences were analyzed in SPSS 19.0 (IBM,Stamford, CT, USA). At-test was used to determine whether significant differences existed, andPvalues < 0.05 were considered statistically significant. The least significant difference (LSD) test also was used to determine whether significant differences existed.Multivariate statistical methods were performed in SIMCA (V13.0,Umetrics, Malmo, Sweden). Principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) were used to classify the effect of different bee pollen interventions on the mice with MetS.Due to a large number of assessment indexes with different units, first,the data were dimensionally reduced in SPSS 19.0.
3. Results and discussion
3.1 Nutritional composition of different bee pollen samples
In this study, the nutritional profiles of bee pollen, WB and YB were determined, and detailed information is shown in Table 2. The contents of most nutritional components significantly improved after wall breaking. Wall breaking is beneficial for the release of protoplasm from the exine. 24-Methylenecholesterol and stigmasterol were the primary phytosterols in rape bee pollen, with contents of 2.85 and 1.98 mg/g, respectively. Notably, the contents of 24-methylenecholesterol and stigmasterol in WB increased by 43.9%and 89.9%, respectively, compared to those in bee pollen. Phytosterols are well-established LDL-C-lowering agents that effectively prevent cardiovascular diseases [17]. After wall breaking of the pollen, the level of polyunsaturated fatty acids (PUFAs) increased by 6.3%, with a 9.5% increase in linolenic acid, compared to that in bee pollen.Consumption ofn-3 PUFAs can reduce MetS risk [18]. Rape WB is a good dietary source for functional lipids, which is related to a low incidence of MetS.

Table 2Nutritional components analysis of bee pollen samples.
In the fermentation process, WB showed additional advantages in the transformation of nutrients [8]. Following yeast fermentation,YB had a 73.0% reduction in fructose and an 89.1% reduction in glucose compared to WB [8]. Microorganisms and enzymes degrade bee pollen proteins to small molecules, such as free amino acids and oligopeptides. Some oligopeptides have blood pressure-lowering and antioxidant functions [19]. Phenolic compounds exist in a free form or are covalently bound to other macromolecules, such as proteins, fatty acids and cellulose. The bioavailability and biological activity of free phenolic compounds can be potentiated [20]. Yeast fermentation led to a 15.4% increase in free phenolic compounds compared to those in WB. The level of DF increased by 23.4% after yeast fermentation.A high fiber intake is associated with a 30% reduction in the risk of MetS [18]. The contents of 24-methylenecholesterol and stigmasterol in YB increased by 13.7% and 8.8%, respectively, compared to WB.The effect of YB on MetS is associated with all beneficial components working together, and whether the change in components following yeast fermentation has a prominent effect on MetS has unfortunately never been reported before.
3.2 MetS feature parameters obtained from mice
HF diets can cause metabolic disorders in a growing body,including body weight gain, adiposity, dyslipidemia, obesity, insulin resistance and hypertension [21]. In this study, to induce the MetS animal model, an HF diet was utilized to feed mice for 16 weeks. The initial body weights were not significantly different in the different groups. As shown in Table 3, after 16 weeks, the final body weight,body mass index (BMI), final systolic blood pressure (SBP), and glucose,insulin, TC, TG and LDL-C levels of mice fed the HF diet increased by at least two standard deviations, and the HDL-C level was significantly reduced (P< 0.05) compared to that in the Ctrl group.
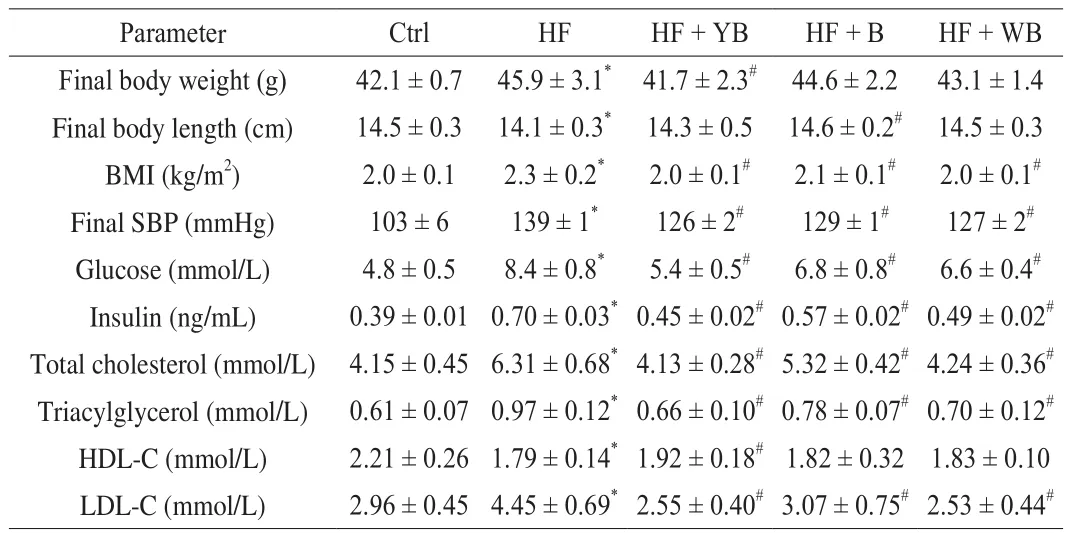
Table 3Effect of bee pollen supplementation on metabolic parameters in mice fed a high fat diet.
According to the diagnostic criteria of MetS [1], the MetS animal model was induced by an HF diet. The mice fed the HF + B, HF +YB and HF + WB diets exhibited alleviated MetS symptoms,especially those supplemented with the YB diet. Surprisingly, the body weight and TC level of the HF + YB group mice were similar to those of the mice fed the Ctrl diet. Bee pollen not only is rich in nutrients but also has a variety of active components [22]. The YB had a 70.6% reduction in fructose and an 87.1% reduction in glucose,while phenolic compounds and DF were increased by 5.4% and 24.1%, respectively, compared with those in bee pollen. Abundant polyphenols fromSchisandra chinensisbee pollen could attenuate body weight gain in obese mice [22]. Diets with high polyphenols and DF are strongly related to a lowered occurrence of MetS and enhanced health [23,24].
WB also showed a better effect on alleviating MetS than bee pollen. After pollen wall breakage, the levels of some nutritional components, such as protein, free amino acids, vitamins, small molecule sugar and linolenic acid, significantly increased;however, the increase in sterol content was surprising, with 24-methylenecholesterol and stigmasterol contents increasing by 43.9% and 89.9%, respectively. By regulating the lipid-associated plasma protein fraction profile and reducing inflammation,supplementing diets with phytosterol can prevent obesity,cardiovascular disease and other related chronic diseases [25]. There have been many reports on the function of phytosterol; nevertheless,to the best of our knowledge, the bioactivity of bee pollen phytosterol has not been reported. Fructose and glucose are the major carbohydrates in bee pollen, with proportions ranging from 13% to 55% depending on different plant origins [8]. Excessive consumption of sugar, especially high-fructose corn syrup, increases the risk of obesity and MetS, which can indirectly influence cancer development by changing numerous physiological and endocrine systems in the body [26].Yeast fermentation can significantly reduce the levels of fructose and glucose in bee pollen. This activity may be one important reason why YB is more effective than bee pollen or WB in alleviating MetS.
3.3 Different tissue weight and viscera coefficients obtained from mice fed different diets
HF feeding led to lipid accumulation and caused changes in visceral organs, such as hyperplasia, congestion, edema or atrophy.The viscera coefficient refers to the ratio of organ weight to body weight, and is a direct assessment of the extent of lesions in the internal organs. As shown in Table 4, HF diet consumption had a significant effect on liver and lung weight; however, only the liver coefficient exhibited a significant increase (P< 0.05). After 16 weeks of YB intervention, the mouse liver weight was reduced by 13.2%compared to that of the mice fed the HF diet, and was closer to that of the Ctrl group mice than that of the bee pollen or WB intervention mice. The liver coefficient of the mice fed YB was also reduced by 8.0% compared to that of the mice fed the HF diet. Consistent with the bodyweight change, the liver weight decreased with the consumption of YB. There was also a decreasing trend in the liver coefficient of the mice fed bee pollen, but the difference was not significant. Excess lipids that cannot be absorbed by adipose tissue are metabolized in the liver. Consequently, a long-term HF diet can induce the development of nonalcoholic fatty liver and affect the biological function of the liver [27]. The liver histopathology result (Fig. 1) showed that in the HF group, the hepatocytes were irregular with fat infiltration, and in other groups mice hepatocytes were closely arranged and had no vacuolation. Feeding mice with YB can alleviate the hepatic harm caused by an HF diet.
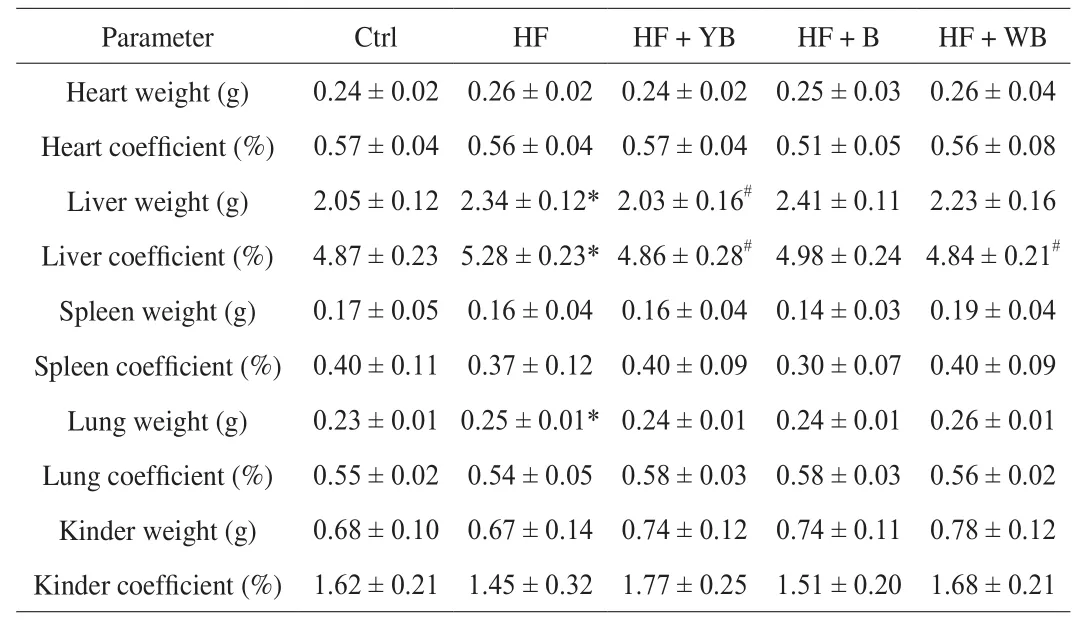
Table 4Organ weights and coefficients of mice fed different treatment diets at 16 weeks.
3.4 Impact of bee pollen product intervention on an oxidant status marker in liver
MetS is a combination of a series of clinical risk factors, including elevated blood pressure, low HDL-C and high TG and blood glucose levels, accompanied by oxidative stress, which plays an important role in the pathogenesis of MetS by triggering or aggravating the body’s biochemical reactions [28]. In our study, oxidant status in the liver was evaluated by CAT, GST and SOD assays. Levels of MDA and protein carbonyls, as the products of lipid and protein oxidation,respectively, were also determined. The results are shown in Table 5.There was a significant decrease in CAT, GST and SOD activities in the HF group compared to those in the Ctrl group (P< 0.05). Higher levels of protein carbonyls and MDA were observed in the HF group compared to the Ctrl group (P< 0.05). A long-term HF diet causes excess lipid metabolism resulting in accumulation of aberrantly high levels of free radicals that cannot be completely scavenged by the mouse body. Thus, the antioxidant enzyme system in the body is restricted, with oxidative stress status, which causes oxidative damage to the body.
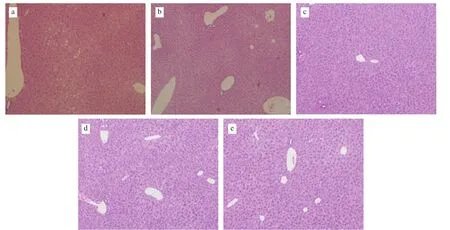
Fig. 1 Hepatic histopathology results of mice fed different treatment diets at 16 weeks (× 100). (a) HF group, (b) Ctrl group, (c) HF + WB group, (d) HF + YB group, (e) HF + B group.
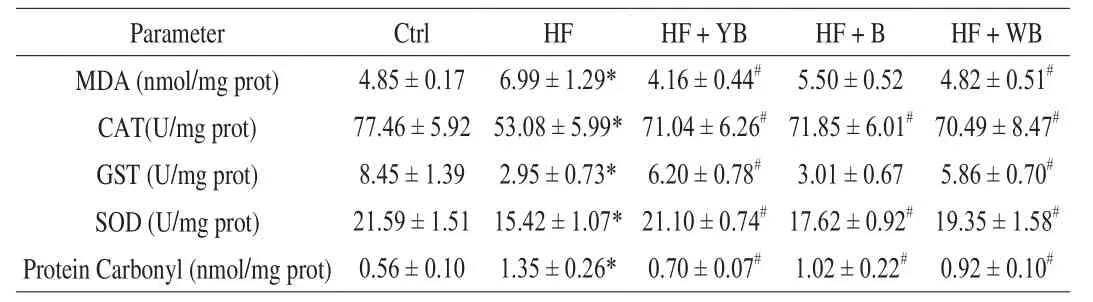
Table 5Hepatic oxidant status markers of mice fed different treatment diets at 16 weeks.
The consumption of bee pollen, WB and YB greatly improved the hepatic levels of CAT and SOD. The consumption of YB and WB was beneficial for recovering hepatic GST activity, but the consumption of bee pollen had no significant effect (P> 0.05). Lower levels of MDA and protein carbonyls were observed in these groups supplemented with YB and WB, compared to the HF group. Consumption of bee pollen decreased the content of hepatic protein carbonyls but did not affect MDA content. These oxidant status parameters of mice fed YB were closer to those of the Ctrl group mice, and YB intervention effectively alleviated oxidative stress caused by MetS.
The antioxidant activity of bee pollen has been corroborated as a free radical scavenger, as well as a lipid peroxidation inhibitor.In the livers of old mice, the MDA content increased by 89.7%,and sulfhydryl groups (SH-Gs) significantly decreased compared to those of adults. Following oral pollen consumption, the MDA content decreased by 81.0%, and the level of SH-Gs was completely normalized.In old mice, bee pollen can alleviate lipid peroxidation and improve the antioxidant defense system and liver function [29]. Phenolic compounds,especially flavonoids and phenolic acids, such as caffeic acid phenethyl ester, quercetin, rutin, apigenin, kaempferol and caffeic acid, are the main components of bee pollen with antioxidant capacity [30]. Other secondary plant metabolites of bee pollen, including vitamin C, vitamin E, carotenoids and glutathione, also contribute to the antioxidant activity[9]. In the present study, the antioxidant activity of bee pollen was again corroborated, and yeast fermentation treatments might be responsible for improving the antioxidant effect.
3.5 Multivariate statistical analysis based on biochemical indexes of mice
To indicate the effect of different bee pollen sample interventions on the mice with MetS, the biochemical indexes, including metabolic parameters and hepatic oxidant status markers, were analyzed by multivariate statistical methods. The PCA scatter plot shows similarities in the data and the relationships between different groups(Fig. 2A). The PCA model was statistically valid when the cumulative variances were more than 75%. In the present study, the variances of the first two PCs were 70.1% and 8.23%, respectively, and their cumulative variances were 78.33%. In a discrete-point map, the HF group was distant from the other four groups, and different bee pollen sample interventions also showed differences. Furthermore, a normal probability scale based on the PCA (Fig. 2B) revealed that the HF + YB group was more similar to the Ctrl group than the HF + WB group or HF + B group.
A loading scatter plot (Fig. 2C) and variable importance in projection (VIP) plot (Fig. 2D) showed the important variables correlated with the effect of different bee pollen sample interventions on the mice fed HF diets. VIP values greater than 1 indicated that the changes in these indexes were significant due to YB, WB or bee pollen intervention. In this study, the indexes with VIP values greater than 1 included HDL-C, GST, CAT and MDA. Among the four parameters, GST, CAT and MDA were associated with oxidative stress, which revealed that YB, WB or bee pollen intervention to attenuate HF diet-induced MetS in mice may be primarily associated with reducing oxidative stress. Additionally, the impact of bee pollen sample intervention on lipid metabolism was also significant, and we will carry out follow-up experiments in subsequent studies.
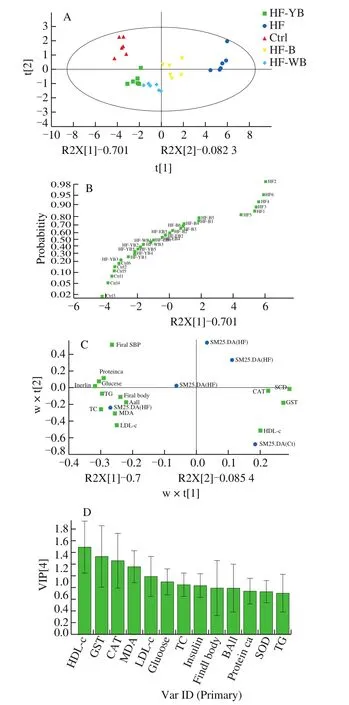
Fig. 2 The results of multivariate statistical analysis for different groups according to biochemical indexes. (A) PCA discrete-point map; (B) normal probability scale plot according to PCA results; (C) PLS-DA loading plot; and(D) variable importance in projection (VIP) plot.
3.6 Impact of bee pollen product intervention on hepatic antioxidant properties
Long-term HF diet feeding can lead to oxidative stress and affect a series of cellular processes in the liver at the transcriptomic level [27].Nuclear factor erythroid 2-related factor 2 (Nrf-2) is a key transcription factor maintaining the redox balance state, which can be activated by modifying reactive cysteines on Kelch-like ECH-associated protein-1(Keap-1) and regulating the transcription of several antioxidative genes, such as HO-1 and nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase quinone oxidoreductase 1 (NQO-1) [31].Keap-1 protein is an important inhibitor of Nrf-2 through obstructing Nrf-2 translocation to the nucleus and facilitating its degradation.Under oxidative stress conditions, Nrf-2 is released from the Keap-1/Nrf-2 complex by modifying cysteines of Keap-1 [31].Therefore, the Nrf-2-Keap-1 cytoplasmic oxidative stress system is central to the transcriptional response. The expression levels of HO-1, NQO-1, Nrf-2 and Keap-1 mRNA under different diets are shown in Fig. 3. After 16 weeks of HF diet feeding, the expression of Nrf-2, Keap-1 and these antioxidative mRNAs regulated by Nrf-2 significantly decreased (P < 0.05) compared to that with normal feeding. Long-term HF diets have a destructive effect on the hepatic Nrf-2 pathway. Compared to the HF group, the YB, WB or bee pollen intervention groups exhibited significantly increased expression of these mRNAs (P < 0.05), and the expression of HO-1, Nrf-2 and Keap-1 mRNA in the HF + YB group was higher than that in the Ctrl group.
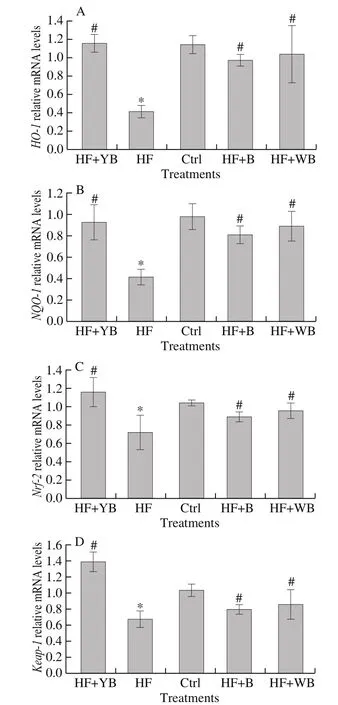
Fig. 3 Hepatic antioxidant gene expression of mice fed different treatment diets at 16 weeks. (A) HO-1 expression levels; (B) NQO-1 expression levels;(C) Nrf-2 expression levels; and (D) Keap-1 expression levels.
Immunohistochemistry analysis for NQO-1 and HO-1 is shown in Fig. 4. The results indicate that the HF diet significantly reduced the hepatic antioxidant intensity, while bee pollen and WB interventions only partly recovered it. Supplementation with YB had the best effect on improving hepatic antioxidant properties. The Nrf-2-Keap-1 pathway is essential in intracellular defense against oxidative stress by regulating the expression of several cellular protective proteins[32]. YB was conducive to building an antioxidant protection system. Cheng et al. [22] demonstrated that Schisandra chinensis bee pollen extract could reduce fat accumulation in the liver and relieve oxidative injury in obese mice, and they suggested that the active ingredients were phenolic compounds. Another piece of evidence is that polyphenol-rich bee pollen ethanol extract prevented hepatic steatosis and degenerative changes caused by HF diets, revealing the hepatoprotective effect of bee pollen [33]. Virgin Cabernet Sauvignon grape seed oil (VGSO) is rich in linoleic, phytosterol and phenolic compounds, and supplementation with VGSO can improve inflammation and oxidative stress in mice fed an HF diet [34].Excessive intake of fructose can lead to MetS, resulting in oxidative stress [35]. Yeast fermentation is an effective method to reduce sugar(fructose and glucose) contents and improve healthy components, such as phytosterols, linoleic compounds, DF and phenolic compounds, in bee pollen. Thus, YB showed the best antioxidant function.
3.7 Impact of bee pollen product intervention on the gut microbial community
The gut is a massive complex ecosystem, including nutrients,the microbiota and host cells, which interact extensively to maintain intestinal homeostasis. The gut microbiota has been closely associated with health and many diseases [36]. HF diet-induced metabolic disorders and oxidative stress have been linked to changes in the intestinal microbiota [37]. In this study, through analysis of gut microbiota 16S rRNA, we obtained an average of 43 468 sequences in the HF group, 56 447 sequences in the HF + WB group, 43 862 sequences in the HF + B group, 59 710 sequences in the HF + YB group, and 46 469 sequences in the Ctrl group. The lengths of these sequences were between 400 and 440 base pairs. According to a Venn diagram (Fig. 5B), 308 OTUs overlapped among the five groups, while individual OTUs in the HF + YB, HF, Ctrl, HF + B and HF + WB groups were 5, 13, 9, 20 and 24, respectively. There was an obvious increase in the HF group (446 OTUs) compared with the Ctrl group (414 OTUs). After YB intervention, the OTU number decreased to 405.
To explore the differences in gut microbial communities among these groups, we carried out PLS-DA on the OTUs. The 30 samples were classified into three groups (Fig. 5C). Compared to the distances among the other four groups, the HF group was very distant from all other groups. The HF + YB, HF + WB and HF + B groups were close in the scatter plot, while the six points from the Ctrl group were colocalized. The effect of consumption of different bee pollen treatments on gut microbial communities (phylum level) is shown in Table 6 and Fig. 5D. Atthe phylum level, Firmicutes, Proteobacteria and Bacteroidetes were the dominant microbiota constituents in the mouse gut and accounted for 79.31% (HF group), 85.86%(HF + WB), 77.69% (HF + B), 91.48% (HF + YB), and 88.19%(Ctrl) of the total microbiota, respectively. The result was similar to that in a previous report [38]. A long-term HF diet led to a significant increase in Firmicutes relative abundance and a sharp decrease in Proteobacteria relative abundance (P < 0.05). Consumption of YB,WB and bee pollen significantly increased the relative abundance of Proteobacteria but had a suppressive effect on that of Firmicutes(P < 0.05). In the HF + WB and HF + YB groups, the relative abundance of Bacteroidetes was significantly increased (P < 0.05).
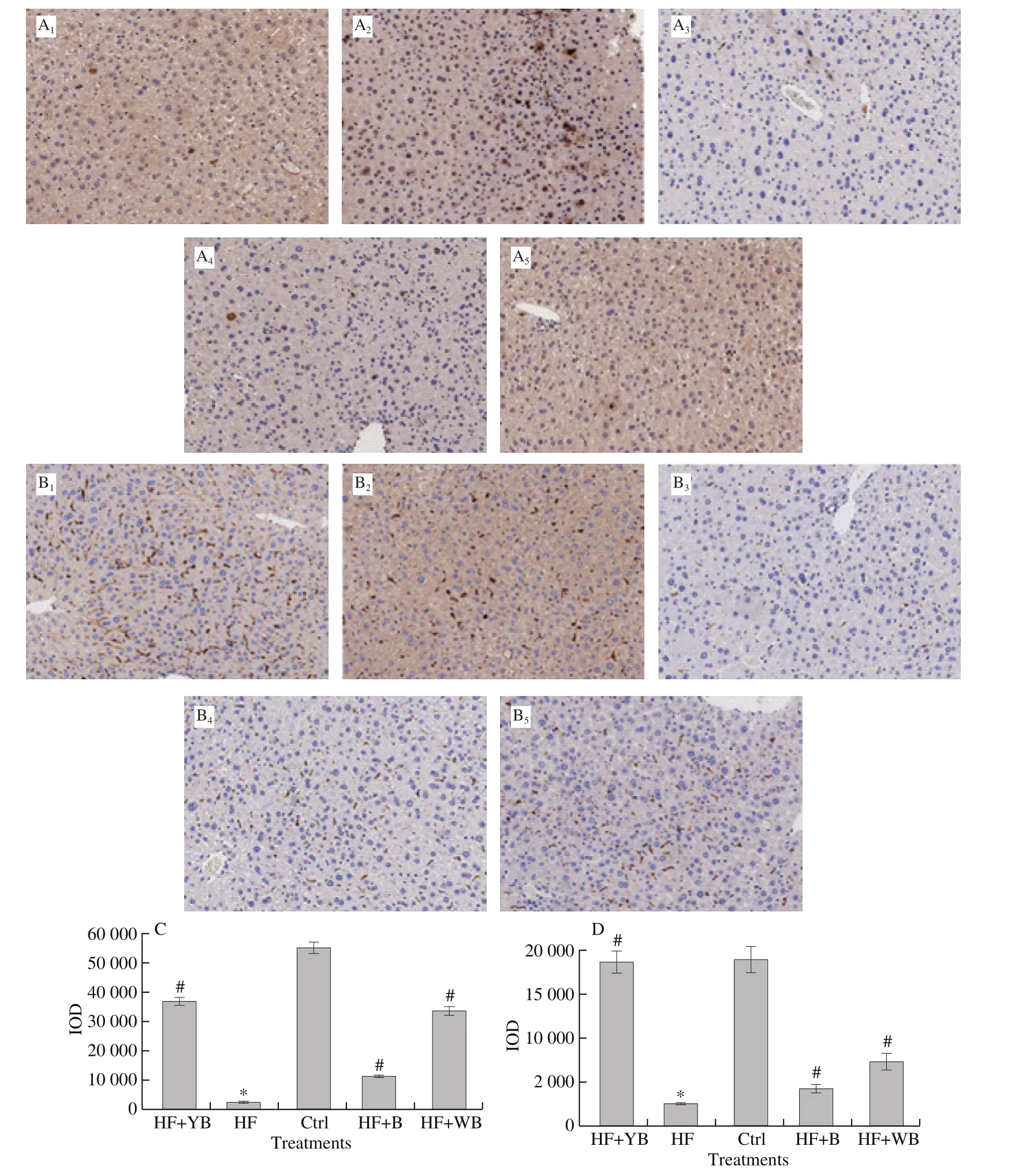
Fig. 4 Results of immunohistochemistry for hepatic antioxidant protein. A-B, magnification, 200 ×. A1-A5 and C are representative results of NQO-1 for different treatments; B1-B5 and D are representative results of HO-1 for different treatments. 1. HF + YB, 2. Ctrl, 3. HF, 4. HF + B, 5. HF + WB.
The HF diet contributes to gut dysbiosis with a high ratio of Firmicutes/Bacteroidetes (F/B) [39]. In our study, the F/B values were 6.94 (HF), 4.32 (HF + WB), 4.84 (HF + B), 3.74 (HF + YB) and 6.12(Ctrl), respectively. Supplementation with YB, WB and bee pollen decreased the F/B value, especially for YB. Table 7 shows the primary composition of gut microbial communities at the genus level. We noticed that intestinal microbes were more abundant in the Ctrl, HF +WB, HF + YB and HF + B groups than in the HF group. Possibly due to low relative abundance, Lactobacillus and Lactococcus were not observed in the HF group (Table 7). Compared to a normal diet,consumption of bee pollen, YB and WB increased Lactobacillus and Lactococcus abundances, and YB intervention was beneficial for the colonization of probiotics in the intestines of mice. Lactobacilli can maintain intestinal homeostasis by inducing the release of some cytokines, functioning to modulate blood glucose content and blood pressure [40]. Lactobacillus has a hypocholesterolemic function in animals fed a high cholesterol diet [41]. Zhou et al. [42]found that in vitro fermentation of polysaccharides from pollen of Chinese wolfberry enhanced the production of short-chain fatty acids and modulated gut microbiota composition, increasing the relative abundances of the genera Prevotella, Dialister, Megamonas,Faecalibacterium, andAlloprevotella. The changes in gut microbiota were inconsistent with our results, which might indicate that a single component of bee pollen had a different effect on shaping the gut microbiota compared with the whole. According to our results, YB intervention had positive effects in modulating the intestinal microecosystem in mice with MetS.

Table 6Primary composition of fecal microbiota in HF animal model on phylum level.

Table 7Primary composition and relative abundance of fecal microbiota in HF animal model on genus level.
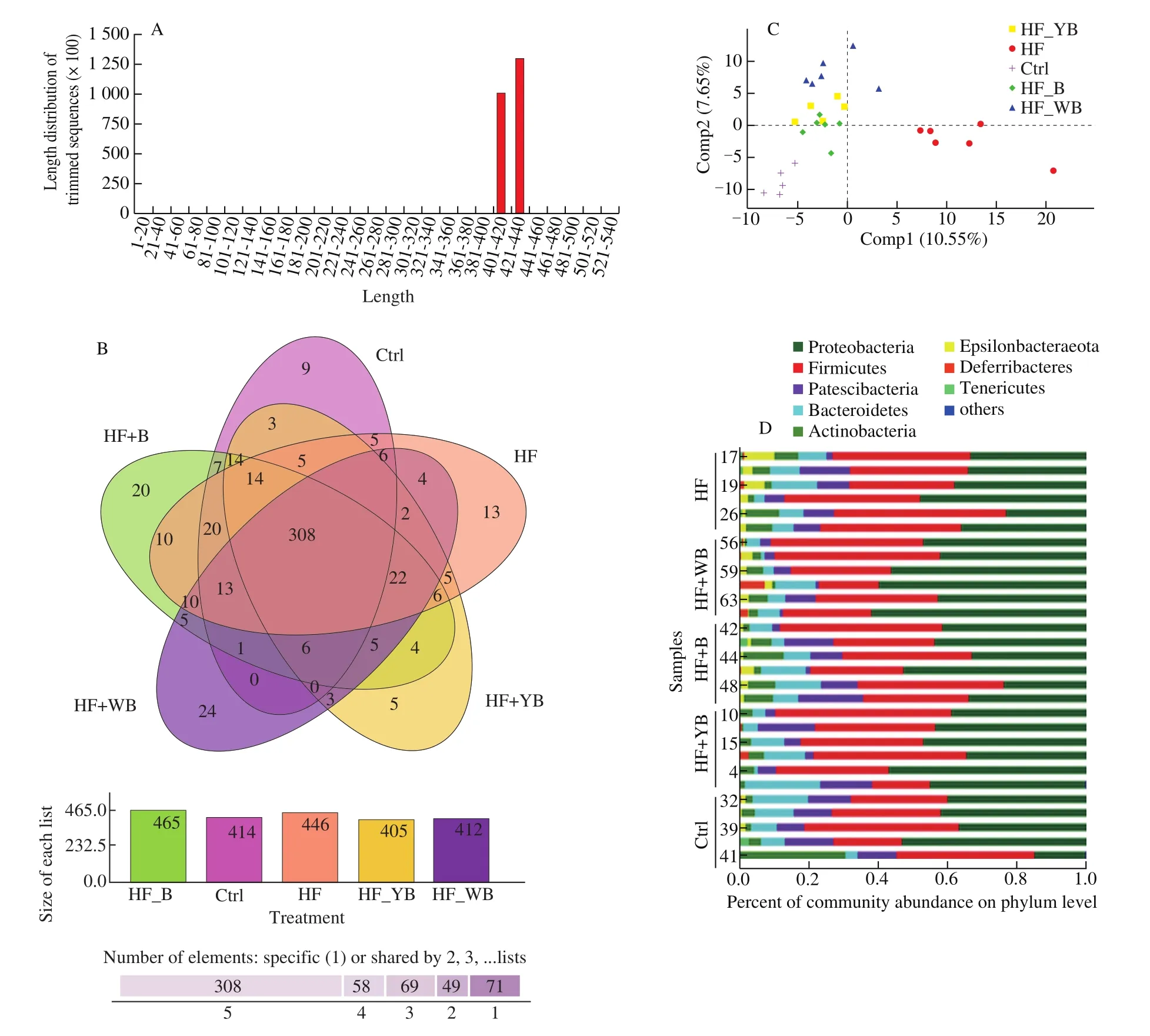
Fig. 5 Effects of bee pollen, WB, YB administration on the gut microbiota change. (A) DNA sequence numbers and lengths; (B) Venn diagram of the differential numbers of OTUs in each group; (C) PLS-DA scatter map based on unweighted UniFrac metrics; and (D) compositions of cecal microbiota in mice at the phylum level.
4. Conclusions
Overall, daily consumption of bee pollen, WB and YB intervention for 16 weeks ameliorated MetS in ICR mice fed HF diets, as determined by evaluating MetS serum parameters and hepatic oxidant status markers. The impact of bee pollen products on MetS may be associated with restoring the Nrf-2-Keap-1 pathway to alleviate oxidative stress. In addition, gut microbial community analysis showed that supplementation with bee pollen, WB and YB could reduce the F/B ratio and increase Lactobacillus and Lactococcus abundance in the mouse gut. YB, with fewer carbohydrates and more free phenolic compounds, phytosterol and DF, showed a better effect on relieving MetS and regulating the intestinal flora than bee pollen or WB. This study provides evidence to support the potential use of YB to prevent MetS and promote the deep processing of bee products in the future.
conflict of interest statement
The authors declare no conflict of interest.
Acknowledgements
We are grateful to Bingyan Wei from Shanxi Medical University for his assistance with the animal experiment. This study was supported by the National Natural Science Foundation of China (No.31972628 and 31472155), a special fund (NYCYTX-43), and the fund of the Beijing Laboratory for Food Quality and Safety, Beijing Technology and Business University.
- 食品科学与人类健康(英文)的其它文章
- Moringa oleifera Lam. leaf extract mitigates carbon tetrachloride-mediated hepatic inflammation and apoptosis via targeting oxidative stress and toll-like receptor 4/nuclear factor kappa B pathway in mice
- Potential of peptides and phytochemicals in attenuating different phases of islet amyloid polypeptide fibrillation for type 2 diabetes management
- Zein as a structural protein in gluten-free systems: an overview
- Spectrum-effect relationship of immunologic activity of Ganoderma lucidum by UPLC-MS/MS and component knock-out method
- A red pomegranate fruit extract-based formula ameliorates anxiety/depression-like behaviors via enhancing serotonin (5-HT) synthesis in C57BL/6 male mice
- Purification, characterization and hypoglycemic activity of glycoproteins obtained from pea (Pisum sativum L.)

