Zein as a structural protein in gluten-free systems: an overview
Xinrui Zhang, Chunxia Dong, Yayun Hu, Mengnan Gao, Guangzhong Luan
College of Food Science and Engineering, Northwest A&F University, Yangling 712100, China
Keywords:
Zein
Gluten-free
Network
Glass transition
ABSTRACT
Zein, a class of alcohol-soluble prolamines in maize endosperm, is mainly composed of α-zein, β-zein, and γ-zein. It has been recognized as a structural protein for various gluten-free systems since it can form glutenlike viscoelastic network. The formation of viscoelastic zein network can make up for the structural defect of gluten-free doughs caused by the lack of gluten. To make the most of structural functionality of zein in glutenfree foods, it is important to clearly elucidate the fundamental properties of zein network. In this article, these properties have been discussed, analyzed and summarized from the relationship between protein network and structural functionality of zein, the feature and formation mechanism of zein network, factors affecting zein network and the applications of zein network in improving the quality of gluten-free food. In addition, this article also looks forward to potential research areas on zein network.
1. Introduction
Celiac disease (CD) is an immune-mediated systemic disorder occurring in genetically susceptible individuals that are triggered by the ingestion of gluten [1]. As no successful treatment has been reported, predisposed individuals require total lifelong avoidance of gluten, and thus forbids the consumption of wheat, barley and rye products [2]. Not only CD patients but people who suffer from nonceliac gluten sensitivity or avoid gluten for reason of lifestyle follow a gluten-free diet. Overall, the demand for gluten-free products worldwide have been steadily increasing because of increased awareness of the disease and changing eating habits. However, there are many problems with gluten-free breads, including crumbling texture, poor color, poorly developed dough structure, and other quality defects, that are due to the absence of gluten. Gluten, the main storage proteins in wheat, have a key role in the viscoelastic dough making capability of wheat flour, which is because gluten can aggregate to form protein network [3]. The unique extensional rheology of gluten network gives wheat doughs the viscoelasticity to retain gas during proofing and baking, which results in the spongy,foam-like structure of wheat breads [4]. Therefore, finding suitable replacements for gluten could solve fundamentally the intrinsic poor quality of gluten-free products when other approaches that are being used to improve the quality of these products have failed [5].
In previous studies, zein had been shown to be able to form a wheat-like dough without structural defects for the production of gluten-free breads [6-9]. Besides the physical similarity between zeinstarch doughs and wheat doughs—best characterized as cohesiveness,extensibility and viscoelasticity— the structure and fibrous protein networks showed similarity as well [7]. Even though the storage proteins from all gluten-free grains have been isolated and studied,there is essentially no evidence that any of them, except zein, have the structural functionality [9]. Therefore, zein is believed to have great promise in the replacement for gluten as a structural protein in glutenfree systems. The viscoelasticity, cohesiveness, and extensibility of zein-based doughs are critical features for producing leavened products. These characteristics allow zein-based doughs to hold CO2produced during dough fermentation to produce breads in different shapes [10]. Consequently, in terms of possessing the correct functional attributes necessary for gluten-free dough formation and bread making applications, maize zein used to replace gluten in gluten-free systems is nevertheless highly desirable, although it has certain limitations such as workability and aging at room temperature [11].
Researchers have different opinions on whether zein as a substitute for gluten is safe for celiac patients. Cabrera-Chávez et al. [12] reported that the use of maize in gluten-free products must be reevaluated for some patients with celiac disease, because several peptides in zein were found to be able to bound to the human leukocyte antigen molecules involved in CD pathogenesis by an in silico analysis. Although zein peptides are immunogenic for rare celiac patients and induce a similar innate response, but to a lesser extent than gliadin peptides of gluten [13]. Barre et al.[14] reported that zein could be recognized as a safe food for celiac patients since there was no immunotoxic epitopes in zein con firmed by another in silico analysis. These conclusions indicate that there is still controversy about whether zein is safe for a few celiac patients,but the fact that zein is safe for most patients with celiac disease is undisputed. Therefore, it is still of great significance to study the structural functionality of zein to improve the quality of glutenfree foods. Based on the theoretical background introduced in this section, the present review focused on the current landscape of zeinbased gluten-free systems. The composition and structural chemistry of maize zein were briefly examined. Researches into the structural functionality and protein network of zein were reviewed. The major section reviewed researches into the feature of zein network formation. Given the absence of a de fined formation mechanism for zein network, the mechanism was discussed and inferred. The measures to optimize zein network was introduced from four aspects.The application of zein as a structural protein in gluten-free foods was reviewed. Lastly, potential directions for future research on zeinbased gluten-free systems was given.
2. The chemical basis of zein
Considering the similarities between maize zein and wheat gluten on their structural functionality in doughs, a comparison of zein and gluten in composition and structure is necessary before stepping into the question of how zein mimics gluten in non-gluten dough systems.
2.1 Compositions of zein
Zein is a class of alcohol-soluble prolamine storage proteins organized in protein bodies that located in the maize endosperm. It was first isolated from whole white maize and was named by John Gorham in 1821 [15]. Different from the high-molecular-weight of gluten, zein was found to be a relatively low molecular protein.Based on the solubility and sequence homology, zein is divided into 4 different classes: α-zein (19 and 22 kDa), β-zein (14 kDa),γ-zein (16 and 27 kDa) and δ-zein (10 kDa) [16]. α-Zein, β-zein, and γ-zein constitute 75%-80%, 10%-15%, 10%-15% of the total zein,respectively [17]. β-Zein, γ-zein, and δ-zein are only extractable in the presence of a reducing agent. δ-Zein accounts for only a small proportion of the total zein [18]. Commercial zein is mainly composed of α-zein and contains a small amount of β- and γ-zein. Their different solubility behaviors are the result of their various amino acid sequences [16]. β- and γ-zein contain more cysteines than α-zein[19]. Unlike gluten with a significant amount of hydrophilic amino acids, zein contains polar and non-polar amino acids, which makes it has a unique amphiphilic character [20]. Thus, zein is soluble only in limited types of solvents such as aqueous alcohols and alkaline solutions [21].

Fig. 1 Proposed three-dimensional (3-D) structural models of zein according to Zhang et al. [26]. (a) Cylindrical model (adapted from Coleman et al. [20]); (b)ribbon-like model (Z22) (adapted from Argos et al. [27]); (c) hairpin model (Z19) (adapted from Forato et al. [28]); and (d) superhelical structural model (Z19)(adapted from Momany et al. [29]).“R” means repeat unit.
2.2 Structure of zein
The structure of zein is closely related to its physicochemical properties and the self-assembly mechanism in various systems.Unlike gluten, α-zein consists of highly homologous repeat units and has high content of α-helix structures [22]. β-Zein has few α-helix structures, with most of the sequence being β-sheet and aperiodic structure (β-turn and random coil) [23]. γ-Zein contains 33% α-helix structures and 31% β-sheet structures in its physiological state [24].δ-Zein does not contain any repeated sequences or have a clearcut domain structure. The secondary structure of zein is solvent dependent [25]. Several structure models of zein monomers have been proposed over the years, such as the cylindrical model, ribbonlike model, hairpin model, and super helical structure model, etc.(Fig. 1) [26]. More detailed protein structure models of zein predicted by Argos et al. [27] were shown in Fig. 2. However, there is not a consensus on the structure of zein. Despite the discrepancy between those models, there is a general agreement that the monomers consist of linear stacks of rod-shaped helical repeat units.
Overall, there is a huge difference between zein and gluten in composition and structure. This means zein could form viscoelastic doughs by different methods.
3. Zein network
3.1 Protein network and structural functionality of zein
A great number of research has demonstrated that protein network forming is an important factor in forming a viscoelastic dough, in both gluten and gluten-free systems. It is generally accepted that wheat gluten provides cohesiveness, viscoelasticity, and extensibility to doughs through building up a 3-D network [3,31]. These unique rheological characteristics of wheat doughs are responsible for retaining gas during leavening and baking, resulting in the uniform,and foam-like structure of wheat breads [4]. There is no doubt that gluten network is important in determining the bread-making properties of wheat flour. Similarly, protein network is vital for gluten-free systems as well. The reason why zein has structural functionality compared to other gluten-free proteins is that it can form the fibrous protein network when mixed with water above its glass transition temperature (Tg) [7]. Zein network that exists in gluten-free doughs can transmit extensibility and viscoelasticity to the dough,which gives the dough bread-making properties (Fig. 3). Above all,the decisive prerequisite for the structural role of zein in gluten-free doughs is the formation of viscoelastic protein network [6,34,35].

Fig. 2 Cartoon illustrations of structure models of zein according to Li et al. [30].
3.2 Feature of zein network formation
3.2.1 Glass transition and network formation of zein
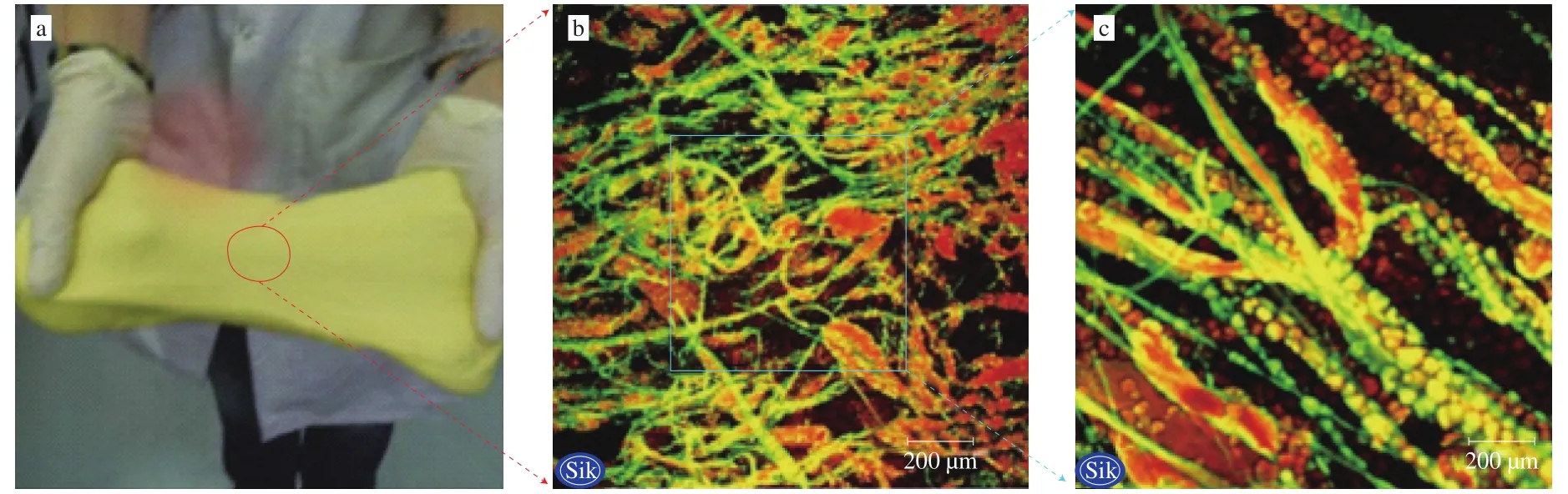
Fig. 3 Zein-starch doughs at the macroscopic and microstructural levels. a, the photograph (adapted from Sly et al. [32]); b and c, confocal laser scanning microscopy(CLSM)-images with scale bars representing 200 and 50 mm, respectively (adapted from Andersson et al. [33]). Zein is green and starch is red in the images.
Zein network was firstly observed in the zein-starch system [6].In this system, zein, starch, and water were mixed above Tg(e.g. at 30-40 °C) of zein, resulting in a viscoelastic, cohesive and extensible dough [6]. Subsequent researches also showed viscoelastic zein network could be formed only when zein was hydrated and mixed above its Tg, which is called the mixing method [7-9]. Of particular note is that, although water is a necessary element for zein network formation in the mixing method, excess water contributes less since zein network has a finite amount of water [36]. Under the study of other scholars, a stable viscoelastic zein network had been also formed in another aqueous system [9,37,38]. Zein was dissolved in glacial acetic acid solution above its Tgand simple coacervation was performed by the rapid addition of cold water under low shear. Zein precipitated as a network of hydrated fibrils which could be handkneaded into a viscoelastic mass (Fig. 4). This approach can be named as the coacervation method in this study because coacervation is the key step for zein network formation.
It is worth noting that, regardless of the approaches, system temperature above the Tgof zein is necessary for zein network formation. In other words, the zein network formation is dependent on its glass transition, which is similar to the first step in the formation of gluten network mentioned below. There has been a growing number of publications proving that zein was unable to form a viscoelastic network at common room temperature which was usually below its Tg[7,34,35,37,39]. When the temperature of a system was below the Tgof zein, zein network had lost its viscoelastic nature quickly [39].The addition of water to zein above its Tgresulted in a transition from the glassy to the rubbery state. This increased the reactivity of zein and enhanced its propensity for aggregation, which were critical to the zein network formation [37]. In general, the glass transition is not only a sufficient condition for zein to form a network, but also a necessary condition.

Fig. 4 Zein viscoelastic masses at the macroscopic (A) and microstructural levels (B). A, the photograph; B confocal laser scanning microscopy (CLSM)-images with scale bars representing 100 mm (adapted from Taylor et al. [40]).
3.2.2 Contribution of different subclasses
Veraverbeke et al. [31] and Schober et al. [35] had reported that the functional zein was composed of α-zein and very low contents of β- and γ-zein, while the non-functional zein contained a high percentage of β- and γ-zein. Commercial zein dominated by α-zein has been shown the ability to form viscoelastic zein network.Compared with commercial zein, total zein was not easy to form viscoelastic network when mixed with water at 50 °C [8]. Hitherto, all studies on the structural functionality of zein had involved only on the α-zein class [9]. The structural functionality of β- and γ-zein are much weaker than that of α-zein.
β- and γ-zein differ from α-zein in two aspects: hydrophobicity and the content of cysteines. Firstly, they are more hydrophilic than α-zein, indicated by their solubility in more dilute aqueous isopropanol [18]. The hydrophobicity of zein plays an important role in the formation of zein network and the structural functionality of zein, which will be introduced below. Higher hydrophily of β- and γ-zein result in the weaker hydrophobicity of these zeins,which would be no benefit to the formation of zein network [35,41].Secondly, the cysteine content of β- and γ-zein was 4.44% and 12.84%, respectively, which was much higher than that of α-zein(1.22%) [19]. The existence of cysteines can promote the formation of disulfide bonds under oxidizing conditions. disulfide bonds contribute to a high molecular weight and abundant cross-linking [18,42]. Large disulfide-linked polymers formed by β- and γ-zein may prevent viscoelastic zein network formation [40]. Furthermore, the increasing number of disulfide bonds of zein can lead to a higher Tgand may be not conducive to zein network formation. This explained why the non-functional zein contains a high percentage of β- and γ-zein [9,43].
More recently, Oguntoyinbo et al. [38] had successfully prepared zein network from total zein comprising α-, β- and γ-zein.Oguntoyinbo et al. [38] used the coacervation method to dissolve total zein in glacial acetic acid, and then quickly added cold water to form the total zein network. This indicated glacial acetic acid treatment can help β- and γ-zein obtain structural functionality. Besides, γ-zein has been proved to be essential to maintain the flexibility of zein network [40].
3.2.3 Secondary structure change of zein during zein network formation
When zein produced network using the mixing method, the content of β-sheet structures increased, from 30% to 48%, at the expense of a reduction in the α-helical domains [39]. This suggested that the protein loses its native structure [39]. The same change on secondary structure had also been observed by Sly et al. [32]and King et al. [8] when both the commercial zein and total zein were hydrated with distilled water to form zein network or slurries(when zein network did not be formed). Intriguingly, the content of β-sheet structures, α-helix and β-turn structures were increased during viscoelastic gluten network formation [44]. Jeong et al.[45] suggested that sufficient structural functionality attributed the formation of β-sheet structure in rice flour-zein doughs. Furthermore,it should be pointed out that the proportion of β-sheet structures decreased significantly from 48% to 30%, when the temperature dropped from 35 °C to 25 °C or shear stress was removed, causing a loss of viscoelasticity of zein network [39]. Together, these researches indicated that β-sheet conformation is critical for zein to form viscoelastic zein network and structural functionality.
Besides, it should be emphasized that β-sheet conformation does not guarantee zein dough formation. For example, the viscoelastic zein network cannot be formed from hydrated total zein with predominant β-sheet conformation [46]. There is a complex interplay of various conditions involved in the formation of zein network of structural functionality, including the composition, hydrophobicity and secondary structure of zein, the addition of ingredients and chemicals, etc. The positive influences of β-sheet conformation on the formation of zein network seems to be offset by the negative effects of β- and γ-zein in total zein.
3.2.4 Role of non-covalent interactions
As is known to all, the stable 3-D gluten network can be stabilized and strengthened by disulfide bonds [47]. Considering the similarity between gluten network and zein network in structural functionality,it can be speculated that disulfide bonds also play an important role in the formation of zein network. However, Schober et al. [35] and Smith et al. [41] had reported that hydrophobic interactions rather than disulfide bonds are the key to zein network formation.
Oom et al. [37] first proposed disulfide cross-links of kafirin(prolamin like zein) monomers were probably the reason for the stiffening behavior of kafirin network, which causes it to lose structural functionality. Schober et al. [35] also reported that the disulfide bond was detrimental to the viscoelasticity of zein network.The negative effects of disulfide bonds could be attributed to its preventative effect on the aggregation of the α-zein helical structures[35]. At present, the mechanism of disulfide bond affecting zein network formation is not clear, which needs more research.
Schober et al. [35] proposed that for zein network formation,hydrophobic interactions rather than disulfide bonds play a central role based on the fact α-zein was easier to form network and more hydrophobic than β- and γ-zein. Smith et al. [41] found that the ability of zein to form network changed with hydrophobic interactions, but remained the same as the disulfide bonds decreased. Interestingly,too strong non-covalent interactions also have some harmful effect on zein network formation [41]. Since hydrophobic groups of zein can be buried by the too strong non-covalent interactions, zein would be not capable of gathering and forming viscoelastic zein network.Conversely, weaker non-covalent interactions between zein have some beneficial effect on the formation of zein network, possibly by promoting the unfolding of zein proteins. This unfolding of zein may allow for increased interactions between zein by exposing regions of the protein that were previously buried [41].
3.3 Formation mechanism of zein network
Unlike the formation mechanism of gluten network has been clarified, little work has been completed to elucidate the formation mechanism of zein network. The lack of fundamental knowledge about the formation mechanism of zein network have limited the application of zein as an alternative to gluten in the production of gluten-free systems. Understanding the formation mechanism of gluten network may provide clues on how to make zein into viscoelastic zein network.
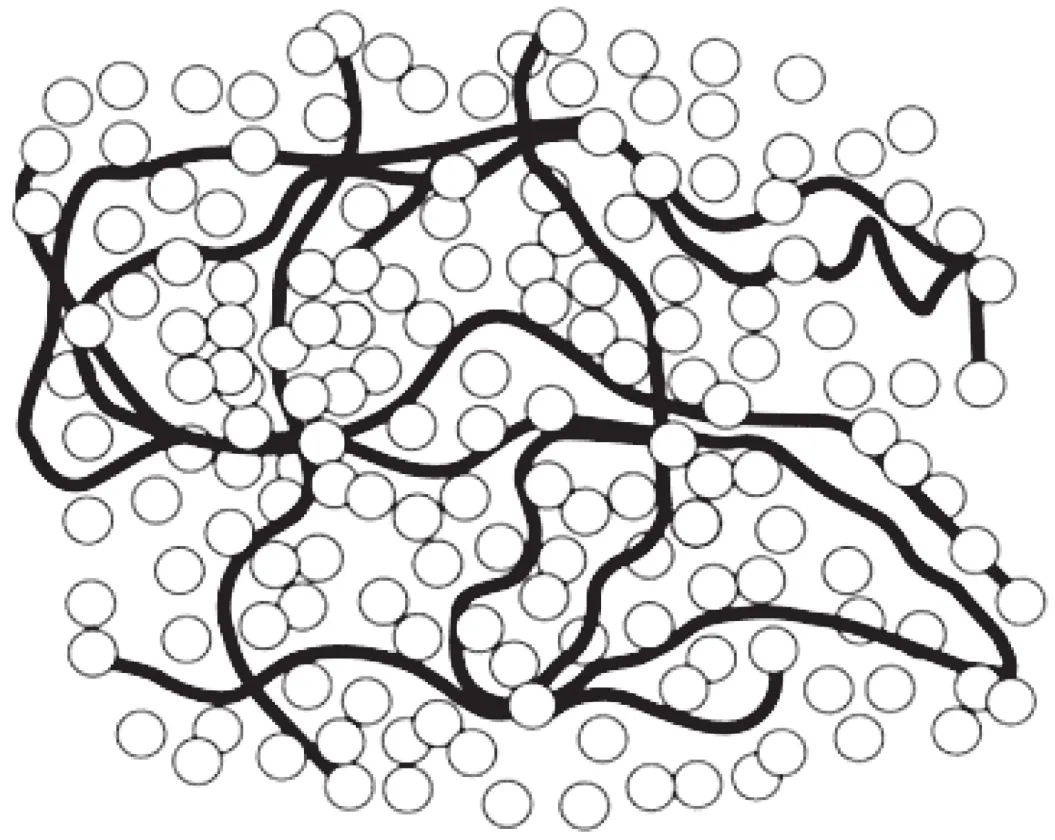
Fig. 5 A model for the molecular structure of gluten network. HMS are approximated by linear polymers, HMS and gliadin are approximated by spheres (adapted from Belton [48]).
The formation mechanism of gluten network is reviewed in detail by Belton et al. [48]. A concise molecular model of gluten network has been proposed. In this model shown in Fig. 5, gluten has been divided into linear proteins which include the high molecular weight glutenin subunits (HMS), globular proteins which include low molecular weight glutenin subunits (LMS) and the monomeric gliadin. During the formation of gluten network, linear HMS develops through alignment of peptide chains and formation of end-to-end disulfide bonds, creating an elastic network that endows gluten network elasticity [48]. Globular LMS and gliadins distribute in elastic HMS network, giving gluten network viscosity and extensibility [49].The first step of gluten network formation is also a glass transition like zein network [48]. Due to at 16% moisture, the Tgof gluten is below room temperature and continues to drop linearly with higher moisture contents, gluten network can be formed at room temperature [50]. Similar changes in secondary structure, the increase of β-sheet, occur in both zein network and gluten network [51]. However, different from zein network,gluten network is mainly linked by disulfide bonds [52].

Fig. 6 Schematic diagram of the formation mechanism of amyloid fibrils (adapted from Brundin et al. [55]).
Since there are no covalently-bounded large protein complexes found in zein and zein network, zein must form network via a different mechanism. Erickson et al. [53] reported that the formation mechanism of zein network may be the same as the formation mechanism of amyloid fibrils because they appear to share some similarities mainly in the development of amorphous aggregates and rapid precipitation into insoluble, β-sheet-rich, fibrous aggregates.Amyloid fibrils are an intensively studied category of extracellular protein deposited and associated with neurodegenerative disorders[54]. To find out the formation mechanism of zein network, the mechanism of amyloid fibrillogenesis could be utilized as the basis for understanding of the formation of the viscoelastic zein network.The formation mechanism of amyloid fibrils is shown in Fig. 6 [55].In the first step of amyloid fibrillogenesis, protein changes from native state to unstable intermediates. Then, aggregation nucleus are formed when a critical concentration of the unstable intermediates is reached. Finally, fibrillation proceeds through a nucleation dependent pathway, resulting in a rapid extension and propagation of the fibrous network. Amyloid fibrillogenesis can be regarded as a self-assembly of amyloid fibrils driven by non-covalent interactions [56]. Zein is capable of self-assembling due to its amphipathicity and noncovalent interactions which are the main driving force in zein network formation [57]. Therefore, there is a certain possibility that amyloid fibrillogenesis occur in the formation of zein network, which can be a new direction for researches on gluten-free systems.
4. Impacting factors on the zein network
Research showed that there were some defects about zein network, which would affect the use of its structural functionality[6,7,8,39,50]. zein network rapidly loses its viscoelasticity in the absence of shear [39]. Total zein is not easy to form viscoelastic network by the mixing method [8]. Zein-based doughs have a limited ability to retain gas [7]. Compared with gluten, zein has a higher Tg[6,50]. Network formation is crucial to the structural functionality of zein, finding approaches that can promote the formation and strengthen the rheology of zein network can improve these defects.These methods can be summarized from four aspects, which are respectively the addition of co-proteins, plasticizers, hydrocolloids,and the increase of system temperature. In what follows, we briefly outline all the above strategies.
4.1 Co-proteins
Co-proteins are a class of proteins that can change the structure and improve the viscoelastic properties of a protein-based material[39,53,58]. Mejia et al. [59] first reported that the content of β-sheet conformation of zein could be increased with the addition of casein(co-protein) resulting in an improvement of zein-starch dough in viscoelasticity [39,58]. As mentioned before, the β-sheet conformation is a critical element of highly viscoelastic zein network, thus it is easy to understand the effect of co-protein on the gluten-free system based on zein. So far, there are few co-proteins that have been identified.Therefore, investigations on co-proteins from different sources should be conducted.
4.2 Plasticizers
Recently, researches had found that the addition of acetic acid or lactic acid facilitated the formation of zein network with uniform ordered fibril structures and improved extensibility and gas holding capacity of zein-starch dough [8,32]. Other researchers also reported that in the existence of acetic acid, total zein can form zein network using the coacervation method [32,38]. According to Berta et al. [11],the most effective way to decrease the Tgof zein was to add citric acid, which also influenced the viscoelasticity of zein network. These studies showed a fact that dilute organic acids as plasticizers have positive effects on zein network formation, which can be attributed to the plasticizing effect of plasticizers.
Plasticizer is a type of low-molecular-weight chemicals that can modify the Tgof protein by means of increasing the flexibility of protein [47]. As mentioned above, the Tgof zein decreased with the increase of moisture content due to the plasticizing effect of water [60].However, with the increase of moisture content of zein, its Tgwill drop but not fall much below the room temperature [6]. Therefore,under the condition of excessive water, zein network and zein-based dough still need a temperature higher than the room temperature.Plasticizers are also used to improve the flexibility of zein films because they are brittle and do not extend well [61]. Plasticizers commonly used in zein films include glycerin, ethylene glycol, fatty acids, monoglycerides, acetyl glycerides, etc [61].
The plasticizing effect of plasticizers can be explained by three theories: lubrication theory—the plasticizer is considered to be a lubricant that promotes movement between large molecules; gel theory—plasticizers block reactions between monomers, including hydrogen bonds, van der Waals forces and ionic forces; free volume theory—plasticizers may reduce the Tgby increasing the free volume of the polymer [62]. The basic idea in these theories is that plasticizers exist between polymer chains and reduce the binding force between the chains [63]. It is worth noting that in addition to plasticization,acetic acid also had a protonation effect on zein, which would lead to partial unfolding, swelling and complete solvation of zein [64]. These changes were thought to enable total zein to form zein network with the addition of water [38].
4.3 Hydrocolloids
Schober et al. [7] found that there was a remarkably increase in viscosity and extensibility of zein network and zein-starch dough when hydroxypropyl methylcellulose (HPMC) was added into zein.Simultaneously, zein network existed in the form offiner fiber. These changes of zein network and zein-starch dough caused by a softening effect of HPMC were verified in the reports by Schober et al. [7] and Andersson et al. [33]. They all suggested that the softening effect of HPMC on zein network and zein-starch dough can be attributed to a lubricating effect as HPMC forms viscous and lubricant-like solutions. Moreover, because HPMC might hinder the connection of protein chain, interactions between protein chains could become weaker. This contributes to the above-mentioned tendency of zein network and zein-starch dough [7].
More recently, there are a growing number of research have found that hydrocolloids can be used as improvers in gluten-free doughs and breads [65]. So, in addition to HPMC, the effect of other hydrocolloids on zein network and zein-starch dough should be studied and discussed, which can be a new direction to improve the structural functionality of zein.
4.4 Temperature
The increase of temperature could also promote zein network formation [34]. The hydrophobic interactions accelerate with increasing temperature, which facilitates the formation of zein network [35].
The four approaches are summarized based on the existing research results, but there are certainly some aspects that are not covered, such as effects of starch, enzyme, fiber, physical or chemical treatment on zein network. Therefore, further work need to be done to promote the formation and strengthen the rheology of zein network,which will help the application of zein in various foods.
5. Application of zein in gluten-free systems
The viscoelasticity of zein network can be obtained after mixing with water above its Tg[6]. Several researches have been conducted to further develop the application of zein on gluten-free systems. Due to the structural functionality of zein, the viscoelasticity of glutenfree doughs and the quality of gluten-free foods can be significantly improved.
Schober et al. [7] had reported that the zein-starch dough was suitable for the production of hearth-type gluten-free rolls, as its capacity to hold the shape during proofing and baking. The addition of zein can enhance the mixing stability of the zein-rice dough, because the water absorption significantly increased as the proportion of zein in the mixtures increased [45]. The rheology of sorghum dough and the loaf volume of sorghum bread were improved after zein was added [33]. The bubble structure in gluten-free doughs became more stable after zein was added [11]. Rice-zein based dough that can be slit into long and thin noodle strands were successfully produced without gluten [66].
These research indicated that zein has great potential in the development of gluten-free foods. Hence, more work should be done to expand the application of zein in gluten-free foods.
6. Conclusions and future directions
Zein has gluten-like structural functionality, since it can form viscoelastic network. Thus, zein has great potential to replace gluten and can be widely used to improve the structural quality of gluten-free foods. However, more studies are needed on zein network-forming behavior, as its formation mechanism cannot be clearly revealed. It is worth noting almost all researches on structural functionality of zein were based on commercial zein that comprised essentially only the α-zein class. Even though β- and γ-zein are also important parts of zein as well as α-zein, only three researches studied the structural functionality of β- and γ-zein. Two of them showed β- and γ-zein were harmful to zein network formation due to their great ability to form disulfide bonds [8,35]. However, another study showed β- and γ-zein had obtained structural functionality after dissolving in glacial acetic acid solution [38], which implied β- and γ-zein dissolved in glacial acetic acid solution accompanied by structural changes.Further studying these structural changes of β- and γ-zein to explain why β- and γ-zein can form protein network would be as a new idea for structural functional research of zein.
- 食品科学与人类健康(英文)的其它文章
- Moringa oleifera Lam. leaf extract mitigates carbon tetrachloride-mediated hepatic inflammation and apoptosis via targeting oxidative stress and toll-like receptor 4/nuclear factor kappa B pathway in mice
- Potential of peptides and phytochemicals in attenuating different phases of islet amyloid polypeptide fibrillation for type 2 diabetes management
- Spectrum-effect relationship of immunologic activity of Ganoderma lucidum by UPLC-MS/MS and component knock-out method
- A red pomegranate fruit extract-based formula ameliorates anxiety/depression-like behaviors via enhancing serotonin (5-HT) synthesis in C57BL/6 male mice
- Purification, characterization and hypoglycemic activity of glycoproteins obtained from pea (Pisum sativum L.)
- Effects of filleting methods on composition, gelling properties and aroma pro file of grass carp surimi

