Anthocyanin profiles and color properties of red wines made from Vitis davidii and Vitis vinifera grapes
Ynlun Ju, L Yng, Xiofeng Yue, Yunkui Li, Rui He, Shenglin Deng,Xin Yng, Yulin Fng,b,*
a College of Enology, Northwest A&F University, Yangling 712100, China
b Shaanxi Engineering Research Center for Viti-Viniculture, Yangling 712100, China
Keywords:
Spine grape
Vitis vinifera L.
Wine analysis
Wine color
Anthocyanin
ABSTRACT
Spine grape (Vitis davidii Foex.) is an important wild grape species native to China. Fifteen red spine grape clones and three red Vitis vinifera grape varieties were used to evaluate the differences in the anthocyanin profiles and color properties of wines made from V. davidii and V. vinifera grapes. Among spine wines,‘Junzi #2’ wine had the highest total phenolic and total anthocyanin, ‘Xiangzhenzhu’ wine had the highest total flavonoids, and ‘Junzi #1’ wine had the highest total tannin. The anthocyanin compositions of all of the spine wines were dominated by Mv-3,5-diglucoside. The total individual anthocyanin contents in spine wines, except ‘Gaoshan #5’, ‘Junzi #5055’, ‘Junzi #5061’, and ‘Junzi #5044’, were significantly higher than in V. vinifera wines. Most of the spine wines had a stronger red intensity and a brighter chroma with a bluer hue than V. vinifera wines. Correlation analysis revealed that the color properties were closely related to the anthocyanin composition. These results suggest that wines made from spine grapes may be useful for the wine industry for their color properties and high individual anthocyanin contents.
1. Introduction
Anthocyanins have many bioactive functions and play an important role in the quality of red grapes and wines [1,2].Anthocyanins are the main source of color in wines and play an important role in the stability of wine color [3]. Anthocyanins can be combined with substances such as tannins to maintain the stability of the color and structure of the wine, which, in turn, give the wine a good taste and color, attracting consumers [3,4].
Anthocyanins are synthesized by the flavonoid pathway and are widely present in different plant tissues [5,6]. In grape skins,anthocyanins are synthesized during ripening from the veraison stage[7]. Anthocyanins in grapes are mainly divided into delphinidin,cyanidin, petunidin, peonidin, and malvidin derivatives [8]. The anthocyanin composition and content of grapes and wines are affected by many factors, such as variety, light, moisture, yeast strain, and fermentation conditions [5,9]. Difference in grape varieties is the direct factor for different anthocyanin components and content in wine. It was found from many studies that anthocyanidin-3-O-monoglucoside is the most abundant in Vitis vinifera varieties, while diglucoside anthocyanins are mainly found in other species, such as Vitis amurensis, Vitis davidii, Vitis quinquangularis, and Vitis rupestris and its hybrids [8,10].
Spine grape (V. davidii Foex.), which is native to China, belongs to the East Asian Vitis spp., and are mainly grown in the subtropical areas (Yangtze River Basin and Yunnan-Guizhou Plateau) of China[11]. Because the one- or two-year-old canes are densely covered with 1-2 mm spines, they are called spine grapes. Spine grapes have been found in different regions of China, such as Yunnan Province,Jiangxi Province, Hunan Province, and Chongqing Municipality[12,13]. In addition to discovering red spine grapes clones such as‘Xiangzhenzhu’, ‘Junzi #1’, and ‘Junzi #2’, the researchers found white spine grape clones called Baiputao [10,13]. The researchers found that spine grapes are rich in phenolics and have a typical aroma[10,12,13], but compared withV. viniferagrapes, spine grapes are mostly used as table grapes, so the development and use of spine grapes are a good commercial prospect. Although we previously reported the phenolic characteristics and antioxidant properties of spine wines [14,15], few studies on spine grapes have been made to date. Studying the characteristics of spine wines is necessary to provide a theoretical basis for commercial use of spine wines.
This study evaluated the anthocyanin profiles and color properties of wines made fromV. davidiiFoex. andV. viniferaL. grapes. The relationship between wine color and anthocyanin components was also analyzed. From a practical standpoint, the results of this study might provide a theoretical basis for further development of spine wines.
2. Materials and methods
2.1 Chemicals and standards
Folin-phenol, gallic acid, rutin, (+)-catechin, andp-DMACA were obtained from Sigma-Aldrich (St. Louis, MO, USA). Standards for anthocyanin compounds were purchased from Sigma-Aldrich(St. Louis, MO, USA). The purities of these standards were > 97%.Formic acid and acetonitrile (HPLC grade) were purchased from Fisher Co. (Fairlawn, NJ, USA). All other chemicals (analytical grade) were supplied by Tianjin Bodi Chemical Reagent Co., Ltd.(Tianjin, China).
2.2 Vinifications
Fifteen red spine grape clones (V. davidiiFoex.) and three redV. viniferaL. grape varieties (including ‘Cabernet sauvignon’,‘Shiraz’, and ‘Marselan’) were collected at commercial maturity from different regions and used for winemaking (see Table 1 for sample information). The naming of each grape clone is determined based on our previous research results, which indicated that the collected samples were from different clones or varieties at the genetic level using the molecular marker method (SRAP) [16]. The vinification method has been previously reported [17]. Briefly, the grapes were sorted and crushed; after pressing, the grape musts were transferred to a 50-L fermenter, and 50 mg/L of SO2and 20 mg/L of pectinase were added. After 24 h, 0.2 g/L of active dry yeast (Saccharomyces cerevisiaestrain Rhone 2323, Lalvin, Denmark) was added. Then,appropriate amount of sucrose was added according to the alcohol of wine. Fermentation was carried out at 23-25 °C. Cap was pressed three times a day, while the temperature and must density were recorded. After alcoholic fermentation, wine samples were collected and stored at -40 °C. Three replicate vinifications were carried out for each grape sample.

Table 1Information of materials.
2.3 Analysis of the general parameters of the wines
Residual sugar, titratable acidity, volatile acids, pH, and alcohol content were determined according to the methods of Wang et al.[18]. The Folin-Ciocalteu method [19] was performed to determine total phenolic content (TPC). Total flavonoid content (TFOC) was determined according to the method of Ivanova et al. [20]. The total flavanol content (TFAC) was estimated byp-DMACA method reported by Li et al. [21]. Total anthocyanin content (TAC) was detected according to the pH differential method reported by Ju et al.[5]. The total tannin content (TTC) was estimated using the methyl cellulose precipitable (MCP) assay [22].
2.4 UPLC-MS analysis of anthocyanin profiles
The analysis of anthocyanin compounds was carried out according to Bindon et al.[23]. The wine samples were filtered through a 0.45 μm inorganic filter and directly subjected to UPLC-MS analysis.Qualitative and quantitative analysis of the samples was performed using an Agilent 1100 series system (Agilent Corporation, CA, USA).A reversed phase column (ZORBAX SB-C18, 3.5 × 250 mm i.d.,1.8 μm) was used.
Chromatographic conditions: mobile phase A: formic acid:acetonitrile:water = 2:6:92; mobile phase B: formic acid:acetonitrile:water = 2:54:44. The mobile phase elution procedure was as follows: 0-0.5 min, 0%-5% B; 0.5-0.6 min, 5%-15% B;0.6-2.5 min, 15% B; 2.5-2.6 min, 15%-18% B; 2.6-5 min, 18% B;5-5.5 min, 18%-5% B; 5.5-8 min, 5%-0% B. The flow rate of the mobile phase was 0.5 mL/min; the column temperature was 30 °C;the detection wavelength was 525 nm; the wavelength was scanned at 200-900 nm; and the injection volume was 30 μL. Mass spectrometry conditions: electrospray ionization source (ESI), positive ion mode.The ion scan range was 100-1000m/z; the atomizer pressure was 35 psi; the dry gas flow rate was 10 L/min; and the dry gas temperature was 350 °C.
Anthocyanin compounds were identified according to the retention time of corresponding external standards, and their concentrations were calculated by building the calibration curves of the area ratio of target compounds to the corresponding external standard against the concentration ratio.
2.5 Wine color analysis
A UV-2450 spectrophotometer (Shimadzu, Japan) was used to analyze wine color according to the methods of Han et al.[15] and Li et al.[24]. briefly, distilled water was used as a blank. The wine samples were filtered through a 0.45 μm filter, and the samples were scanned with a UV-2450 spectrophotometer in the visible light region from 400 nm to 780 nm using a 2-mm path-length quartz cuvette. The illuminant D65 and a 10° observer angle were used to calculate the values ofL*,a*andb*. The calculation formulas for the parametersL*,a*, andb*were performed by the methods of Li et al.[24] and Ayala et al.[25]. The chroma (C*ab), tone (hab), and color difference(ΔE*ab) were calculated using the methods of Wang et al.[18] and Han et al.[15]. Three replicates were carried out for each sample.
2.6 Statistical analysis
Data were reported as mean ± standard deviation (SD) values for the triplicate experiments. Tukey multiple range tests with a significance level at 0.05 or 0.01 were used to analyze the data by SPSS 22.0 software (Inc., Chicago, IL, USA). A wine feature color map was drawn using Fireworks 8 software (Macromedia Co. CA, USA). A heat map of correlation was created by MetaboAnalyst 3.0 [26].
3. Results and discussion
3.1 Physicochemical parameters
The basic physicochemical parameters of the wine samples are shown in Table 2. The titratable acidity of the wine determines the wines pH and affects its color and stability [14]. The titratable acidity of the wine samples ranged from 6.01 g/L to 8.34 g/L; the ‘Gaoshan#2’ wine had the highest titratable acidity content, reaching 8.34 g/L, followed by ‘Junzi #5055’ and ‘Cabernet sauvignon’.The lowest titratable acidity value was found in Junzi #5049 wine at about 6.01 g/L. The titratable acidity of the wine samples varied widely, and the titratable acidities of most spine wines were lower than those of wines made fromV. viniferagrapes. In general, the pH ofV. viniferawine ranges from 2.8 to 3.8 [27], and pH plays an important role in the stability and sensory quality of the wine. The pH values of spine wines ranged from 2.97 (‘Gaoshan #2’ wine) to 4.17 (‘Junzi #1’ wine), which were not significantly different fromV. viniferawines.
Wines volatile acid content can reflect the health of the wine and whether there is a change in the quality of the wine [27,28].As reported,V. viniferawine volatile acid (calculated as acetic acid) ranges from 0.37 g/L to 0.61 g/L [27]. As shown in Table 2,the volatile acid for the wine samples ranges from 0.35 g/L (Junzi#5044 wine) to 0.51 g/L (‘Gaoshan #4’, ‘Gaoshan #5’ and ‘Cabernet sauvignon’ wine).
According to the National Standard of China [29], the alcohol content of wine is ≥ 7.0%. The alcohol content of wines made from spine grapes ranged from 13.5% to 14.8% (Vol), which is statistically similar toV. viniferagrape wines (Table 2).

Table 2Basic physicochemical parameters of wine samples. Three V. vinifera cultivars (‘Cabernet sauvignon’, ‘Shiraz’ and ‘Marselan’) and fifteen spine cultivars/clones(‘Xiangzhenzhu’, ‘Gaoshan #2’, ‘Gaoshan #4’, ‘Gaoshan #5’, ‘Zhilan’, ‘Junzi #1’, ‘Junzi #2’, ‘Junzi #5015’, ‘Junzi #5044’, ‘Junzi #5049’, ‘Junzi #5059’, ‘Junzi#5055’, ‘Junzi #5061’, ‘Junzi #5063’) were studied.

Table 3Phenolics in wine samples. Three V. vinifera cultivars (‘Cabernet sauvignon’, ‘Shiraz’ and ‘Marselan’) and fifteen spine cultivars/clones (‘Xiangzhenzhu’, ‘Gaoshan#2’, ‘Gaoshan #4’, ‘Gaoshan #5’, ‘Zhilan’, ‘Junzi #1’, ‘Junzi #2’, ‘Junzi #5015’, ‘Junzi #5044’, ‘Junzi #5049’, ‘Junzi #5059’, ‘Junzi #5055’, ‘Junzi #5061’, ‘Junzi#5063’) were studied.
3.2 Analysis of phenolics
As p henolics, flavonoids, anthocyanins, and tannins play important roles in the antioxidant activity of grapes and wines [14]. As Table 3 shows, the phenolic compositions—TPC, TFOC, TFAC, TAC and TTC—ofV. davidiiandV. viniferagrape wines were determined.The TPC of the wine samples ranged from 613.33 GAE mg/L to 2 389 GAE mg/L. The TPC was significantly lower for allV. davidiiFoex. varieties and clones than for ‘Cabernet sauvignon’and ‘Shiraz’, and there were consequences for the wine quality.The results of these studies were consistent with previous reports,which found that the TPC ofV. viniferagrape varieties ranged from 1 402 mg GAE/L to 3 130 mg GAE/L [30]. ‘Cabernet sauvignon’wine had the highest TPC value, while wine made from ‘Junzi #5044’clone grapes had the lowest TPC value. The varietal difference in TPC betweenV. davidiiandV. viniferagrape wines suggested that‘Cabernet sauvignon’ wine might have better antioxidant activity than‘Xiangzhenzhu’, ‘Gaoshan #2’, and ‘Junzi #5044’ wines. A significant difference was detected in TAC betweenV. davidiiandV. viniferagrape wines. Wines made from ‘Xiangzhenzhu’, ‘Gaoshan#4’, ‘Junzi #2’, ‘Junzi #5015’, and ‘Junzi #5063’ clone grapes had higher TAC thanV. Viniferagrape wines. A recent study reported that some grape species native to China, includingV. davidiiandV. amurensis, had higher TAC thanV. viniferagrapes [10]. These indicated that spine wines might have the similar health benefits since its phenolic and anthocyanin contents were competitive withV. viniferagrape wines.
The TFAC, TFOC, and TTC contents were significantly lower inV. davidiigrapewines than inV. viniferagrape wines. No significant difference was detected in TFAC content amongV. viniferawines.The TFAC ofV. davidiiwine samples ranged from 20.1 mg/L to 51.92 mg/L. The highest TFOC was found in Shiraz wine, followed by ‘Cabernet sauvignon’. These results were in agreement with those obtained by other studies for Malbec wine from Argentina[31]. Wine made from ‘Junzi #5044’ clone grapes had the lowest TFOC. The TTC of wine samples ranged from 141.19 mg/L to 1 182.86 mg/L. ‘Cabernet sauvignon’ had the highest TTC value,while wine made from ‘Junzi #5049’ clone grapes had the lowest TTC value. A previous study reported that the non-anthocyanin compounds in grape skins, including flavanols and tannins, could be converted to anthocyanins during grape development [32], which might explain the significant differences in TFAC, TFOC, and TTC betweenV. davidiiandV. viniferagrape wines.
3.3 Anthocyanin profiles
Anthocyanins play an important role in the color of grapes and wines [33]. Anthocyanins in grape skins are transferred into wine during fermentation [7]. Han et al.[15] reported that the anthocyanin profiles were similar in different clones of the same variety, but the contents were different. Anthocyanins that have been identified in grapes and wines are mainly pelargonidin (Pg), delphinidin (Dp),cyanidin (Cy), petunidin (Pt), peonidin (Pn), and malvidin (Mv)derivatives [10]. A total of 17 anthocyanins were detected in the wine samples (Table 4). Pg was only detected in ‘Shiraz’ and ‘Junzi#5015’ wines, and was not detected in other wines. The content of the Pg derivative, Pg-3,5-diglucoside, in ‘Junzi #5015’ wine was the highest at 0.08 mg/L and the lowest in ‘Junzi #5064’ and ‘Junzi#5064’ wines at 0.02 mg/L. Dp was detected in all of the wines, and the Dp contents in the spine wines were significantly lower than in‘Cabernet sauvignon’, ‘Shiraz’ and ‘Matheran’. Among spine wines,‘Xiangzhenzhu’ had the highest Dp content at 1.99 mg/L, and the lowest was detected in ‘Junzi #5064’. Three Dp derivatives were detected, including Dp-3,5-diglucoside, Dp-3-glucoside, and Dp-3-rutinoside. The Dp-3,5-diglucoside content in spine grape wines was significantly different. The Dp-3,5-diglucoside content in wine made from ‘Junzi #2’ was the highest at 6 mg/L, and was lowest in ‘Junzi#5055’ wine at 0.11 mg/L. The Dp-3-glucoside contents in spinewines were significantly lower than in ‘Cabernet sauvignon’, ‘Shiraz’,and ‘Matheran’ wines. The lowest Dp-3-glucoside contents were detected in ‘Junzi #5044’ and ‘Junzi #5055’ wines, and the highest in‘Xiangzhenzhu’, reaching 1.17 mg/L. Dp-3-rutinoside was detected in 7 kinds of spine wines, and the Dp-3-rutinoside content in the spine wine was significantly lower than inV. viniferawine. The Cy-3,5-diglucoside contents in spine wines were distinct. Wine made from the ‘Junzi #5055’ grape had the lowest content of Cy-3,5-diglucoside,while ‘Junzi #2’ wine had the highest at 0.88 mg/L. Cy-3-glucoside was only detected in six kinds of wines, and the Cy-3-glucoside contents in spine wines were significantly higher than in ‘Cabernet sauvignon’ and ‘Matheran’ wines. The highest Cy-3-glucoside content was detected in ‘Shiraz’ wine (0.42 mg/L), followed by‘Zhilan’. ‘Matheran’ wine had the lowest Cy-3-glucoside content. Pt was detected in 14 wine samples, and the Pt contents in spine wines were significantly lower than in ‘Cabernet sauvignon’, ‘Shiraz’, and‘Matheran’. Pt-3-glucoside was detected only in ‘Junzi #2’ wine, and the content was significantly lower than inV. viniferawine. Mv and their derivatives were the main anthocyanins in all wine samples.‘Matheran’ wine had the highest Mv content, followed by ‘Junzi#2’ and ‘Junzi #1’. Many researchers reported that 3,5-diglucosides were only found in non-viniferaand hybrid (vinifera× non-vinifera)cultivars [34,35]. The Mv-3,5-diglucoside was the most abundant anthocyanin in spine wine, and the content was significantly higher than inV. viniferawine (Tables 4 and Table 5). These results could be explained by the reports that the activity of methyltransferase enzyme involved in the biosynthesis of Mv-3,5-diglucoside in spine grapes was significantly higher than inV. viniferagrapes [36].The highest Mv-3-galactoside content was detected in ‘Matheran’wine, followed by ‘Junzi #2’ and ‘Junzi #1’. Previous studies reported that the expression levels of key genes, including flavonoid 3’-hydroxylase (F3’H) or flavonoid 3’5’-hydroxylase (F3’5’H),determined the 3-substituted and 3,5-substituted anthocyanin content[34]. The activity levels of key enzymes therefore determined the concentrations of Mv derivatives in grapes [37], so there was a difference in Mv derivative contents in the wine samples. Alcalde-Eon et al. [38] reported that the anthocyanin profile ofV. viniferagrape wine was mainly composed of anthocyanidin monoglucosides with malvidin-3-O-glucoside and its derivatives. However, this study found that the anthocyanin profiles of spine wines were mainly composed of anthocyanin diglucoside (Table 4). Previous report found that the Mv-3,5-diglucoside was the second highest content anthocyanin inV. viniferagrape wine [39]. Our results also found that Mv-3,5-diglucoside was the second largest anthocyanin inV. viniferagrape wines, but the content of this type of anthocyanins in several spine wines was significantly more than that inV. viniferawines. Pt-3-glucoside and Cy-3-glucoside were only detected in several spine wine samples (Table 4). These wines showed the trace level of these anthocyanins, but these anthocyanins could be detected inV. viniferawines [39].

Table 4Anthocyanin profiles in wine samples (mg/L). Three V. vinifera cultivars (‘Cabernet sauvignon’, ‘Shiraz’ and ‘Marselan’) and fifteen spine cultivars/clones (‘Xiangzhenzhu’, ‘Gaoshan #2’, ‘Gaoshan #4’, ‘Gaoshan#5’, ‘Zhilan’, ‘Junzi #1’, ‘Junzi #2’, ‘Junzi #5015’, ‘Junzi #5044’, ‘Junzi #5049’, ‘Junzi #5059’, ‘Junzi #5055’, ‘Junzi #5061’, ‘Junzi #5063’) were studied.
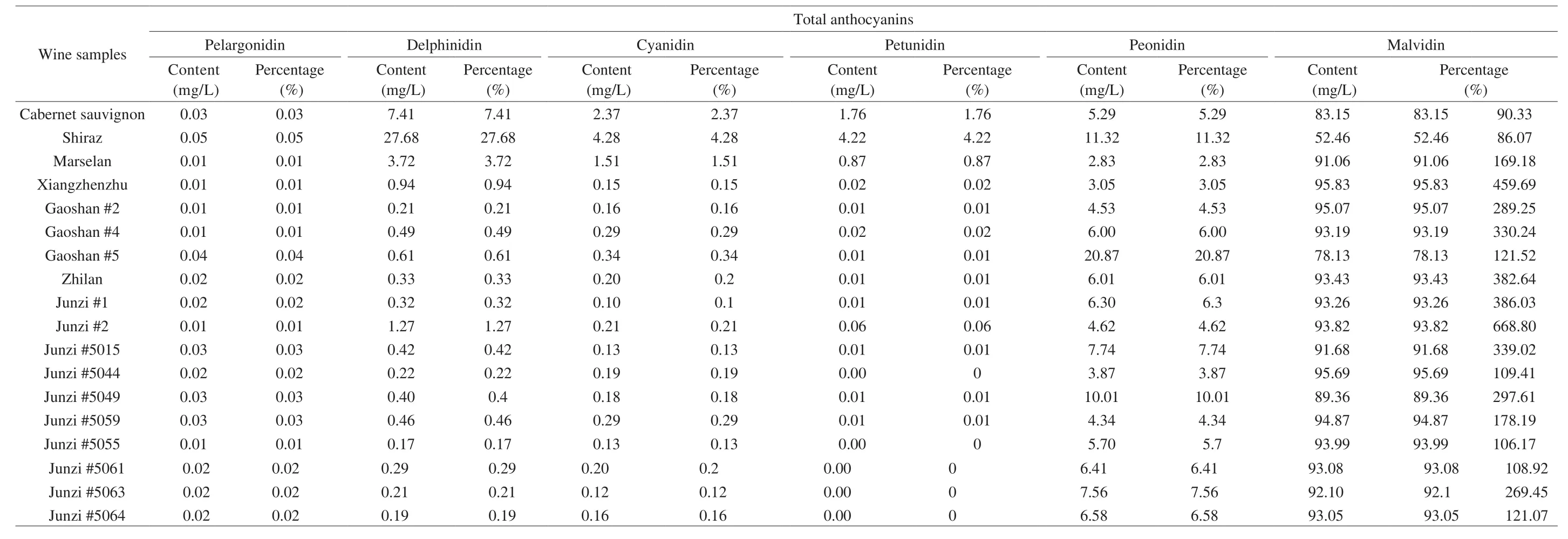
Table 5Contents of different types of anthocyanins in wines. Three V. vinifera cultivars (‘Cabernet sauvignon’, ‘Shiraz’ and‘Marselan’) and fifteen spine cultivars/clones (‘Xiangzhenzhu’, ‘Gaoshan #2’, ‘Gaoshan #4’,‘Gaoshan #5’, ‘Zhilan’, ‘Junzi #1’, ‘Junzi #2’, ‘Junzi #5015’, ‘Junzi #5044’, ‘Junzi #5049’, ‘Junzi #5059’, ‘Junzi #5055’, ‘Junzi #5061’, ‘Junzi #5063’) were studied.

Table 6CIELAB parameter values of wine. Three V. vinifera cultivars (‘Cabernet sauvignon’, ‘Shiraz’ and ‘Marselan’) and fifteen spine cultivars/clones (‘Xiangzhenzhu’,‘Gaoshan #2’, ‘Gaoshan #4’, ‘Gaoshan #5’, ‘Zhilan’, ‘Junzi #1’, ‘Junzi #2’, ‘Junzi #5015’, ‘Junzi #5044’, ‘Junzi #5049’, ‘Junzi #5059’, ‘Junzi #5055’, ‘Junzi#5061’, ‘Junzi #5063’) were studied.
As shown in Table 5, the anthocyanins detected in this study were classified into 6 categories—Pg, Dp, Cy, Pt, Pn, and Mv derivatives—consistent with the results of Liang et al.[10]. Mv derivative contents were the highest in the wine samples, accounting for more than 80% (except for ‘Shiraz’ wine, where they accounted for 52.46%).The total amounts of individual anthocyanins in spine wines were significantly higher than in ‘Cabernet sauvignon’ and ‘Shiraz’. The wine made from ‘Junzi #2’ had the highest total amount of individual anthocyanins, followed by ‘Xiangzhenzhu’.
3.4 Analysis of color properties

Fig. 1 Color and CIELAB characteristic of wine samples. The colored circle below the glass represents the CIELAB characteristic of wine samples. The number below the glass represents the wine samples.
In CIELAB color space, color can be decomposed into three orthogonal independent parameters,L*,a*, andb*.L* indicates the lightness and darkness of the color, whilea* andb* indicate the hue.A separate analysis of these two types of parameters facilitates the visual representation of color features [24]. In wine, as the L* value increases, the brightness of the wine is higher [15,24]. The brightness of spine wine was significantly higher than that of V. vinifera wine(Table 6, Fig. 1). Among spine wines, the color of ‘Junzi #5044’wine was the brightest, with the highest L* value (81.85), and ‘Junzi#2’ wine was the darkest, with the lowest L* value (36.88). For color evaluation, a* represents the red and green color, with values ranging from -100 (green) to 100 (red). The a* values of spine wine differed significantly, and the red component of the color was relatively large.As Table 6 and Fig. 1 show, ‘Gaoshan #2’ wine was the most red and had the highest a* value (72.07), and ‘Junzi #5044’ wine was the least red and had the lowest a* value (23.35). Wines made from‘Xiangzhenzhu’, ‘Gaoshan #2’, ‘Gaoshan #4’, ‘Zhilan’, and ‘Junzi#2’ grapes had a deeper red color and a brighter chroma with higher a* and C*abvalues. In color evaluation, b* indicates the degree of yellow and blue, with values ranging from -100 (blue) to 100 (yellow).The b* values of spine wine were significantly lower than V. vinifera wines. The blue component was relatively larger, so this may be a characteristic of spine wine [15]. V. vinifera wine was marked by more yellow color than spine wine (i.e., higher b* value), which is similar to the results found by Han et al. [15]. All spine wine samples had lower b* and habvalues, which indicates that these samples exhibited a stronger blue color. All wine samples exhibited different color properties, and the color differences among spine wines were significantly higher than those among V. vinifera wines, with a higher ΔE*abvalue. The total color difference ΔE*abincludes the contributions of the three components L*, a* and b*, and characterizes the overall color difference between wine samples.The larger the value is, the more significant the difference is.Generally, the color difference with ΔE*abvalue above 6 will bring a strong sense of color difference [40]. Color properties could be affected by many factors, including grape variety, origin, and vineyard management [41]. It has been reported that spine wines might be more attractive to consumers due to their brighter color,purer chroma, and stronger red intensity [42].

Fig. 2 Feature color of wine samples. (A) The position of the color spots are arranged according to L*. (B) The position of the color spots are arranged according to ΔE*ab. The number represents the wine samples as in Fig. 1.
The traditional CIELAB color analysis method could only give relevant parameters, without giving the feature color and visually representing the color of the wine samples [24]. As shown in Fig.2, the wine samples were represented by a numbered round spot(numbered as in Fig. 1), and the position of the round spot was arranged according to L* and ΔE*ab. The contributions of L*, a*, and b* parameters were combined to give the corresponding coloration of the round spot, thereby giving each wine a characteristic color under natural observation conditions (natural white light, 10° observer angle). The ‘Junzi #5044’ (NO.14), ‘Junzi #5055’ (NO.16), ‘Junzi#5059’ (NO.17), ‘Junzi #5061’ (NO.18) and ‘Zhilan’ (NO.8) wine.
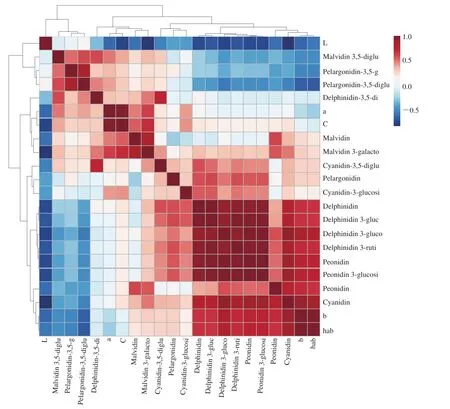
Fig. 3 Correlations of color properties of wine samples with anthocyanins compounds in wines.
Samples had a brighter chroma (Fig. 2A). However, ‘Shiraz’(NO.2), ‘Marselan’ (NO.3) and ‘Xiangzhenzhu’ (NO.4) wine samples exhibited a darker color (Fig. 2A). All spine wines showed more differently color properties than V. vinifera wines, with a higher ΔE*ab(Fig. 2B).
3.5 Correlation of color properties and anthocyanin compounds in wines
The anthocyanin content and composition play important roles for wine color. To clarify the contribution of anthocyanin compounds to wine color, the correlations of color properties and anthocyanin compounds were analyzed. As Fig. 3 shows, the L* values of all samples were significantly negatively correlated with all types of individual anthocyanins. The L* value was also negatively correlated with a*, b*, and C*ab. These results are consistent with those of Han et al. [15] using the PLSR analysis method. A previous study reported that a* and C*abvalues were positively related to anthocyanins in spine wines [15]. In this study, a* and C*abvalues were significantly positively related to the Cy, Cy-3-glucoside, Mv, Mv-3-glucoside,Mv-3,5-diglucoside, Pt, Dp-3,5-iglucoside, Pn-3,5-diglucoside, Pg,and Pg-3,5-diglucoside contents in all wine samples. The a* value was also significantly positively correlated with the hue C*ab. These results indicate that these individual anthocyanins might make an important contribution to the brightness of wine color [41]. The concentrations of Cy, Pt, Pn, Dp, Pn-3-glucoside, Dp-3-rutinoside, Pt-3-glucoside, Dp-3-glucoside, Cy-3-glucoside, and Mv-3-glucoside in wine samples were significantly positively correlated with b* and habvalues, which suggests that these anthocyanins might give a bluer hue to wines [43]. However, the b* and habvalues had a negative correlation with Dp-3,5-diglucoside, Pn-3,5-diglucoside, Pg-3,5-diglucoside, and Mv-3,5-diglucoside in wine samples. Interestingly,the 3,5-substituted anthocyanins had a negative correlation with L*, b*, and habvalues, and a positive correlation with a* and C*abvalues. Torskangerpoll et al. [44] reported that acylation of anthocyanins might contribute to color shifts and the variation in hue. Because the diglucoside anthocyanins in wines were more abundant and stable than the monoglucosides, the diglucoside anthocyanins appear to play a more important role in wine color stability and chroma diversity [10,44].
4. Conclusions
There were no significant differences in the alcohol content, pH,or volatile acids among V. davidii and V. vinifera wines. The total phenolic, total flavonoid, total flavanol and total tannin contents of the spine wines were lower than in V. vinifera wines. Spine wines were rich in individual anthocyanins, with Mv-3,5-diglucoside being the characteristic anthocyanin. Regarding individual anthocyanins,‘Junzi #2’ had the highest Mv-3,5-diglucoside and Mv-3-galacoside content, while ‘Matheran’ wine contained the highest Mv and Pt content. Most of the spine wines had a stronger red intensity and a brighter chroma with a bluer hue than V. vinifera wines. These color properties mainly resulted from anthocyanin profiles, such as Mv-3,5-diglucoside, Pn-3,5-diglucoside, Pg-3,5-diglucoside,and Dp-3,5-diglucoside. The results suggest that red spine grape clones, such as ‘Junzi #2’, could be used as a red cultivar resource for winemaking.
conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
This work was supported by the Fundamental Research Funds for the Central Universities (Grant No. 2452019208); the National Key R&D Program on Monitoring, Early Warning and Prevention of Major National Disasters (Grant No. 2017YFC1502806), and the China Agriculture Research System for Grapes (Grant No. CARS-29-zp-6). We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
- 食品科学与人类健康(英文)的其它文章
- Moringa oleifera Lam. leaf extract mitigates carbon tetrachloride-mediated hepatic inflammation and apoptosis via targeting oxidative stress and toll-like receptor 4/nuclear factor kappa B pathway in mice
- Potential of peptides and phytochemicals in attenuating different phases of islet amyloid polypeptide fibrillation for type 2 diabetes management
- Zein as a structural protein in gluten-free systems: an overview
- Spectrum-effect relationship of immunologic activity of Ganoderma lucidum by UPLC-MS/MS and component knock-out method
- A red pomegranate fruit extract-based formula ameliorates anxiety/depression-like behaviors via enhancing serotonin (5-HT) synthesis in C57BL/6 male mice
- Purification, characterization and hypoglycemic activity of glycoproteins obtained from pea (Pisum sativum L.)

