Effects of common prebiotics on iron status and production of colonic short-chain fatty acids in anemic rats
Fn Zhng, Ken K.L. Yung, Chi KongYeung
a Division of Science and Technology, BNU-HKBU United International College, Zhuhai, Guangdong, China
b Department of Biology, Hong Kong Baptist University, Hong Kong, China
c Animal Science Department, California Polytechnic State University, San Luis Obispo, California, USA
Keywords:
Iron
Non-digestible oligosaccharides
Short-chain fatty acids
Prebiotics
Rat
ABSTRACT
Prebiotics may enhance iron absorption, and one plausible mechanism involves the production of shortchain fatty acids (SCFA) in the colon by intestinal microflora. The objectives of this study were to determine the effects of common commercially-available prebiotics including fructooligosaccharide (FOS),inulin, FOS-inulin mixture, galactooligosaccharide (GOS), and lactulose on the iron status of anemic rats,and to monitor changes in the production of colonic SCFA. Anemic Sprague-Dawley rats receiving a lowiron diet (12 μg Fe/g diet) were supplemented with or without prebiotics (5% m/V in drinking water) for 5 weeks. Hemoglobin concentration in rats supplemented with GOS after 3 weeks (4.3 g/dL) was significantly higher than rats without supplementation (3.7 g/dL), while FOS also significantly increased hemoglobin concentration after 4 weeks (4.1 g/dL vs. 3.7 g/dL). All other prebiotics showed no effects.Anemic rats showed lower overall SCFA production in the colon than normal rats, and only FOS significantly increased the production of the three main SCFA (acetic acid, propionic acid and isobutyric acid) identified in anemic rats, with other prebiotics showing no noticeable trends. Our results suggest that GOS and FOS may slightly improve iron status of anemic rats, but the role of SCFA in the colon is not clear.
1. Introduction
According to the Food and Agriculture Organization (FAO) of the United Nations, a prebiotic is “a non-viable food component that confers a health benefit on the host associated with modulation of the microbiota” [1]. A modified concept by Gibson et al. [2] suggests that a dietary prebiotic is “a substrate that is selectively utilized by host microorganisms conferring a health benefit”. Prebiotics, generally speaking, are non-digestible oligosaccharides that selectively stimulate the growth and activities of specific species of bacteria in the colon, usually bifidobacteria and lactobacilli, with benefits to health [3,4].
Only few compounds of carbohydrates have been regarded as prebiotics, most noticeably, short- and long-chain β-fructans such as fructooligosaccharide (FOS) and inulin, galactooligosaccharide(GOS), and lactulose [5]. FOS and inulin are fructans because they both contain GpyFn (α-D-glucopyranosyl-[β-D-fructofuranosyl]n-1-D-fructofuranoside) and FpyFn (β-D-fructopyranosyl-[β-D-fructofuranosyl]n-1-D-fructofuranoside) [6]. The number of fructose units could range from 2 to more than 70, and fructans contain less than or equal to 10 units are typically considered FOS [6]. While inulin is naturally found in hundreds of different plant foods [7], FOS is usually obtained through hydrolysis of inulin by inulinase [8]. Both inulin and FOS (or their mixtures) are commercially available as ingredients for foods and beverages [9].
GOS, naturally present at a low level in breast milk, is a galactosecontaining oligosaccharide with a terminal glucose unit. Commercially produced GOS is derived from lactose by the transglycosylation action of β-galactosidases. The degree of polymerization of GOS is usually 2-8, with types of linkages being mainly β(1-4), β(1-2),and β(1-6) [10,11]. Lactulose is a synthetic disaccharide that could also be manufactured from lactose by the action of β-galactosidases,but under carefully controlled processing conditions, resulting in the final form of 4-O-β-D-galactopyranosyl-β-D-fructofuranose [12,13].Although non-digestible oligosaccharides generally contain at least three monosaccharide units, lactulose has similar prebiotic properties and therefore, is also conventionally included in the prebiotic class [14].
The effect of prebiotics on mineral absorption including calcium and magnesium have been studied both in animals and humans[11,15-20]. Studies on iron were fewer and results were inconsistent and sometimes contradictory [21-25]. Most research was carried out with fructans including FOS, inulin and FOS-inulin mixture, and to a lesser extent, GOS and lactulose. There is a lack of studies that examine the effects of all these acknowledged prebiotics together in a single experiment.
Iron deficiency affects an estimated 2 billion people worldwide,with the prevalence of iron deficiency anemia at 30%-40% in children and women of childbearing age in developing countries [26,27]. It has been suggested that dietary prebiotics may have an enhancing effect on iron absorption, and one plausible mechanism is the increased production of short-chain fatty acids (SCFA) and other organic acids[28]. An accumulation of these acidic compounds could lead to a decline in pH of the luminal environment, which could enhance iron absorption by reducing iron to its soluble ferrous form, solubilizing elemental iron to its ionized form, and releasing iron from proteincomplexes [16,29].
The objectives of this study were to determine the effects of common commercially-available prebiotics including FOS, inulin,FOS-inulin mixture, GOS and lactulose on the iron status of anemic rats, and to monitor changes in the production of colonic SCFA.
2. Materials and methods
2.1 Animals
Weanling 2-week-old male Sprague-Dawley rats with a mean body weight of < 40 g were purchased from Laboratory Animal Unit of The Chinese University of Hong Kong (Shatin, Hong Kong,China). Upon arrival, they were housed in a temperature-controlled room in polycarbonate cages with stainless-steel cover, on a 12 h dark-light cycle.
2.2 Diets
Commercial purified AIN-93G rodent diets, modified to contain either 12 μg Fe/g diet (low-iron diet) or 45 μg Fe/g diet (regular diet), were prepared by Trophic Animal Feed High-tech Co. Ltd (Nantong, China).
2.3 Chemicals and reagents
Chemicals and reagents used in tissue non-heme iron analysis and SCFA analysis were obtained from Sigma-Aldrich Inc. (Shanghai,China) or Thermo Fisher Scientific (Guangzhou, China) unless stated otherwise. Hydrochloric acid (HCl), Trichloroacetic acid (TCA),sodium 3-(2-pyridyl)-5,6,-bis(4-phenylsulfonate)-1,2,4-triazine(ferrozine), propyl chloroformate (PCF), and DL-2-Methylbutyric acid were purchased from Tokyo Chemical Industry Co. Ltd (Shanghai,China). FOS (BENEO Orafti®P95 Oligofructose, powder), inulin(BENEO Orafti®GR, powder, DP ≥ 10), FOS-inulin mixture (BENEO Orafti®Synergy1, oligofructose-enriched inulin, powder) were obtained from the Food Ingredients Division of Guangzhou DPO Co. Ltd(Guangzhou, China). GOS (King-Prebiotics, powder) was obtained from New Francisco Biotechnology Co. Ltd (Yunfu City, China). Lactulose(Duphalac®) was obtained from K.L. Medicine (Hong Kong). Water used in this study was purified with the Milli-Q Reference Ultrapure water purification system (EMD Millipore, Billerica, MA).
2.4 Experimental design
Upon arrival, 80 weaning Sprague-Dawley rats were divided into 10 groups (n = 8) with equal mean body weights across groups.During the acclimation period, 7 groups received the low-iron diet while the other 3 groups received the regular diet for 14 days (Table 1).All rats had free access to their diets and water. On Day 14, one group from each diet treatment was sacrificed to obtain baseline data on hemoglobin concentration, as well as liver, spleen, kidney and heart non-heme iron levels to con firm iron-deficiency anemia.
At the onset of the feeding trial (Day 15), the 2 remaining“Normal” groups were kept on the same regular diet supplemented with or without an FOS-inulin mixture (Synergy1), while the other 6“Anemic” groups were kept on the same low iron diet with or without supplementation of prebiotics (Table 1). All prebiotics were provided by dissolving in water at 5% (m/V). Rats had free access to their respective test diets and water for 35 days (Day 15-49).

Table 1Experimental design.
Rats were observed daily during the whole study for signs of abnormalities. The body weight of each rat was measured every week,and water consumption was recorded every 1-2 days. Blood samples were drawn weekly for hemoglobin concentration measurements.At the end of the feeding trials, all rats were sacrificed. Rats were first anaesthetized through exposure to diethyl ether. Liver, spleen,kidney and heart were harvested after laparotomy, rinsed with a saline solution (9 g/L), and stored at -80oC until tissue non-heme iron analysis. Rat colon samples (with the colon contents) were also collected, rinsed with a saline solution (9 g/L), and stored at -80oC until SCFA analysis by GC-MS.
The experimental protocol was approved by The Government of The Hong Kong Special Administrative Region, Department of Health (License No. (15-37) in DH/HA&P/8/2/6 Pt. 4). All rat experiments were conducted in animal facilities at Hong Kong Baptist University (HKBU).
2.5 Hemoglobin concentration measurement
Whole blood samples were collected from the tail vein of rats each week during the feeding trial period. A commercial hemoglobin assay kit (Pointe Scientific, Inc., Canton, MI) was used and the manufacturer’s protocol was followed to measure the hemoglobin concentration of the blood samples. briefly, 2.0 mL of commercial hemoglobin reagent and 10 μL of standard (11.5 g/dL) or samples were first mixed in test tubes,and stood for 3 min at room temperature. Absorbance at 540 nm of the samples and the standard was measured by UV-VIS spectrophotometer(Shimadzu UV-1700, Kyoto, Japan). Hemoglobin concentration was calculated using the following formula:
Hemoglobin (g/dL) = (Abs. of sample/Abs. of standard) ×concentration of standard (g/dL)
2.6 Tissue non-heme iron analysis
Tissue non-heme iron levels were determined by the colorimetric method described by Rebouche et al. [30]. briefly, 500 μL of tissue homogenate (prepared in purified water (1:10, m/V) by using a glass homogenizer) from each sample was transferred to a 1.5 mL centrifuge tube and mixed with equal volume of the protein precipitation solution (1 N HCl and 10% TCA solution), followed by heating for 1 h at 95oC. Tubes were then cooled in water for 5 min,vortexed and centrifuged at 12 000 r/min for 10 min. Supernatant of each sample was carefully divided into 2 aliquots of 300 μL (each in a new 1.5 mL centrifuge tube). One tube was mixed with the chromogen solution (0.508 mmol/L ferrozine, 1.5 mol/L sodium acetate and 1.5% (V/V) thioglycolic acid in purified water) for iron chromogenic reaction. The other tube served as the blank was mixed with the sample blank solution (1.5 mol/L sodium acetate and 1.5% (V/V) thioglycolic acid in purified water). After 30 min at room temperature, absorbance was measured at 562 nm by UV-VIS spectrophotometer (Shimadzu UV-1700, Kyoto, Japan).
Standard curves were prepared freshly from iron standard solutions at 0, 2, 4, 6, 8, and 10 μg/mL on the day of tissue analysis.Results were expressed as μg Fe/g tissue (wet weight).
2.7 Short chain fatty acid (SCFA) analysis by GC-MS
2.7.1 Standard solution preparation
Accurately weighed standards of acetic acid, propionic acid,butyric acid, isobutyric acid, pentanoic acid and isopentanoic acid were used to prepare the standard solutions at 8 concentrations: 0.1,1.0, 10, 20, 50, 100, 250, and 500 μg/mL.
2.7.2 Standard pretreatment
One mL of each standard was added to 50 μL of DL-2-methylbutyric acid (the internal standard), vortexed for 2 min and left standing for 2 h. It was then vortexed for 2 more min and centrifuged at 13 000 r/min at 4oC for 20 min. After centrifugation, 500 μL of supernatant was transferred to another centrifuge tube, and 300 μL of purified water, 500 μL of isopropanol/pyridine solution (3:2, V/V)and 100 μL of PCF were added and gently vortexed for 30 s, followed by ultrasonication for 1 min at room temperature for derivatization reaction. A two-step extraction was performed afterwards. First,300 μL of hexane was added to the mixture and vortexed for 1 min,and then centrifuged at 3 000 r/min for 5 min. The upper hexane layer was transferred to a new centrifuge tube. Second, the extraction was repeated by adding another 200 μL of hexane to the mixture residue.The upper hexane layers from the two extractions were combined and mixed with sodium sulfate anhydrous to remove trace of water,vortexed for 30 s and centrifuged at 3 000 r/min for 5 min. The supernatant was drawn for GC-MS analysis.
2.7.3 Sample pretreatment
All sample pretreatment steps were performed at 4oC to preserve the volatile SCFA. The colon samples were first thawed on ice, and colon contents were extruded, weighed and recorded accurately.The colon contents (100 mg) were put into 5 mL glass centrifuge tubes, and 1 mL of 0.005 mol/L NaOH solution and 50 μL of DL-2-methylbutyric acid were added to the tubes. Subsequent steps were the same as for standard pretreatment.
2.7.4 GC-MS analysis conditions
Agilent HP-5 capillary column (30 m × 0.32 mm × 0.25 μm) was used and the injection volume was 1 μL. The initial temperature was held at 60oC for 5 min, then ramped up to 250oC at 10oC/min. Helium was used as carrier gas at the flow rate of 1.0 mL/min. Temperature settings of front inlet, ion source, and transfer line were 280, 230 and 250oC, respectively.
2.8 Statistical analysis
All statistical analyses were done using IBM SPSS Statistics(Statistical Product and Service Solutions, version 21.0). Differences in body weight, water consumption, hemoglobin concentration, tissue non-heme iron, and colonic SCFA content in rats with or without prebiotic supplementation were analyzed by the Student’s t-test.P < 0.05 was considered significant.
3. Results and discussion
3.1 Acclimation and iron-deficiency anemia
After 14 days of acclimation, rats received the regular diet showed a normal iron status with a hemoglobin concentration of 14.0 g/dL, and those received the low-iron diet showed a hemoglobin concentration of 5.0 g/dL. Anemic rats also showed much lower tissue non-heme iron levels in liver, spleen, kidney and heart than normal rats (Table 2), con firming that the low-iron diet induced irondeficiency anemia.
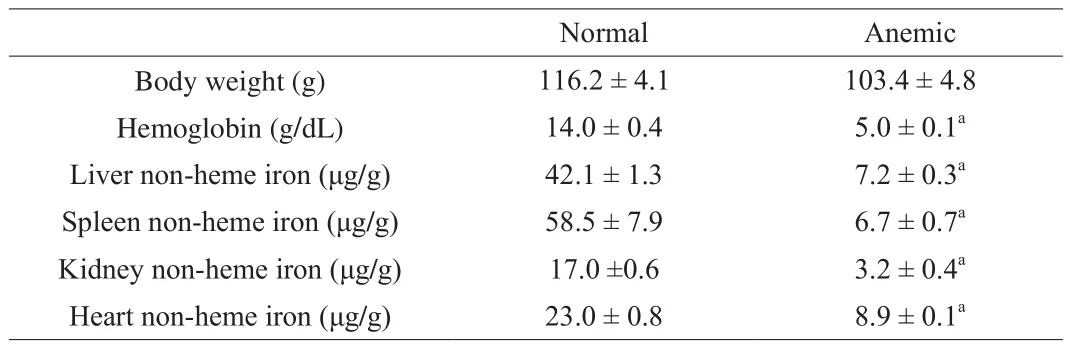
Table 2Body weight, hemoglobin concentration and tissue non-heme iron levels.
3.2 Weight gain and water intake during the feeding trial
In general, all rats gained weight during the feeding trial, although the rate was much slower in anemic rats from Day 22 to Day 49(Table 3). Anemic groups supplemented with Synergy1 or inulin had lower body weight when compared to the anemic group without supplementation on Day 49 (and also on Day 35 for the anemic with Synergy1). There were no significant differences in body weight for other anemic rat groups at any time points or between the 2 normal rat groups (Day 14, Day 21, Day 28, Day 35, Day 42 and Day 49).
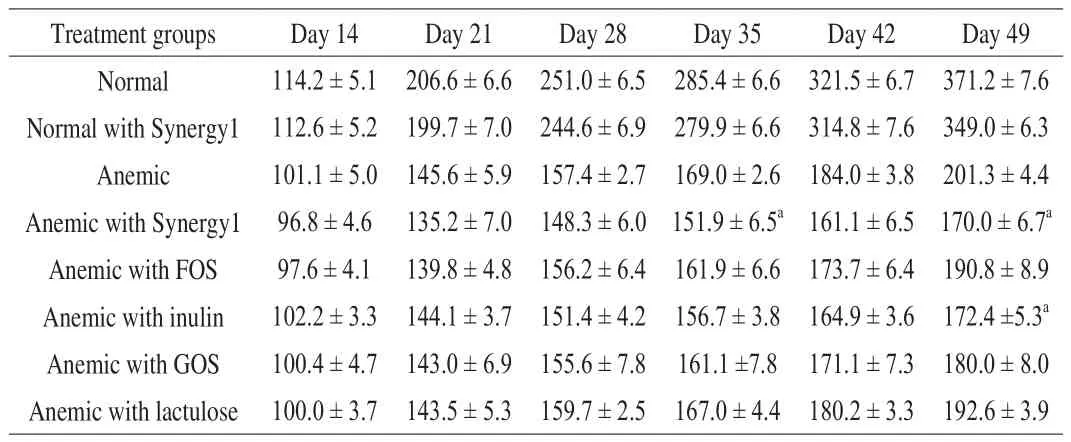
Table 3Body weight (g, mean ± SEM) of normal and anemic rats during the feeding trial.
The average daily water intake showed significant differences among the normal groups and the anemic groups. The normal rat group with Synergy1 consumed less water in the last two weeks(Day 36-49) than the normal group without supplementation(Table 4). Among the anemic groups, the groups supplemented with FOS, GOS or lactulose had lower water intake than the one without supplementation (Table 4). The lactulose group had the lowest water intake during the whole feeding trial, followed by the FOS group which started to show lower intake from the second week (from Day 22)and the GOS group which started from the third week (from Day 29).Nevertheless, none of these groups showed any significant differences in body weight when compared to rats without supplementation(Table 3). In general, prebiotics did not affect the weight gain in normal or anemic rats even if some prebiotics (FOS, GOS and lactulose) caused less water consumption.

Table 4Daily water intake (mL, mean ± SEM) of normal and anemic rats during the feeding trial.
Rats took in roughly 20-30 mL of water daily, and at 5%m/V,it would be equivalent to an intake of 1-1.5 g prebiotics per day during the feeding trial. This level of prebiotics intake was comparable to other published studies [16,31,32]. The level of supplementation in these studies ranged from 2%-10% in diet (m/m)or in drinking water (m/V), with a corresponding intake of 1-1.6 g prebiotics/day based on calculations from their data on feed consumption or water intake.
3.3 Hemoglobin concentration and tissue non-heme iron levels
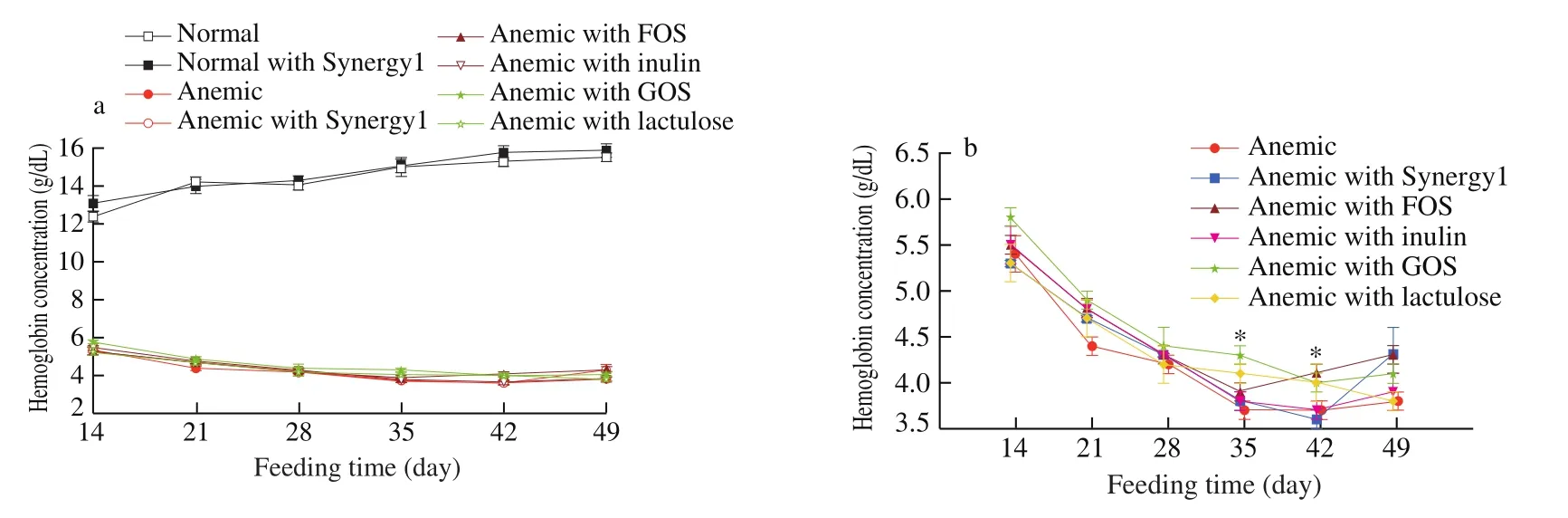
Fig. 1 Weekly changes in hemoglobin concentration (g/dL, mean ± SEM) of (a) normal and anemic rats, and (b) anemic rats only. * Significant difference (P <0.05) when compared to anemic rats without prebiotic supplementation at the same time points (GOS on Day 35, and FOS on Day 42).

Fig. 2 Rat tissue non-heme iron levels (μg/g, mean ± SEM) after the feeding trial (Day 49) in (a) liver; (b) spleen; (c) kidney; (d) heart. *Significant difference(P < 0.05) when compared to anemic rats without prebiotic supplementation.
Our previous study showed that anemic rats fed a regular diet were able to recover in two weeks with and without FOS,suggesting that FOS did not boost iron status in rats already with adequate iron intake [33]. Synergy1, a mixture of 50% shortchain FOS and 50% long-chain inulin, had been reported to be more efficient than inulin or FOS alone in enhancing calcium and magnesium absorption [34,35], and thus was chosen to be included in this study for rats on the regular diet. Normal rats with and without Synergy1 showed no differences either in hemoglobin concentration(Fig. 1a) or tissue non-heme iron levels (Fig. 2). Taken together,prebiotics had no effects on rats with normal iron status, but did not negatively affect iron status either.
Anemic rats on the low-iron diet continued to show low hemoglobin concentration throughout the feeding trial, ranging 3.7-5.8 g/dL (Fig. 1b). Anemic rats with FOS showed a mild but significant increase (P < 0.05) in hemoglobin concentration after 4 weeks (Day 42) compared to anemic rats without supplementation(Fig. 1b). These results are consistent with our previous study that FOS supplementation mildly improves the iron status of anemic rats with a low iron intake [33].
Other anemic rat groups supplemented with prebiotics (Synergy1,inulin, GOS or lactulose) showed somewhat higher values in hemoglobin concentration in general when compared with the anemic group without supplementation (Fig. 1b), but only the GOS group was statistically significant after 3 weeks (Day 35). The GOS group also showed a slight but significant increase (P < 0.05) in liver non-heme iron level (Fig. 2a).
3.4 SCFA analysis of rat colon contents
Rat colon contents collected after sacrifice were further analyzed for SCFA composition using GC-MS. As shown in Fig. 3, the individual SCFA (acetic acid, propionic acid, butyric acid, isobutyric acid, pentanoic acid and isopentanoic acid) in the standard mixture were well separated, validating the conditions used for SCFA detection.

Fig. 3 Chromatogram of the SCFA standard mixture. The retention times for acetic, propionic, butyric, isobutyric, pentanoic, and isopentanoic acids were 2.360, 3.512, 4.160, 4.506, 6.846, and 7.098 min, respectively.
Colon contents from normal and anemic rats were firstly analyzed after two weeks of acclimation (on Day 14). While acetic acid, butyric acid and pentanoic acid in anemic rats were significantly lower than normal rats, propionic acid and isobutyric acid showed no differences between the 2 groups (Table 5). Isopentanoic acid in anemic rats was too low to be detected.
Han et al. [36] reported that rats fed on a normal basal AIN-93 diet showed a relatively higher amount of acetic acid, followed by propionic acid and butyric acid. The same trend was observed in this study. Iron is an essential nutrient for the growth of gut micro flora. It has been shown that luminal iron concentration affects the microbiota composition in the colon [37], and iron-deficiency anemia reduces the relative abundance and diversity of gut microbiota [38]. As proliferation of gut micro flora could be dependent on the presence of bioavailable iron in the colon, our results showed that a low-iron diet led to an overall lower SCFA production.
At the end of the feeding trial (on Day 49), groups supplemented with prebiotics showed significant differences in SCFA concentrations(Table 6). For normal rats, the group with Synergy1 had significant higher acetic and propionic, but lower butyric and pentanoic acid concentrations than the group without supplementation. While for anemic rats, the groups supplemented with Synergy1 or inulin were significantly lower in acetic, propionic and butyric acid concentrations than the group without supplementation, with the inulin group showing overall the lowest SCFA concentrations. In addition, the groups with FOS and GOS had lower butyric acid concentration,and the group with lactulose had lower propionic and butyric acid concentrations.

Table 5SCFA in rat colon contents (μg/g, mean ± SEM) after the acclimation period(Day 14).
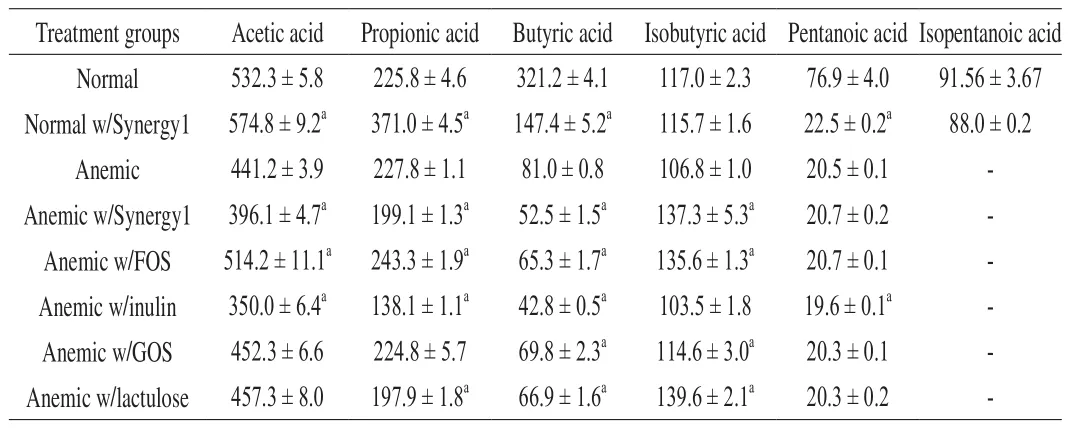
Table 6SCFA in rat colon contents (μg/g, mean ± SEM) after the feeding trial (Day 49).
All anemic groups with prebiotics (except inulin) showed a significantly higher concentration in isobutyric acid, but no differences in pentanoic acid when compared to the group without supplementation (Table 6). The isopentanoic acid concentration for all anemic groups supplemented with prebiotics was too low to be detected. Only FOS significantly increased the concentrations of all three main SCFA (acetic acid, propionic acid and isobutyric acid),when compared to no supplementation. When FOS reaches the colon,presumably it is hydrolyzed and fermented by the resident micro flora,including bifidobacteria and lactobacilli, to produce SCFA and other organic acids (e.g., lactic acid). Rossi et al. [39] showed that acetate and lactate were the major fermentation products in fecal cultures by investigating 55Bifidobacteriumstrains on FOSin vitro.
Propionate has been shown to form a soluble complex with iron to make iron more absorbable [40]. Therefore, higher propionic acid in the colon content of anemic rats with FOS should be supportive of a higher hemoglobin concentration. It is also believed that the fermentation and production of SCFA cause morphological,physiological and molecular changes in the intestine, such as the proliferation of epithelia cells which increases the absorptive area[29]. In the study by Kleessen et al. [41], FOS supplementation led to an increase in intestinal villi and a deeper mucosal crypt in bacterial colonized rats but not in the germ-free group.
While an enhancing effect on rat irons status in anemic groups supplemented with FOS was seen in our results, anemic groups with other common fructans (inulin and Synergy1) showed no such improvement. Although inulin and FOS are the most studied prebiotics and both have been reported to be utilized by different strains ofBifidobacteriumin the gut [42], not all inulin derivatives have the same effects on intestinal microflora and may in fact influence different segments of the large intestine [43]. Inulin and FOS are processed differently in the digestive tracts, thus affecting different types of microbial populations, leading to different digestive or metabolic impacts, including the types of SCFA produced and pH changes in intestine [44,45]. It has also been reported that the structure and chain length of the fructans may exert different effects on mineral absorption. Short-chain FOS is more preferable to bifidobacteria as a substrate for growth because it is more readily fermentable for its lower molecular weight [46,47]. Furthermore, while longer chain inulin may need to reach the distal ileum or the cecum for fermentation, FOS can be fermented not only in the large intestine but also to a greater extent in the small intestine (jejunum and ileum)[48]. Therefore, FOS might provide a more noticeable effect than inulin on iron absorption due to greater fermentation in different parts of the intestinal tract. Sakai et al. [49] reported that 7.5% FOS feeding could increase iron absorption and promote recovery from post-gastrectomy anemia in gastrectomized rats, but 7.5% inulin supplementation did not aid in the recovery. Petry et al. [23] also demonstrated that inulin did not influence fractional iron absorption in women with low iron status.
As for Synergy1, which is a combination of short-chain FOS and long-chain inulin, the enhancing effect was not significant. Similar results were also observed in the study by Freitas et al. [22], when 10% feeding of FOS resulted in a significant higher hemoglobin concentration in rats than the control group, but 10% Synergy1 led to lower value than the control group. Synergy1 had been reported to be more efficient and to have higher activity than FOS or inulin in calcium absorption [34,35], but this enhancing effect on iron absorption was barely found. Yasuda et al. [50] showed that 4%Synergy1 improved hemoglobin synthesis in young pigs on a corn and soybean meal, but because of the diet they used, the observed effect of Synergy1 could be a co-effect of Synergy1 and soybean oligosaccharides. Soybean oligosaccharides are potential prebiotics that have been shown to also stimulate bifidobacteria [51,52].
Although GOS mildly increased hemoglobin concentration in anemic rats after 3 weeks of supplementation, the main SCFA increases (as in anemic rats with FOS) were not observed. Studies on the effect of GOS on mineral absorption were mainly found on calcium and magnesium, seldom on iron. Maawia et al. [53]demonstrated an enhancing effect of 5% GOS feeding on iron,calcium and magnesium after 3 weeks in rats, but van den Heuvel et al. [54] showed that 15 g of GOS, inulin or FOS supplementation to healthy men for 3 weeks had no effect on iron or calcium absorption.There is, nevertheless, evidence supporting GOS as a bifidobacteriapromoting substrate. Rowland and Tanaka [55] demonstrated the bifidogenic properties of GOS when germ-free rats inoculated with human feces was fed with 5% (m/m) GOS for 4 weeks, resulting in a significant increase in bifidobacteria and lactobacilli. Fanaro et al. [56]showed that GOS was bifidogenic when incorporated into a followon formula with no observed side effects in infants of 4-6 months old. In addition, GOS supplementation could increase crypt depth and cell density in the proximal and distal colon [57], providing a larger area for absorption. Therefore, GOS could have a similar effect as FOS in anemic rats, but the role of colonic SCFA in enhancing iron absorption is not clear.
Lactulose could be well-utilized by bifidobacteria as a substrate for fermentationin vitro[58]. It was also shown to stimulate bifidobacteria in rats fed with infant formula containing 0.5%-1% lactulose for 5 weeks with a decrease in fecal pH [59]. However,a follow-up study by the same researchers showed no significant differences in absorption and retention of nitrogen, calcium,phosphorus or iron in rats fed with infant formula with or without 0.5%-1% lactulose [60]. Nevertheless, enhanced calcium and magnesium absorption, as well as decreased cecal and ileum pH, had been observed in rats with higher concentration of lactulose (5%-10%)in the test diet [61-63]. These studies suggest that lactulose has the potential to enhance iron absorption, but further studies are warranted to con firm the dosage required.
4. Conclusions
Supplementation of FOS or GOS might slightly improve the iron status of anemic rats on a low-iron diet, while other prebiotics including inulin, FOS-inluin mixture or lactulose showed no effects.The main colonic SCFA identified in the current study were acetic acid, propionic acid and isobutyric acid in anemic rats (or butyric acid in normal rats), and iron status as well as prebiotic supplementation could change the composition of SCFA in the colon. However,the role of SCFA in enhancing iron absorption and the mechanism involved with the enterocyte need to be further investigated.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgment
The technical assistance provided by the staff at HKBU animal facilities is grateful acknowledged. This project was funded by the National Natural Science Foundation of China (award no. 31171664).
- 食品科学与人类健康(英文)的其它文章
- Moringa oleifera Lam. leaf extract mitigates carbon tetrachloride-mediated hepatic inflammation and apoptosis via targeting oxidative stress and toll-like receptor 4/nuclear factor kappa B pathway in mice
- Potential of peptides and phytochemicals in attenuating different phases of islet amyloid polypeptide fibrillation for type 2 diabetes management
- Zein as a structural protein in gluten-free systems: an overview
- Spectrum-effect relationship of immunologic activity of Ganoderma lucidum by UPLC-MS/MS and component knock-out method
- A red pomegranate fruit extract-based formula ameliorates anxiety/depression-like behaviors via enhancing serotonin (5-HT) synthesis in C57BL/6 male mice
- Purification, characterization and hypoglycemic activity of glycoproteins obtained from pea (Pisum sativum L.)

