Neuroprotective effects of Ginkgo biloba dropping pills in Parkinson’s disease
Dingyi Yu,Pengli Zhng,Junying Li,Ting Liu,Yodn Zhng,Qingqing Wng,Jining Zhng,Xioyn Lu,**,Xiohui Fn,*
aPharmaceutical Informatics Institute,College of Pharmaceutical Sciences,Zhejiang University,Hangzhou,310058,China
bZhejiang University-Wanbangde Pharmaceutical Group Joint Research Center for Chinese Medicine Modernization,Hangzhou,310058,China
Keywords:
Ginkgo biloba dropping pills
Parkinson’s disease
Neuroprotection
Akt/GSK3β
Bax/bcl-2
A B S T R A C T
Parkinson’s disease(PD)is the second most common neurodegenerative disease in the world;however,it lacks effective and safe treatments.Ginkgo biloba dropping pill(GBDP),a unique Chinese G.biloba leaf extract preparation,exhibits antioxidant and neuroprotective effects and has a potential as an alternative therapy for PD.Thus,the aims of this study were to evaluate the effects of GBDP in in vitro and in vivo PD models and to compare the chemical constituents and pharmacological activities of GBDP and the G.biloba extract EGb 761.Using liquid chromatography tandem-mass spectrometry,46 GBDP constituents were identified.Principal component analysis identified differences in the chemical profiles of GBDP and EGb 761.A quantitative analysis of 12 constituents showed that GBDP had higher levels of severalflavonoids and terpene trilactones than EGb 761,whereas EGb 761 had higher levels of organic acids.Moreover,we found that GBDP prevented 6-hydroxydopamine-induced dopaminergic neuron loss in zebrafish and improved cognitive impairment and neuronal damage in methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced PD mice.Although similar effects were observed after EGb 761 treatment,the neuroprotective effects were greater after GBDP treatment on several endpoints.In addition,in vitro results suggested that the Akt/GSK3βpathway may be involved in the neuroprotective effects of GBDP.These findings demonstrated that GBDP have potential neuroprotective effects in the treatment of PD.
1.Introduction
Parkinson’s disease(PD)is the second most common neurodegenerative disease globally and it leads to severe behavioral and cognitive consequences[1].The global incidence of PD is approximately 10-18 per 100,000 persons per year,and the number of individuals over the age of 50 with PD is expected to double between 2005 and 2030[2].Prominent death of dopaminergic neurons in substantia nigra pars compacta with Lewy bodies containingαsynuclein,ubiquitin,and neurofilament aggregation are the main pathological features of PD.This leads to a movement disorder characterized by classical Parkinsonian motor symptoms(tremor,rigidity,slowness,balance problems)and numerous non-motor symptoms,including cognitive impairment,psychiatric symptoms,and sleep disorders[3].Although the exact pathogenetic mechanisms of PD remain unknown,recent studies have shown that PD results from the complicated combination of genetic and environmental factors[4].Mutations in genes like SNCA,LRRK2,GBA,and VPS35areknowntocausePDinanautosomaldominantmanner,and mutations in genes like Parkin,PINK1,DJ-1,and VPS13C are known to cause PD in an autosomal recessive manner.Meanwhile,environmental toxins,such as 6-hydroxy-dopamine(6-OHDA)and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine(MPTP),impair mitochondrial function and cause oxidative stress,leading to PD[5].
As the pathogenesis of PD is complex,current therapies mainly focus on two methods of slowing or stopping the underlying neurodegenerative process.One method enhances the function of dopaminergic neurons by increasing dopamine(DA)concentration,stimulating DA receptors,or inhibiting DA uptake[1].The other method decreases the excitability of cholinergic nerves by blocking striatal cholinergic receptors[6].However,the currently-used drugs employing these methods have many adverse reactions.For instance,DA agonists and levodopa induce nausea,daytime somnolence,and edema[1].DA agonists are also commonly associated with hallucinations[7]and the long-term use of levodopa can result in motor complications and dyskinesias[8].Clozapine is the most effective drug for treating psychosis in PD,but it can lead to potentially life-threatening agranulocytosis[9].Moreover,nonmotor symptoms often have limited treatment options[10].Thus,there is increasing interest in the development of PD treatments with higher efficacy and fewer side effects.
Noticeably,several herbal medicines,such as Ginkgo biloba L.,Panax ginseng C.A.Mey.,and Valeriana officinalis L.,have neuroprotective and antioxidant effects;thus,they may be alternative therapies for PD with higher efficiency and fewer side effects[6,11].G.biloba is one of the most universally used herbal supplements in the world[12].A standardized G.biloba leaf extract,EGb 761,has been used as an antioxidant and a neuroprotective agent to treat various conditions,such as cerebrovascular insufficiency,degenerative dementia,and neurosensory disorders[13].Recent studies in rodent PD models have demonstrated the possible application of EGb 761 in the treatment of PD,since it attenuates the loss of striatal DA levels and prevents neurodegeneration of the nigrostriatal pathway[14].A decrease in levodopa toxicity has also been observed after EGb 761 treatment[15].
Various commercial G.biloba preparations are available,with a standard content of 24% flavonoids and 6% terpene trilactones.However,differences in the preclinical and clinical efficacy of these preparations have been demonstrated[16].One reason for this phenomenon is that these commercial G.biloba preparations have different contents and proportions of active constituents[16].G.biloba dropping pill(GBDP)is a unique G.biloba leaf extract preparation produced in China,with antioxidative and neuroprotective effects in various conditions[17].A previous study identified 21 constituents that differ between EGb 761 and GBDP,mostly belonging to the organic acid and flavonol families[18].Moreover,quantitative analysis of these constituents shows that EGb 761 has higher levels of organic acids compared with GBDP,whereas GBDP has higher levels of flavonoids[18].Nevertheless,the chemical constituents of GBDP have not yet been determined,and little is known about whether GBDP is an effective PD therapy and whether it differs from EGb 761 with respect tothe mechanism and treatment effect.
The aims of this study weretoidentify the chemical constituents of GBDP and to explore the effects and mechanisms of GBDP in the treatment of PD using in vitro and in vivo experiments.We compared the chemical constituents of GBDP and EGb 761 and their protective effects against PD.Forty-six constituents were identified in GBDP,including terpene trilactones,flavonoids,bioflavonoids,and organic acids.Using principal component analysis(PCA)and partial least squares-discriminate analysis(PLS-DA),differences in the chemical profiles of GBDP and EGb 761 were detected.Quantitativeanalysisshowed thatthelevelsof12 constituents(including ginkgolide A,ginkgolide B,ginkgolide C,and bilobalide)differed between GBDP and EGb 761.Moreover,GBDP significantly increased the viability of 1-methyl-4-phenyl-pyridinium(MPP+)-treated SH-SY5Y cells and showed neuroprotective effects in both a 6-OHDA-induced PD model in zebrafish and an MPTP-induced PD model in mice.Although similar effects were observed with EGb 761 treatment,GBDP showed greater protective effects on several endpoints,such as cognitive function improvement,compared with EGb 761.Additionally,the effects of GBDP on PD may be partly mediated by the inhibition of apoptosis by the Akt/GSK3βsignaling pathway.The findings from this study may contribute to the application of GBDP in the treatment of PD.
2.Material and methods
2.1.Chemicals and reagents
GBDPs(batch numbers:A01J180506,A01J150717,A01J151244,A01J151245,A01J151246,A01J151247,A01J151248,A01J151249,and A01J150931)were provided by Wanbangde Pharmaceutical Group Co.,Ltd(Wenling,China).Ginaton® (EGb 761®)tablets(batch numbers:9970716,7890715,7990815,0820617,7710615,6631014,and 7930715)were purchased from Dr.Willmar Schwabe GmbH&Co.KG(Karlsruhe,Germany).Each uncoated GBDP weighed approximately 60 mg and consisted of 16 mg of G.biloba leaf extract and 44 mg of polyethylene glycol 4000,whereas each EGb 761 tablet contained 40 mg of G.biloba leaf extract and excipients,including croscarmellose sodium,silica,hypromellose,lactose monohydrate,and polyethylene glycol 1500.Moreover,GBDP was prepared as the solid dispersions with polyethylene glycol 4000 by melting method.Solid dispersion is an efficient technology to improve solubilization and bioavailability of insoluble drugs including G.biloba leaf extract[19],whereas PEG 4000 is a commonly used carrier matrix for solid dispersion[20,21].It has been demonstrated that G.biloba extract solid dispersions have higher dissolution and faster dissolution rate than natural extract[22].EGb 761 were removed from the film,and then EGb 761 and GBDP were pulverized to homogeneous powders for the following experiments.Detailed information of the other chemicals and reagents is described in the Supplementary Material.
2.2.Qualitative liquid chromatography tandem-mass spectrometry(LC-MS)analysis
2.2.1.Sample preparation
Sample preparationwas performed as previously described[16].A brief description of the method is included in the Supplementary Material.
2.2.2.LC conditions
An Agilent 1200 high performance liquid chromatography(HPLC)system(Agilent Technologies,Santa Clara,CA,USA)with an Agilent Zorbax SB-C18column(1.8μm,4.6 mm × 100 mm)was used for chromatographic separation.The mobile phase was composed of 0.01% formic acid-water(A)and acetonitrile(B),with a gradientelution as detailed inTable S1.The flowrate of the mobile phase was 0.6 mL/min.The column temperature was maintained at 30°C and sample injection volume was 10 μL.The diode array detector scan wavelength was set from 190 to 400 nm.
2.2.3.MS parameters and analysis
Qualitative analysis of the GBDP solution was performed by liquid chromatography-ion trap mass spectrometry(LC-IT-MS)using a Finnigan LCQ DECA XPplusmass spectrometer(Thermo Fisher Scientific,Waltham,MA,USA),with positive and negative electrospray ionization sources.Analysis was performed as previously described[16,23].The procedure is described in the Supplementary Material.
2.2.4.Multivariate statistical analysis of the chemical profiles of GBDP and EGb 761
The chemical profiles of eight batches of GBDP and six batches of EGb 761 were compared based on the relative intensity of the 46 identified constituents.The peaks were aligned to generate a 2-dimensional data table,in which rows and columns represent the samples and the relative peak areas of the 46 constituents,respectively.The resulting data table was imported into SIMCA-P software(version 12.0,Umetrics AB,Malmo,Sweden)for multivariate analysis,using both the unsupervised and supervised pattern recognition methods,i.e.,PCA and PLS-DA.Variable importance plots in PLS-DA were also constructed.

Table 1 Details of the 46 constituents of GBDP.
Table 1(continued)

Table 1(continued)
2.3.Quantitative LC-MS analysis
2.3.1.Sample preparation and LC conditions
The sample preparation and chromatographic separation conditions were the same as described in subsections 2.2.1 and 2.2.2,respectively.The reference solution containing ginkgolide A,ginkgolide B,ginkgolide C,bilobalide,quercetin,kaempferol,isorhamnetin,6-hydroxykynurenicacid,quercetin-3-O-glucoside,kaempferol 3-O-rutinoside,apigenin 7-O-glucoside,and rutin was prepared in 70% methanol.
2.3.2.MS parameters and analysis
Electrospray ionization and an API 4000 triple quadrupole mass spectrometer(AB Sciex Pte.Ltd.,Redwood City,CA,USA)were used for MS analysis in negative ion detection mode with multiple reaction monitoring scan.The ionization source conditions were set at:CAD,6;CUR,35;IS,-4500V;TEM,500°C;GS1,55;GS2,60.The optimized parameters of parent ion,daughter ion,declustering potential,entrance potential,collision energy,and collision cell exit potential are shown in Table S2.
2.3.3.Method validation
The method was validated as previously described[16].The procedure is described in the Supplementary Material.
2.4.Zebrafish culture
Zebrafish husbandry was based on a previous study[24].Wildtype AB and melanin allele-mutant albino zebrafish,provided by Hunter Biotechnology Co.,Ltd(Hangzhou,China),were raised in water(200 mg instant sea salt/1 L of reverse osmosis water;conductivity,480-510μs/cm;pH 6.9-7.2;hardness,53.7-71.6 mg/L CaCO3)at 28°C with a 14 h light/10 h dark cycle,and fed dry flakes once a day and live brine shrimp twice daily.Feeding management met the requirements of the Association for Assessment and Accreditation of Laboratory Animal Care International Certification.
2.5.Zebrafish locomotion behavioral assay
Three hundred thirty wild-type AB zebrafish larvae at 4 days post fertilization(dpf)were randomly divided into 9 experimental groups:vehicle control(10% dimethyl sulfoxide(DMSO),n=60);model(n=60);positive control(1.5μg/mL nomifensine,n=30);GBDP-treated(125,250,and 500μg/mL;n=30);and EGb 761-treated(125,250,and 500μg/mL;n=30)groups.The 500μg/mL dose was the maximum nonlethal concentration for both GBDP and EGb 761.Both GBDP and EGb 761 were dissolved in 10% DMSO.Zebrafish larvae in the GBDP-and EGb 761-treated groups were cotreated with 250μM 6-OHDA and various concentrations of GBDP or EGb 761 for 48 h.Positive control zebrafish larvae were treated with 250 μM 6-OHDA and 1.5 μg/mL nomifensine.At 6 dpf,10 zebrafish were randomly selected in each group for behavioral monitoring using a digital video tracking system(ZebraLab V3,Viewpoint Life Sciences,Civrieux,France)in a 96-well plate.The swimming pattern and total distance travelled were recorded over a period of 30 min.
2.6.Tyrosine hydroxylase(TH)immunostaining of zebrafish
The TH immunostaining procedure is described in the Supplementary Material.
2.7.Mouse model
Animal experiments were approved by the Animal Care and Use Committee of Zhejiang University School of Medicine.Male C57BL/6 mice(6-8 weeks old,18-20 g)were purchased from Shanghai SLAC Laboratory Animal Co.,Ltd(Shanghai,China)and housed under a 12 h light/dark cycle in a controlled environment with food and water provided ad libitum.After 3 days of acclimation,mice were randomly divided into four groups according to body weight,i.e.,vehicle control,model,EGb 761,and GBDP groups(n=5).Mice in the model,GBDP,and EGb 761 groups were intraperitoneally administered MPTP(30 mg/kg/day)for 5 consecutive days,while mice in the vehicle control group were administered the same volume of saline.From day 6 to day 19,mice in the GBDP and EGb 761 groups received oral administration of GBDP or EGb 761 at 50 mg/kg daily(the equivalent dose of G.biloba leaf extract one person receives,i.e.,240 mg daily).Mice in the other groups received carboxymethyl cellulose sodium at 0.1 mL/10 g body weight/day for 14 days.On day 19,a pole test was used to measure motor coordination.From day 20 to day 24,the morris water maze(MWM)test was performed to determine the learning and recall capacity of the mice after 4 days of training,which is a typical timeframe for mice to reach asymptotic performance[25].The pole and MWM tests are described in detail in the Supplementary Material.After the MWM test,three mouse brains from each group were randomly selected,dissected,and fixed in 10% neutralbuffered formalin.Hematoxylin and eosin(HE)staining was performed on the brain sections after embedding in paraffin.
2.8.Cell culture
Human neuroblastoma SH-SY5Y cells(Cell Bank of Shanghai Institutes for Biological Sciences,Chinese Academy of Sciences,Shanghai,China)were cultured in Dulbecco’s modified eagle medium supplemented with 10%(V/V)heat-inactivated fetal bovine serum,100 U/mL penicillin,and 100μg/mL streptomycin in a humidified atmosphere incubator at 37°C with 95% air and 5% CO2.When cells reached 90% confluency,they were detached from the flasks using 0.25% trypsin-EDTA.The subcultivation ratio was set at 1:4.
2.9.Cell viability assay
SH-SY5Y cells wereseeded into 96-well plates at a densityof 4×103cells/well.Firstly,cells wereexposed todifferentconcentrations of GBDP or EGb 761 for 24 h to evaluate their cytotoxicities.The dosages of the two preparations were set at the equivalent dose of 15,30,60,90,120,and 150μg/mL G.biloba leaf extract.Cells were then incubated with 2 mM MPP+and EGb 761,2 mM MPP+and GBDP,or 2 mM MPP+alone for 24 h.The protective effects of different concentrations of GBDP and EGb 761(60,90,and 120μg/mLG.biloba leaf extract)were determined.After 24 h of incubation,100μL of 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide(MTT)solution was added to each well,to a final concentration of 0.5 mg/mL.After 4 h,the medium was removed and formazan crystals in the viable cells were dissolved with 100μL of DMSO.The absorbance of the solution was measured at 580 nmusing InfiniteM1000 Promicroplatereader(Tecan,Zurich,Switzerland).Cell viability in the treatment groups was quantified as a percentage of the vehicle control.For the inhibitor experiment,SH-SY5Y cells were pretreated with a GSK3β-Specific inhibitor(CHIR-99021,10μM)for 2 h.
2.10.Western blot analysis
Cells were seeded into 6-well plates at a density of 3×104cells/well.After treating with 2 mM MPP+and GBDP or EGb 761(90μg/mL G.biloba leaf extract)for 24 h,the Western blot analysis was performed,as described in detail in the Supplementary Material.
2.11.Statistical analysis
Data are expressed as means±standard deviation and were analyzed using Prism 6.0(GraphPad,San Diego,CA,USA).Multiple groups were analyzed by one-way analysis of variance(ANOVA),followed by Dunnett’s multiple comparison test.A Student’s t-test was used to analyze two groups.P<0.05 was considered significant.
3.Results
3.1.Qualitative LC-MS analysis of GBDP and EGb 761
To obtain accurate and comprehensive MS data for chemical identification,LC-IT-MS was used to obtain multistage MS data and liquid chromatography quadrupole time-of-flight mass spectrometry(LC-Q-TOF-MS)was employed to acquire high resolution MS data.Figs.S1A and S1B show representative base peak chromatograms in negative and positive ion modes of LC-IT-MS,respectively.The base peak chromatogram in LC-Q-TOF-MS negative ion mode is shown in Fig.S1C.The main constituents of GBDP were primarily deduced from molecular formulae generated by ultra-performance liquid chromatography quadrupole time-of-flight mass spectrometry,compound cleavage regularity analysis,and compound database alignment.Moreover,the structures were further corroborated and confirmed by MSnfragmentations on HPLC-IT-MS,which allowed the establishment of neutral losses related to functional groups and substitutions.Forty-six constituents,including 5 terpene trilactones,9 quercetin glycosides and their aglycones,6 kaempferol glycosides and their aglycones,3 isorhamnetin glycosides and their aglycones,2 myricetin glycosides and their aglycones,1 syringetin glycoside,2 apigenin glycosides and their aglycones,1 luteolinglucoside,1 genistein,2 bioflavonoids,4 organic acids,and 10 other classes were identified or tentatively characterized(Table 1).The chemical profiles of GBDP and EGb 761 were further compared based on the relative intensityof the 46 identified constituents using PCA(Fig.S2A).For further comparison,PLS-DA was performed and variable importance plots were constructed.Variable importance in projection(VIP)is one of the most frequently used methods for variable selection and the VIP plot indicates the most important variables in the model as a whole[26].Generally,a VIP value>1 in the first component of the PLS-DA model is used to select the candidates[27].Astheresults,GBDPwasclearlyseparatedfromEGb761 in the PLS-DA plot(Fig.S2B).Moreover,based on VIP values>1(Fig.S2C),23 variables were selected as the important constituents for clustering,including 6-hydroxykynurenic acid(1),epi-radicinol(36),ginkgolide A(37),yadanziolide S(26),kaempferol(41),ginkgolide B(38),isorhamnetin(42),quercetin(39),zizybeoside I(2),myricetin(31),quercetin 3-O-[2-O’’-(E)-p-coumaroyl][b-D-glucopyranosyl(1->3)-a-L-rhamnopyranosyl(1->6)]-b-D-glucoside (5),kaempferol 3-O-α-L-(β-D-glucopyranosyl)-(1 → 2)-rhamnopyranoside(24),prunin(27),quercetin 3-O-Glc 7-O-Rha(14),quercetin 3-O-2′′-(6′′-p-coumaroyl)-glucosyl-rhamnoside(29),picrodendrin E(34),bilobalide(30),apigenin 7-O-glucoside(25),ginkgolide C(23),GA-1(44),4-O-(4-hydroxyprenyl)-caffeic acid 4′-O-β-D-glucopyranoside(18),kaempferol 3-O-2′′-(6′′-p-coumaroyl)-glucosyl-rhamnoside(33),and luteolin 3′-O-β-D-glucoside(22).These were the main constituents with the greatest differences between GBDP and EGb 761.

Fig.1.The 12 main constituents of Ginkgo biloba dropping pill(GBDP)and EGb 761.Data were analyzed using Student’s t-test,*P<0.05.
3.2.Quantitative LC-MS analysis of GBDP
3.2.1.Method validation
Calibration curves of all compounds showed good linearity within the test range(r2>0.999,Table S3).Good injectionprecision was obtained,with peak area RSDs of less than 6.0%.Furthermore,intra-and inter-day variations were less than 6.0% for all analytes,and the overall recovery ranged from 93.85% to 104.85%(Table S3),indicating that the method was suitable for determining the contents of the selected 12 constitutes.
3.2.2.Comparison of the contents of 12 constituents in GBDP and EGb 761
Many studies have demonstrated that flavonoids and terpene trilactones are the main active constituents of G.biloba leaf extract preparations[28-31].Thus,we chose the 11 flavonoids and terpene trilactones present at high concentrations in the preparations for quantitative analysis.6-hydroxykynurenic acid was also quanti fied,as a previous report identified that organic acid content may differ between EGb 761 and GBDP[18].The concentrations of these 12 constituents were determined in 8 batches of GBDP and 6 batches of EGb 761,according to the validated method.The concentrations of ginkgolide A,ginkgolide B,ginkgolide C,bilobalide,quercetin,quercetin-3-O-glucoside,kaempferol,kaempferol 3-O-rutinoside,and apigenin 7-O-glucoside were significantly higher in GBDP than EGb 761,whereas the concentration of6-hydroxykynurenic acid was significantly lower in GBDP(Fig.1).However,there was no difference in concentrations of rutin or isorhamnetin between GBDP and EGb 761.
3.3.GBDP rescued 6-OHDA-induced locomotor impairment in zebrafish
According to the results of LC-MS analysis,differences in the chemical constituents existed between GBDP and EGb 761;thus we further explored whether GBDP could benefit PD therapy.The effects of GBDP on PD were first evaluated in the zebrafish model using locomotor analysis with the parameters of swimming trajectory and total distance travelled.A hydroxylated analogue of the neurotransmitter DA,6-OHDA,is toxic due to its high affinity for dopaminergic plasma membrane transporters.Consistent with that in previousstudies[27],zebrafish locomotoractivity was significantly reduced after 6-OHDA treatment,as evidenced by decreases in the swimming trajectory area(Fig.2A)and total distance travelled(Fig.2B).In contrast,the swimming trajectory and total distance travelled were markedly increased after the administration of GBDP,EGb 761,or the positive control,nomifensine,to 6-OHDA-treated zebrafish.significant protective effects were observed after treatment with either GBDP or EGb 761 at doses of 125 and 250μg/mL.However,at 500μg/mL,only GBDP significantly increased the total distance travelled(Fig.2B).

Fig.2.GBDP rescued 6-hydroxy-dopamine(6-OHDA)-induced locomotor impairment in zebrafish.(A)A Viewpoint Zebrabox system was used to test locomotive behavior.The green plot and the red line represent the movement trajectories recorded by the Viewpoint Zebrabox system.(B)Total distance travelled in 30 min.Data were analyzed by one-way ANOVA followed by Dunnett’s test.###P<0.0001,compared with the control group;*P<0.05,**P<0.001,***P<0.0001,compared with the 6-OHDA group.n=10 per group.Nom indicates the positive control nomifensine-treated group.
3.4.GBDP prevented 6-OHDA-induced dopaminergic neuron loss in zebrafish
TH immunostaining was used to determine the viability of dopaminergic neurons in zebrafish after GBDP and EGb 761 treatments.Exposure to 400μM 6-OHDA for 48 h resulted in a significant loss of dopaminergic neurons in zebrafish(Fig.3).However,treatment with 250 or 500 μg/mL GBDP or 250 μg/mL EGb 761,significantly ameliorated 6-OHDA-induced dopaminergic neuron loss.Other doses of GBDP and EGb 761 showed no protection(Figs.3A and B).

Fig.3.GBDP protected against 6-OHDA-induced dopaminergic neuron loss in zebrafish.(A)Representative images of DA neurons in the zebrafish brain,indicated by tyrosine hydroxylase immunostaining.Red arrow:dopaminergic neurons in the zebrafish brain.(B)The area of the dopaminergic neurons calculated for each group.Data were analyzed by one-way ANOVA followed by Dunnett’s test.###P<0.0001,compared with the control group;*P<0.05,**P<0.001 compared with the 6-OHDA group.n=10 per group.
3.5.GBDP improved MPTP-induced cognitive impairment and ameliorated MPTP-induced neuronal damage in mice
Numerous cognitive impairments,including deficits in learning and memory,are common clinical symptoms of PD.Thus,the effect of GBDP on MPTP-induced cognitive impairment in mice was assessed using the MWM test.After 19 days of treatment,the four groups demonstrated no differences in pole test results,indicating that the athletic ability of the PD mice would not influence the results of the MWM test(Fig.4A).After 4 days of training,the GBDP group showed significantly more site crossings than the MPTP group in the probe trial(Fig.4B),suggesting that GBDP improved the spatial memory of PD mice.The EGb 761 group showed a nonsignificant trend towards more site crossings than the MPTP group.

Fig.4.GBDP improves 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine(MPTP)-induced cognitive impairment in mice.(A)T-turn time in the pole test on day 19.(B)Site crossings in the morris water maze(MWM)test.(C)GBDP protected dopaminergic neurons in the MPTP-induced mouse model of Parkinson’s disease.Brain sections were processed for HE staining.Black arrow:nerve fiber bundles are loose and lightly stained and the fiber components are significantly reduced;yellow arrow:a large number of neurons were atrophied and the nuclei were intensely stained;green arrow:glial cells show slight hyperplasia.**P<0.001.Data were analyzed by unpaired,two-tailed Student’s t-test.n=5 per group.
Histopathological examinations were performed in PD mice using HE staining(Fig.4C).The control group exhibited normal histology in the brain,while damage to striatal neurons and glial cell hyperplasia was detected in the MPTP model group.Striatal neurons in the GBDP group were intact and no glial cell hyperplasia was observed,whereas only a small amount of nerve fiber bundle loss was observed in the striatum of the EGb 761 group,suggesting that these two preparations ameliorated MPTP-induced neuronal damage.
3.6.GBDP protected SH-SY5Y cells from the MPP+-induced decrease in cell viability
Human SH-SY5Y cells,which synthesize DA and noradrenaline and express TH,have been broadly utilized in studies of PD and PD drug mechanisms[32].MPP+is the toxic metabolite of MPTP which causes severe Parkinsonism in humans when intravenously injected[33].Therefore,MPP+-induced SH-SY5Y cell injury was used as an in vitro PD model in this study.GBDP and EGb 761 exhibited no cytotoxicity to SH-SY5Y cells at doses ranging from 15 to 150μg/mL(Fig.S3).MPP+(2 mM)significantly decreased SH-SH5Y cell viability,while GBDP and EGb 761 protected cells from MPP+-induced damage,at doses from 60 to 120μg/mL(Fig.5).

Fig.5.GBDP protected SH-SY5Y cells against 1-methyl-4-phenyl-pyridinium(MPP+)-induced toxicity.GBDP and EGb 761 protected SH-SY5Y cells against the MPP+-induced decrease in cell viability.Data were analyzed by one-way ANOVA followed by Dunnett’s test.###P<0.0001,compared with the control group;**P<0.001,***P<0.0001,compared with the MPP+group.n=3 per group.
3.7.GBDP blocked MPP+-induced apoptosis via the Akt/GSK3β signaling pathway
The phosphorylation levels of Akt and GSK3βwere significantly decreased in SH-SY5Y cells after MPP+treatment,while 90μg/mL GBDP treatment induced a significant increase in the amount of phosphorylated Akt Ser473 and GSK3βSer9(Fig.6C and D).At the same dose,EGb 761 had similar effects,but the effect on phosphorylated GSK3βSer9 levels was not significant.The Bax/Bcl-2 ratio was then determined to test whether GBDP provided neuroprotection through an anti-apoptosis pathway downstream of the GSK3βsignaling pathway.The Bax/Bcl-2 ratio was elevated in MPP+-treated SH-SY5Y cells,whereas a marked reduction in the Bax/Bcl-2 ratio was detected in the GBDP group,but not in the EGb 761 group(Fig.6B).To confirm the role of the Akt/GSK3βpathway in the protective effects of GBDP against MPP+-induced SH-SY5Y cell injury,the Specific GSK3βinhibitor,CHIR-99021,was used.CHIR-99021 significantly abolished the neuroprotective effects of GBDP and EGb 761,while CHIR-99021 did not affect cell viability(Fig.6E),indicating that the Akt/GSK3βpathway played an important role in protecting GBDP against MPP+-induced neuron cell injury.
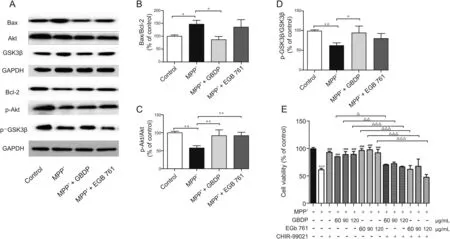
Fig.6.GBDP treatment decreased the Bax/Bcl-2 ratio and increased Akt/GSK3β levels in MPP+-treated human SH-SY5Y cells.(A)Western blot assay of p-Akt,Akt,p-GSK3β,GSK3β,Bax,and Bcl-2.(B-D)Ratio of p-Akt,Akt,p-GSK3β,GSK3β,Bax,and Bcl-2 band intensities.*P<0.05,**P<0.001.(E)SH-SY5Y cells were pre-treated with the GSK3β inhibitor,CHIR-99021(10μM)for 2 h and subsequently exposed to 2 mM MPP+and different concentrations of GBDP or EGb 761.After 24 h,cells underwent an MTTassay.***P<0.0001,compared with the control group.###P<0.0001,compared with the MPP+group.△P<0.05,△△P<0.001,△△△P<0.0001.Data were analyzed by one-way ANOVA followed by Dunnett’s test.n=3 per group.GAPDH:glyceraldehyde-3-phosphate dehydrogenase.
4.Discussion
Current treatments for PD usually induce serious adverse reactions,especially after long-term use.Thus,there is a growing interest in developing alternative therapies with higher efficacy and fewer side effects.This study focused on exploring the neuroprotective effect of GBDP,a unique G.biloba leaf extract preparation produced in China,on Parkinson’s toxin-induced dopaminergic neurodegeneration.The chemical constituents and protective effects against PD were also compared between GBDP and EGb 761.
Chemical analysis identified 46 constituents in GBDP,including terpene trilactones,flavonoids,bioflavonoids,and organic acids.Meanwhile,PCA and PLS-DA identified differences in the chemical profiles of GBDP and EGb 761(Fig.S2).Moreover,quantitative analysis of 12 constituents revealed that although GBDP and EDb 761 followed the same standard(≥24% flavonoids and 6% terpene trilactones),higher levels of several flavonoids and terpene trilactones were detected in GBDP than in EGb 761,whereas EGb 761 had a higher concentration of 6-hydroxykynurenic acid(Fig.1).Previous studies have shown that oral administration of G.biloba extract preparations results in high concentrations of ginkgoflavonoid metabolites and terpene trilactones in the plasma of rodents and humans,and several of these compounds can cross the blood-brain barrier and enter the central nervous system of rats[34-37],indicating their potential importance in mediating the pharmacological effects of G.biloba extract preparations in the brain.Specifically,Rangel-Ordo´n~ez et al.[35]observed that flavonoid metabolites(i.e.,quercetin,kaempferol,and isorhamnetin derivatives)could be detected in the plasma and brain after single and repeated oral administration in rats.These flavonoids were mainly observed in the hippocampus,frontal cortex,striatum,and cerebellum.Ude et al.[36]found that the concentration of ginkgolide A,ginkgolide B,and bilobalide rapidly increased up to 40-98 ng/g in the brain after oral administration,with no difference between the extract and the pure compounds.Furthermore,Cao et al.[38]used G.biloba extract which is the same material and from the same company as GBDP to identify the metabolites from intestinal mucosa of G.biloba extract-treated rats;in this way,53 metaboliteswere identified in intestinalmucosalsamples including terpene lactones,flavone glycosides and their degradation,and part of them could be found in the liver,plasma and brain,indicating these ingredients may be responsible for the pharmacological effects of GBDP.In addition,the activities of the 12 constituents studied here on neuroprotection have previously been demonstrated[28-31,39-46].For instance,ginkgolide homologues have antagonistic effects on platelet-activating factorinduced platelet aggregation and show neuroprotective effects[39].Bilobalide protects against theα-synuclein-induced decrease in cell viability associated with the pathogenesis of PD[40].Antiinflammatory and neuroprotective effects of kaempferol,quercetin,and isorhamnetin have also been previously demonstrated[41-43].6-Hydroxykynurenic acid is a central nervous system amino acid antagonist that acts on N-methyl-D-aspartate to reduce post-ischemic neuronal damage[44].Rutin has been shown to remove the inflammatory component of neurodegeneration[45].Apigenin 7-O-glucoside has anti-inflammatory,antioxidant,and anti-carcinogenic properties[46].Together,these constituents may contribute to the neuroprotective effects of G.biloba,while different levels of these constituents between GBDP and EGb 761 may lead to differences in their efficacy.Future studies should compare the dissolution profiles and pharmacokinetics of GBDP and EGb 761.
The effects of GBDP and EGb 761 against neurodegeneration were further compared in a 6-OHDA-induced zebrafish PD model.6-OHDA treatment induced a locomotordeficit and neuronal loss in zebrafish larvae,which was consistent with that in previous studies[24].Some doses of GBDP and EGb 761 markedly restored dopaminergic neuron loss and improved locomotor activity.However,at 500μg/mL,only GBDP significantly increased locomotor activity and reduced dopaminergic neuronloss.These results indicated that GBDP may have a greater efficacy than EGb 761 for PD treatment.
MPP+/MPTP-induced PD models were also used to assess the neuroprotective effects of GBDP and EGb 761.MPP+significant decreased SH-SY5Y cell survival,as previously reported[47],but this was ameliorated by GBDP or EGb 761 treatment.Moreover,consistent with former studies[48],our data confirmed that MPTP-treated mice had dyskinesia and cognitive impairment.However,GBDP ameliorated MPTP-induced neuronal damage in these mice.By contrast,exposure to EGb 761 had no significant effect on cognitive impairment,but it ameliorated MPTP-induced neuronal damage.A previous study showed that EGb 761 attenuated the decreaseinstriataldopaminelevelsand prevented neurodegeneration of the nigrostriatal pathway in an MPTP mouse model[49].Another study found that EGb 761 improved the MPTP-induced impairment of spontaneous locomotor activity[50].There are some possible reasons for these discrepancies.Firstly,the mice used in our study were 6-8 weeks old,but were 11-13 weeks old in the previous study.Secondly,the EGb 761 dosages and treatment time were different.Thirdly,MWM and HE staining were used in our study,while spontaneous locomotor activity and TH measurement were used in the previous study.Further investigations of the differences in protective effects against PD between GBDP and EGb 761 are needed.
The neuroprotective mechanisms of GBDP and EGb 761 were further explored in MPP+-treated SH-SY5Y cells.The Akt/GSK3β pathway plays a major role in neuronal cell apoptosis and survival[51].Akt directly phosphorylates GSK3βat Ser9 and subsequently inhibits its activity,which then prevents apoptosis[52].Previous studieshave suggested that the levels of p-Akt(Ser473)andp-GSK3β(Ser9)are markedly decreased in invitro and invivo PD models[53].A similar phenomenon was observed in the current study,with MPP+-treatedSH-SY5Y cellsshowingamarked reductioninsurvival.Interestingly,GBDP treatment prevented the down-regulation of p-Akt(Ser473)and p-GSK3β(Ser9)and significantly increased cell viability in MPP+-treated cells.GSK3βinhibitors have recently been shown to bepotential therapeutic agents in PD,indicating that GBDP may be an alternative PD therapy.Moreover,the effect of GBDP on GSK3β was further confirmed using the GSK3β inhibitor,CHIR-99021.Similar effects were observed with EGb 761 treatment,but the effect on p-GSK3βwas not significant.Furthermore,previous studies have shown that dysregulation of members of the Bcl-2 family,such as Bax,is involved in GSK3β-induced neuronal apoptosis[54].Thus,we measured the Bax/Bcl-2 ratio in SH-SY5Y cells after MPP+treatment.In line with previous studies[55],MPP+significantly increased the Bax/Bcl-2 ratio,resulting in a proapoptotic trend in SH-SY5Y cells,but GBDP treatment reversed this effect,suggestingananti-apoptoticeffectofGBDP.However,EGb761 had no significant effect on the Bax/Bcl-2 ratio in MPP+-treated SHSY5Y cells.A previous study showed that EGb 761 prevented the decrease in Bcl-2 levels and the increase in Bax levels in mice with tardive dyskinesia[56].Collectively,these results indicated that the Akt/GSK3βpathway may be involved in the anti-apoptotic effect of GBDP in this in vitro PD model.
5.Conclusions
Taken together,this is the first study to comprehensively analyze the chemical constituents of GBDP,investigate its efficacy as a PD treatment,and compare it with EGb 761.We found that GBDP protected dopaminergic neurons against 6-OHDA and MPTP/MPP+-induced neurotoxicity,and the mechanism might be mediated by the Akt/GSK3βsignaling pathway.Moreover,the contents of 12 main constituents differed between GBDP and EGb 761,and GBDP showed better effects than EGb 761 against PD,especially in an MPTP-induced mice PD model.The findings presented herein provide novel insights into the potential use of GBDP for the treatment of PD.
Declaration of competing interest
The authors con firm that there are no conflicts of interest.
Acknowledgments
The work was supported by the National S&T Major Project(Grant No.2018ZX09201011)and the Key Program from the Sci-Tech Plan of Zhejiang Province(Grant No.2018C03075).We thank Hunter Biotechnology Co.,Ltd.(Hangzhou,China)for technical support of the zebrafish experiments.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2020.06.002.
 Journal of Pharmaceutical Analysis2021年2期
Journal of Pharmaceutical Analysis2021年2期
- Journal of Pharmaceutical Analysis的其它文章
- Current diagnostic and therapeutic strategies for COVID-19
- Chemically modified carbon-based electrodes for the determination of paracetamol in drugs and biological samples
- Development of chromatographic technologies for the quality control of Traditional Chinese Medicine in the Chinese Pharmacopoeia
- Design and preparation of a new multi-targeted drug delivery system using multifunctional nanoparticles for co-delivery of siRNA and paclitaxel
- The effective transfection of a low dose of negatively charged drugloaded DNA-nanocarriers into cancer cells via scavenger receptors
- A dual-signal sensor for the analysis of parathion-methyl using silver nanoparticles modified with graphitic carbon nitride
