A dual-signal sensor for the analysis of parathion-methyl using silver nanoparticles modified with graphitic carbon nitride
Yun Li,Mengqi Wn,Guosheng Yn,Ping Qiu,b,*,Xiolei Wng
aDepartment of Chemistry,Nanchang University,Nanchang,330031,China
bJiangxi Province Key Laboratory of Modern Analytical Science,Nanchang University,Nanchang,330031,China
cInstitute of Translational Medicine,Nanchang University,Nanchang,330088,China
Keywords:
Organophosphorus pesticides
Dual-signal
g-C3N4/AgNPs
Fluorescence
UV-vis spectrophotometry
A B S T R A C T
A highly sensitive and selective method was developed for both UV-vis spectrophotometric and fluorimetric determination of organophosphorus pesticides(OPs).This method used silver nanoparticles(AgNPs)modified with graphitic carbon nitride(g-C3N4).The AgNPs reduced the fluorescence intensity of g-C3N4.Acetylthiocholine(ATCh)could be catalytically hydrolyzed by acetylcholinesterase(AChE)to form thiocholine,which induces aggregation of the AgNPs.This aggregation led to the recovery of the blue fluorescence of g-C3N4,with excitation/emission peaks at 310/460 nm.This fluorescence intensity could be reduced again in the presence of OPs because of the inhibitory effect of OPs on the activity of AChE.The degree of reduction was found to be proportional to the concentration of OPs,and the limit of fluorometric detection was 0.0324μg/L(S/N=3).In addition,the absorption of the g-C3N4/AgNPs at 390nm decreased because of the aggregation of the AgNPs,but was recovered in presence of OPs because of the inhibition of enzyme activity by OPs.This method was successfully applied to the analysis of parathion-methyl in real samples.
1.Introduction
The widespread and long-term use of pesticides has caused serious pollution of agricultural products and the environment,and ultimately endangers ecosystems and human life[1,2].The development of organophosphorus pesticides(OPs)has been rapid since their entry into the market in 1943 because these compounds decompose easily and have a short residual time[3].However,OPs have residual problems,and some varieties cause acute toxicityand delayed neurotoxicity.Since the 1970s,the rate of the research and development of OPs has slowed down considerably.In China,OPs account for 70% of the total pesticides.Excessive or improper application of pesticides is a main cause of food contamination.Acetylcholinesterase(AChE)degrades acetylcholine and also can stop the excitatory effects of neurotransmitters on the postsynaptic membrane and ensure the normal transmission of neural signals in the body,so AChE plays a key role in the biological nerve conduction between cholinergic synapses[4].OPs inhibit the activity of AChE in living organisms,leading to metabolic disorders of acetylcholine,delayed neurotoxicity,movement disorders,coma,paralysis of the respiratory center,and even death[5-7](see Scheme 1).
In recent years,methods using conventional techniques,such as gas chromatography[8],liquid chromatography[9,10],enzyme inhibition methods[5],immunoassays[11-13],and molecularly imprinted polymer(MIP)sensors[14,15],have been developed to detect OPs.Although these methods are effective for the analysis of OPs,there are still some disadvantages,including requiring timeconsuming pretreatment of samples,expensive equipment,and skilled operators.In addition,onsite detection can also be easily affected by externalconditions,including instrumentsand environment.
Currently,optical biosen sors are being developed rapidly[16],and there are many fluorescent sensors now available based on various kinds of nanoparticles,such as upconversion nanoparticles[17,18],non-metallic and metallic quantum dots[19-21],organic dyes[22],and sensors to detect OPs.Bioconjugates based on quantum dot preparations have been developed that can Specifically detect diazinon[23],and fluorescence sensors based on boron nitride quantum dots and gold nanoparticles have been developed that enable the ultrasensitive detection of AChE activity[24].However,these methods require material that takes a long time to synthesize or is formed from a low yielding reaction,which is not suitable for rapid detection.Inthepresentstudy,the fluorescenceofg-C3N4was used to construct a methodusinga combination of ultraviolet and fluorescent double signals to quantitatively analyze OPs.

Scheme 1.Mechanism of detecting organophosphorus pesticides based on g-C3N4/AgNPs.IFE:inner filter effect;AChE:acetylcholinesterase.
g-C3N4,an important polymer semiconductor material,has excellent chemical stability and is environmentally friendly,and has attracted great attention from researchers in recent years[25].g-C3N4is a carbon-based material with a high fluorescence quantum yield[26,27],which can be produced by the direct pyrolysis of carbon-containing and nitrogen-containing organic precursors,such as urea[28],ammonium thiocyanate[29],and melamine[30]in a semi-closed system.Importantly,g-C3N4is the most stable allotrope of carbon nitride and is also a light-stable substance.There have been many reports on pesticide residue analysis using methods based on g-C3N4.Zhang et al.[31]prepared zinc oxide(ZnO)hybridized with graphite-like C3N4(ZnO/g-C3N4)nanoflowers for gas chromatography-mass spectroscopy,which was successfully used for the simultaneous determination of nine pesticide residues in real samples,with satisfactory recoveries of 79.1%-103.5%.Yin et al.[32]proposed a photoelectrochemical(PEC)biosensor based on g-C3N4and CdS quantum dots,which might provide useful information on the carcinogenic mechanisms of pesticides.
In ourwork,adual-signalparathion-methylnanoprobe,combining fluorescence with UV-vis spectrophotometry,was developed to analyze OPs based on g-C3N4modified AgNPs.AgNPs,as fluorescent absorbers,can effectively inhibit the fluorescence of g-C3N4by the inner filter effect(IFE),which results in a decrease of fluorescence.AChE can hydrolyze acetylcholine to form thiocholine(TCh),and the HS groups onTCh can then combine with the AgNPs,causing aggregation of the AgNPs and restoring the fluorescence intensity of g-C3N4.Because of the aggregation of the AgNPs,the absorbance of g-C3N4/AgNPs at 390 nm is decreased from the value for free g-C3N4.However,when OPs are present in the system,the absorbance will be recovered because of the enzyme inhibition of the OPs.Based on this mechanism,a highly sensitive and selective dual-signaling method for detecting parathion-methyl(PM)was established.This detection system can be used to measure PM in vegetable and water samples.
2.Experimental
2.1.Reagents and apparatus
PM was provided by Chem Service(West Chester,PA,USA).Various concentrations were prepared by serial dilution of a stock solution of PM(10μg/L in methanol).Acetylthiocholine iodide(ATCh)and AChE(1323 unit/mg)were purchased from Sigma-Aldrich(St.Louis,MO,USA).ATCh(20mM)solution was newly prepared in dual-distilled water and the service time was less than 3 h.AChE(1 unit/mL)was dissolved with Tris-HCl buffer(pH 7.5,0.02nM).Silver nitrate(AgNO3),sodium borohydride(NaBH4,96%),and sodium citrate(C6H5O7Na3·2H2O)were purchased from Sinopharm Chemical Reagent Co.,Ltd.(Shanghai,China).Melamine was obtained from Aladdin Ltd.(China).All chemicals were analytical grade reagents,all of which were prepared using double distilled deionized water.All glassware was washed three times with aqua regia and double-distilled water.
2.2.Instrumentation
Zeta potentials were obtained using a Nano ZS90(Malvern,UK).The fluorescence spectra were performed on an F-4600 fluorescence spectrometer(Hitachi,Japan).The phosphorescence lifetimes were recorded on a FluoroMax-4 luminescence spectrometer(Horiba,USA).The UV spectra were recorded by using UV-2450 Shimadzu Vis-spectrometer(Hitachi,Japan).Transmission electron microscopy(TEM)measurements were taken on JEM-2100(Japan)and the operational accelerating voltage was at 200 kV.The correlation point and linear resolutions were 0.23nm and 0.14 nm,respectively.A drop of solution on a carbon-coated copper grid to prepare samples of TEM characterized was dried at room temperature.
2.3.Preparation of g-C3N4
g-C3N4was synthesized by the direct thermal treatment of melamine as reported in previous work[30].Melamine(10g)was placed in an alumina cruciblewith a coverand then heated to 600°C witha heating rate of 3°C/min underairconditions,and then kept at that temperature for 2h.After cooling the product to room temperature,yellow g-C3N4powder was obtained.The product was taken out and ground into fine powder using ultrasound treatment of the bulk g-C3N4in water for approximately 20h[33].
2.4.Preparation of AgNPs
According to a literature procedure[34],a 250 mL flat-bottomedflask containing 100 mL of secondary distilled water was placed in an ice-water bath(5°C).Then,AgNO3(250 μL,100mM),BH4Na(6 mL,5mM),and sodium citrate solution(250μL,100 mM)were added tothe flask with rapid stirring.The formation of AgNPs could be preliminarily determined when the color of the solution changed from colorless to light yellow.The reaction was continued for 30 min and the product was placed under dark conditions for 24h until further use.
2.5.Fluorometric detection of PM
Briefly,a series of different concentrations(10μL)of PM and AChE(5μL,1.0 U/mL)were incubated in NaCO3-NaHCO3(45μL,10 mol/L,pH 9.0)at 37°C for 20 min.Next,ATCh(5μL,1.0 mol/L)was added and the mixture was incubated at 37°C for 30 min.Finally,g-C3N4(200μL,3.0mg/mL)and AgNPs(235μL,8.5nmol/L)were added and the resulting solution was made up to 500μL with NaCO3-NaHCO3(10 mmol/L,pH 9.0).The fluorescence emission spectrum was measured and the fluorescence intensity at 460 nm was recorded.
2.6.UV-vis spectrophotometric detection of PM
To assess the possibility of a dual-read assay for OPs,a series of different concentrations of PM(10μL)was added to AChE(6μL,1.0 U/mL)in NaCO3-NaHCO3(138μL,10 mM,pH 10.0)and incubated for 20 min at 37°C.Then,ATCh(6μL,1.0 mmol/L)was added and the solution was made up to 600μL with NaCO3-NaHCO3,and left for 30 min.Finally,g-C3N4(240μL,3.0 mg/mL)and AgNPs(200μL,8.5 nmol/L)were added sequentially.The UV-vis absorption spectrum was measured and the absorption spectrum at 390 nm was recorded.
2.7.Detection of PM in real samples
The application of g-C3N4/AgNPs for the analytical detection of PM in real samples was investigated in water and vegetable samples.Water samples were obtained from Runxi Lake in Nanchang University,Nanchang,Jiangxi Province,China,and food samples were bought from the local market of Nanchang city,Jiangxi Province,China.The pretreatment of these samples was based on methods in previous literature reports[35].First,the samples were spiked with a series of concentrations of PM and kept for 24h.To remove the insoluble matrix,the spiked solution was filtered using a FES membrane(0.22μm).Equal volumes of the filtrate and NaCO3-NaHCO3(10mM,pH 9.0)were mixed and the fluorescence emission and UV absorption spectra of the samples were recorded.
3.Results and discussion
3.1.Characterization of g-C3N4and AgNPs
In this study,AgNPs were used as a fluorophore/absorber pair because their extinction coefficient is 100 times higher than that of gold nanoparticles[36,37].Fig.1A shows the fluorescence emission spectra of g-C3N4and the UV-vis absorption spectra of the AgNPs.It can be seen that there was a strong overlap between the emission spectrum and the absorption spectrum,which provided the necessary condition for the inner filter effect(IFE)[38].IFE refers to the phenomenon that the fluorescence decreases due to the absorption of excitation light or emission light by the phosphor or other light-absorbing substances when the concentration of the phosphor is large orcoexists with otherlight-absorbing substances.The AgNPs prepared by the sodium citrate reduction method were easily absorbed by citrate anions,and the electrostatic repulsion between the AgNPs kept them stable without polymerization.Moreover,it was further supported by the zeta potential value of the AgNPs of-26.4mV,which was consistent with the literature value of the zeta potential of g-C3N4for-28.9mV[38].Therefore,there was no electrostatic attraction between the AgNPs and g-C3N4because both the AgNPs and g-C3N4are negatively charged.In general,the fluorescence lifetime is largely unaffected by the fluorescence intensity.However,if a high-efficiency fluorescence resonance energy transfer(FRET)process is employed,the fluorescence lifetime will change substantially.As shown in Fig.1B,the average fluorescence lifetime of g-C3N4did not change appreciably in the presence of the AgNPs,indicating that energy transferdid not occur between g-C3N4and AgNPs.Fig.1C shows that the fluorescence intensity of g-C3N4decreased gradually as the concentration of the AgNPs increased from 0 to 6 nM.When the concentration of the AgNPs reached 4nM,the maximum reduction in fluorescent intensity was obtained.Therefore,we believe that the decrease in the fluorescence intensity of g-C3N4was because of the IFE rather than FRET,and a 4 nM concentration of AgNPs was used in subsequent experiments.
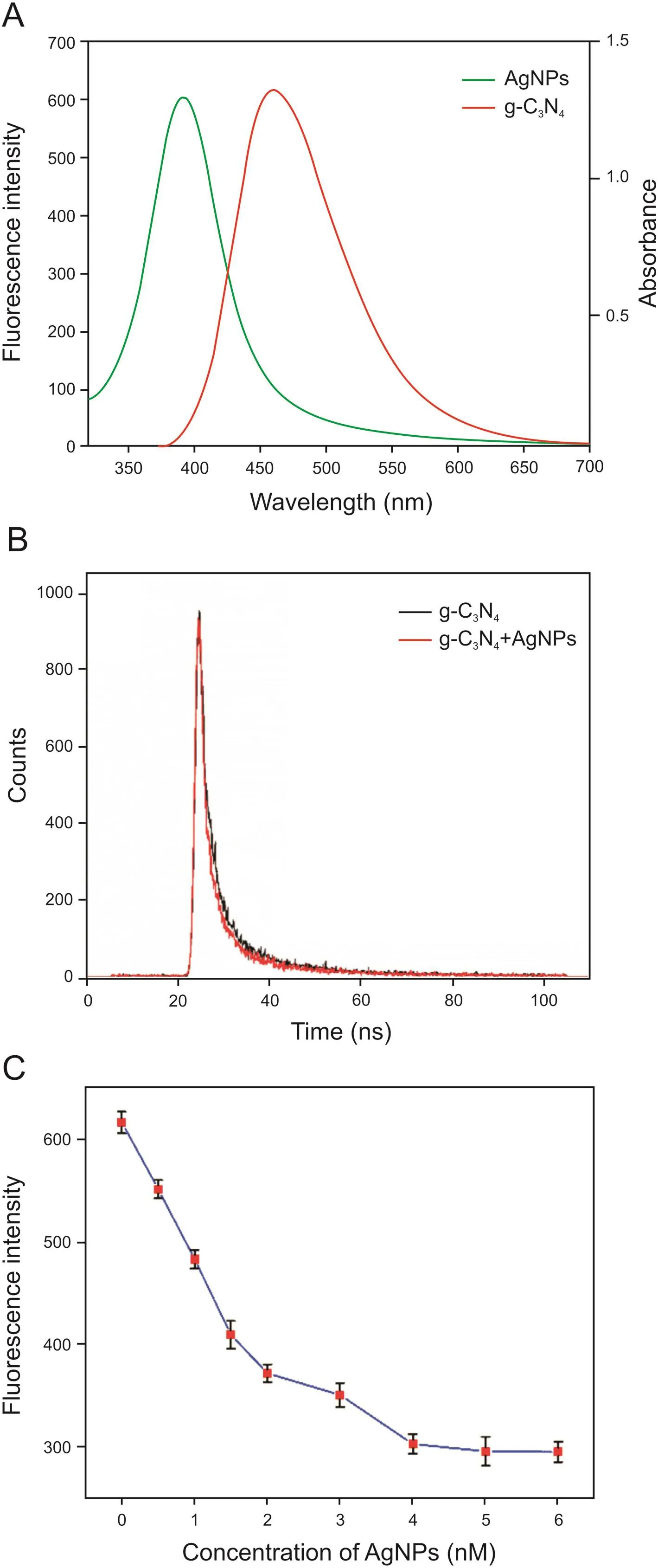
Fig.1.(A)The fluorescence spectra of g-C3N4and the absorption spectra of AgNPs.(B)The fluorescence lifetimes of g-C3N4in presence of AgNPs and g-C3N4in absence of AgNPs.(C)The in fluence of concentration of AgNPs on fluorescence intensity of g-C3N4.
3.2.Mechanism of the dual-signaling determination of PM
It is well known that g-C3N4has strong fluorescence photostability and solubility.Fig.2A shows a comparison of the fluorescence emission spectra of g-C3N4after interaction with different substrates.It is obvious that g-C3N4has strong fluorescence intensity.When the AgNPs were added,the fluorescence intensity decreased,which is because of the IFE of the AgNPs as mentioned above[39].The IFE does not require intermolecular interactions between the absorber and the fluorophore,unlike some widely used conventional sensing mechanisms,such as FRET,thus providing a simple alternative fluorescent nanoprobe[40].AgNPs are effective absorbers in IFE systems because AgNPs have broad absorption bands and a high extinction coefficient in the visible and UV-visible regions[41].

Fig.2.(A)The fluorescence emission spectrum of g-C3N4interacting with different substrates.(B)The absorption spectra of g-C3N4,AgNPs and the mixture with different substrates.
When AChE and ATCh were added to g-C3N4/AgNPs,we found that the fluorescence was recovered.This recovery occurred because ATCh was catalyzed by AChE to produce TCh,and then Ag-S bonds between TCh and the AgNPs were formed,resulting in aggregation of the AgNPs.However,once PM was added to the mixture,the fluorescence of g-C3N4/AgNPs was reduced because of the inhibitory effect of PM on AChE.According to this detection mechanism,we designed a g-C3N4/AgNPs/AChE fluorescent probe to detect OPs.
A similar mechanism can be used to detect PM using UV-Vis spectrophotometry based on g-C3N4/AgNPs.Fig.2B shows the absorption spectra of the AgNPs,g-C3N4,and mixtures of AgNPs and g-C3N4with different substrates.The AgNPs had a strong absorption peak at 390 nm and there was no change in the peak shape after addition of g-C3N4.Addition of AChE and ATCh to the g-C3N4/AgNPs resulted in a sharp drop in absorbance,caused by aggregation of the AgNPs.The absorbance was recovered again when PM was mixed with the solution because of the inhibitory effect of PM on AChE.Interestingly,the absorbance of the solution with g-C3N4was stronger than that without g-C3N4.Therefore,spectrophotometry based on g-C3N4/AgNPs can provide sensitivity high enough for the detection of OPs.
The aggregation behavior of AgNPs in the g-C3N4solution,promoted by TCh generated by the addition of AChE and ATCh,was verified by TEM images.Fig.3A shows a TEM photograph of the dispersion of the AgNPs in a g-C3N4solution.It can be seen that the AgNPs had uniform,spherical,and monodisperse shapes,which were well dispersed in the g-C3N4skeletons.However,as can be seen from Fig.3B,the AgNPs were highly aggregated after addition of AChE and ATCh.Together with the spectral results,these TEM observations confirm our theory of the detection mechanism.

Fig.3.The TEM images of(A)g-C3N4/AgNPs,and(B)AChE and ATCh existing in g-C3N4/AgNPs.Inset:The TEM image of the aggregation state of AgNPs.
3.3.Optimization of the detection conditions for fluorescence analysis
To obtain the optimal sensing response for the detection of PM using g-C3N4/AgNPs for fluorescence analysis,the experimental parameters affecting sensitivity and selectivity were all optimized,including the concentration of g-C3N4and the AgNPs,the pH,and the reaction time.
The optimum values of the reaction parameters could be determined by the reduction efficiency,which was defined as(F0-F)/F0(where F0and Fare the fluorescence intensityof g-C3N4in the presence and absence of the AgNPs,respectively).As the optimal concentration of the AgNPs was previously determined to be 4 nM,the influence of the concentration of g-C3N4was studied.From Fig.4A,it can be clearly observed that the efficiency of the reduction in fluorescence intensity reached its maximum when the concentration of g-C3N4was 1.2 mg/mL.Therefore,a g-C3N4concentration of 1.2 mg/mL was used in further experiments.Then,the influence of pH on the experiment was investigated over a range of pH 5-12.As shown in Fig.4B,the reduction in the fluorescence intensity of g-C3N4/AgNPs reached a maximum at pH 9.0.The reaction time of the fluorescence recovery of g-C3N4/AgNPs also had an in fluence on the response of the nanoprobe.We explored the efficiency of the reduction in fluorescence intensity while varying the reaction time from 0 to 18 min(Fig.4C).The fluorescence intensity of the mixed solution was recorded every 3 minat an excitation wavelength of 310nm.When the time was longer than 12 min,the fluorescence intensity reached a plateau as the reaction reached completeness.Therefore,12 min was used as the measurement time in subsequent experiments.
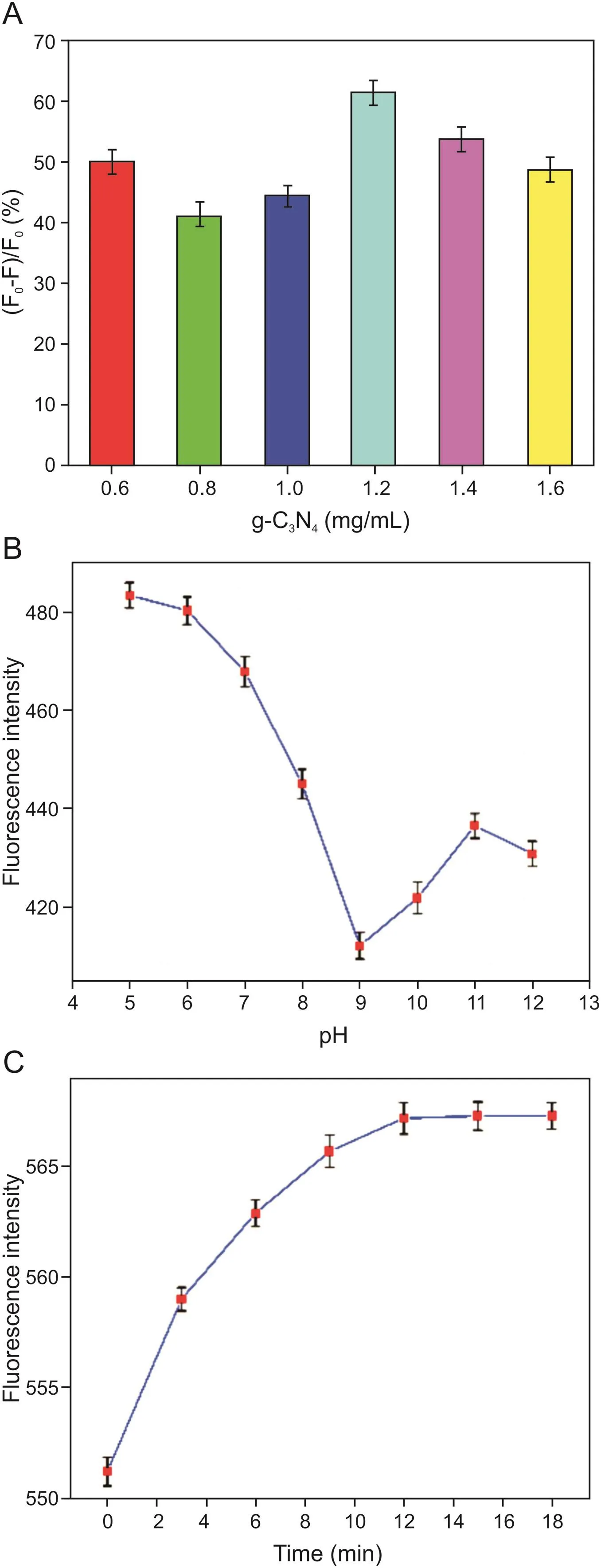
Fig.4.(A)The reduction efficiency of fluorescence intensity of different concentrations of g-C3N4in the presence of AgNPs.(B)The fluorescence intensity of g-C3N4/AgNPs under different pH.(C)The influence of the reaction time of the fluorescence recovery of g-C3N4/AgNPs.
3.4.Optimization of the detection conditions for UV-vis analysis
Similarly,we also optimized the experimental conditions for the UV analysis,as shown in Fig.5.The absorption spectra of g-C3N4/AgNPs in ATCh solution reached a maximum at pH 10(Fig.5A).Because the concentration of ATCh affects the aggregation state of the AgNPs,four different concentrations of ATCh were investigated using reaction time from 0 to 40 min(Fig.5B).It was found that 10μM of ATCh had little effect on the AgNPs,but excessive concentrations of ATCh reduced the absorbance,which may be a result of electrostatic interactions between the positively charged ATCh and negatively charged AgNP molecules leading to aggregation of the AgNPs.

Fig.5.(A)The influence of pH on the absorbance of g-C3N4/AgNPs in ATCh solution.(B)Variation of absorption versus the reaction time for AgNPs with different concentrations of ATCh.
3.5.Specificity of the g-C3N4/AgNPs nanoprobe
To estimate the selectivity of the established method,several potential interferents in water and vegetables were examined.Fig.6 shows the changes in fluorescence intensity and absorbance of the interferents added to the g-C3N4/AgNPs system,with PM(0.5μg/L)as a blank group.It was found that the interferents had little effect on the fluorescence intensity and absorbance of g-C3N4/AgNPs.These results demonstrated that the nanoprobe had a Specific response to PM,regardless of the presence of an interferent.The repeatability of the g-C3N4/AgNPs system was also assessed and the relative standard deviation(n=3)was determined to be less than 4.5%.To analyze the stability,samples containing a PM concentration of 0.5μg/L were stored in the refrigerator at 4°C.After 15 days,the fluorescence intensity was measured and the standard deviation was<2.3%.These results confirmed that g-C3N4/AgNPs systemwas stable and the nanoprobe process had good repeatability.
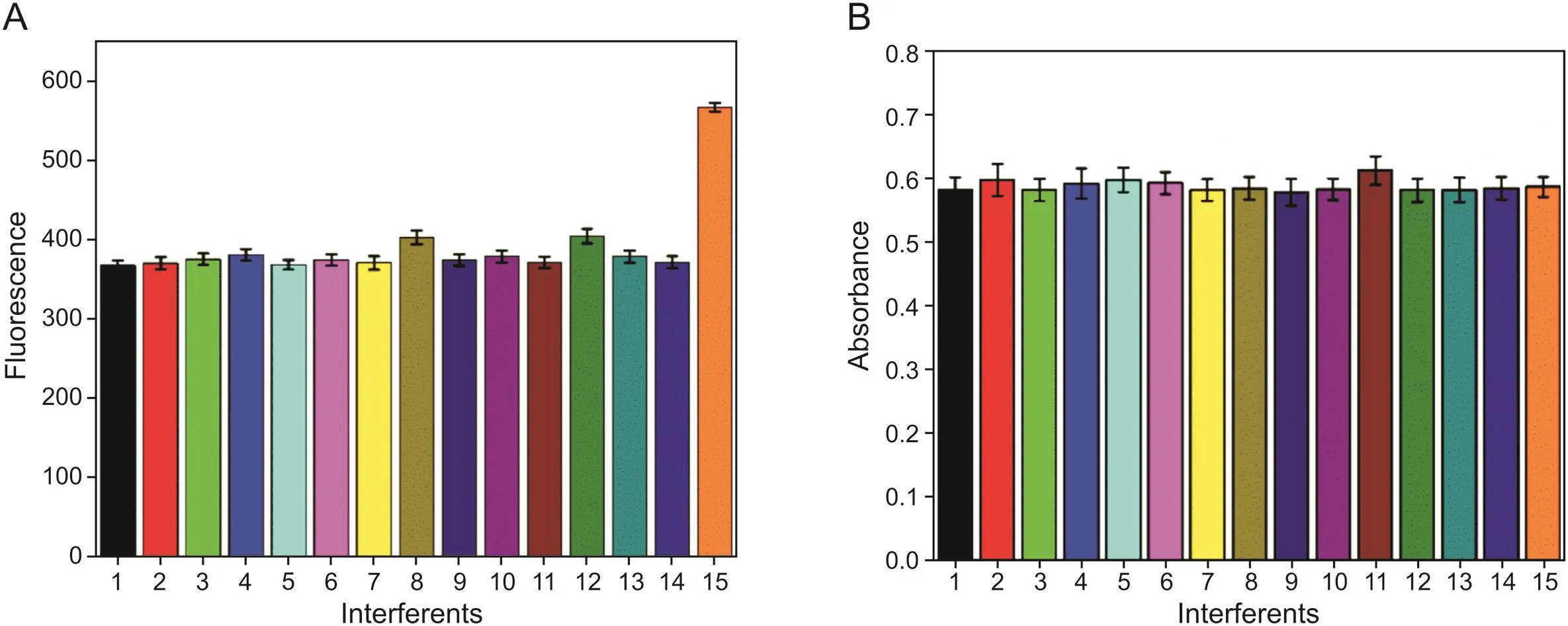
Fig.6.The Specificity of g-C3N4/AgNPs solution with different interferents(1:Cu2+,2:Fe3+,3:Fe2+,4:Al3+,5:Mn2+,6:Ca2+,7:Na+,8:Mg2+,9:K+,10:PO43-,11:HPO42-,12:NO3-,13:Vc,14:VB2,15:PM).(A)Fluorescence,and(B)UV-vis spectrophotometry.
3.6.Dual-signal assay detection of PM
Under the optimal experimental conditions,PM was used as a target analyte using the double reading method.Fig.7A shows the relationship between the PM concentration and the fluorescence intensityovera range of 0.1-1.0μg/L.It can be expressed as follows:y=-325.719x+565.84(R2=0.991)and the limit of detection(LOD)was 0.0324μg/L.It can be seen from Fig.7B that the absorbance of the AgNPs increased with an increasing concentration of PM at 390 nm,and an excellent linear relationship between the absorbance and the PM concentration over the rangeof 0.1-1.0μg/L was observed.The calibration equation can be expressed as y=0.6323x+0.56313(R2=0.990)and the LOD was 0.056μg/L.In addition,a comparison between our work and various other reported methods of analysis[42-47]is shown in Table 1,indicating that our method can achieve relatively high sensitivity and has a low detection limit.

Table 1 Comparison of the present method with other methods for pesticides determination.

Fig.7.Response to PM at increasing concentrations and the linear calibration plot for PM.(A)Fluorescence,and(B)UV-vis spectrophotometry.
3.7.Analysis of PM in real samples
To evaluate the feasibility of the use of the double reading method for real samples,a series of different concentrations of PM in water,apple,and carrot samples was analyzed.The results are shown in Table 2.The results indicated that the percentage of recovery from both spectral methods was in the range of 76%-120% and the relative standard deviation(RSD,n=3)values were found to be<5.3%.These experimental results indicated that the nanoprobe for the detection of PM has a great practical value in food and environmental applications.

Table 2 Detection of PM spiked in real samples.
4.Conclusion
We have constructed an environmentally friendly and sensitive dual-signal nanoprobe(using UV-vis and fluorescence assays)based on g-C3N4/AgNPs to detect organophosphorus pesticides.The mechanism of the experimental method was confirmed by a series of characterizations using TEM images,the fluorescence spectrum,UV-vis absorption spectroscopy,fluorescence lifetime measurements,and zeta potential measurements,and the method was successfully applied to pesticide residue detection in real samples.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China(Grant No.21765015,21808099 to P.Qiu,31860263 to X.Wang)and the Science and Technology Innovation Platform of Jiangxi Province(Grant No.20192BCD40001),China.
 Journal of Pharmaceutical Analysis2021年2期
Journal of Pharmaceutical Analysis2021年2期
- Journal of Pharmaceutical Analysis的其它文章
- Current diagnostic and therapeutic strategies for COVID-19
- Chemically modified carbon-based electrodes for the determination of paracetamol in drugs and biological samples
- Development of chromatographic technologies for the quality control of Traditional Chinese Medicine in the Chinese Pharmacopoeia
- Design and preparation of a new multi-targeted drug delivery system using multifunctional nanoparticles for co-delivery of siRNA and paclitaxel
- The effective transfection of a low dose of negatively charged drugloaded DNA-nanocarriers into cancer cells via scavenger receptors
- Curcumin encapsulated dual cross linked sodium alginate/montmorillonite polymeric composite beads for controlled drug delivery
