Chemically modified carbon-based electrodes for the determination of paracetamol in drugs and biological samples
Wf Boumy,Nwl Toufik,Mouni Achk,Noureddine Brk
aSultan Moulay Slimane University of Beni Mellal,Research Group in Environmental Sciences and Applied Materials(SEMA),FP Khouribga,B.P.145,25000,Khouribga,Morocco
bChouaib Doukkali University,Ecole Nationale des Sciences Appliqu´ees,Laboratoire des Sciences de l’Ing´enieur pour l’Energie,El Jadida,Morocco
cChemical&Biochemical Sciences,Green Process Engineering,CBS,Mohammed VI Polytechnic University,Ben Guerir,Morocco
Keywords:
Paracetamol
Electrochemical sensors
modified electrodes
Pharmaceutical samples
A B S T R A C T
Paracetamol is a non-steroidal,anti-inflammatory drug widely used in pharmaceutical applications for its sturdy,antipyretic and analgesic action.However,an overdose of paracetamol can cause fulminant hepatic necrosis and other toxic effects.Thus,the development of advantageous analytical tools to detect and determine paracetamol is required.Due to simplicity,higher sensitivity and selectivity as well as costefficiency,electrochemical sensors were fully investigated in last decades.This review describes the advancements made in the development of electrochemical sensors for the paracetamol detection and quantification in pharmaceutical and biological samples.The progress made in electrochemical sensors for the selective detection of paracetamol in the last 10 years was examined,with a special focus on highly innovative features introduced by nanotechnology.As the literature is rather extensive,we tried to simplify this work by summarizing and grouping electrochemical sensors according to the by which manner their substrates were chemically modified and the analytical performances obtained.
1.Introduction
Paracetamol(PCT)or acetaminophen is one of the major medicines used for the treatment of numerous cold and fluremedies.It has been used for many decades as an analgesic and as an effective treatment for the relief of fever,headaches and pains.At the therapeutic dose(50-100μM),PCT does not have serious side effects and is an effective substitute for patients who are sensitive to aspirin[1].However,overdosing has frequently a severe cause of fatal hepatotoxicity and nephrotoxicity related to renal failure[2,3].To avoid any complication,very accurate measurement of PCT is vital to controlling drugs and biological samples.
Several conventional techniques have been performed to determine PCT,including high performance liquid chromatography(HPLC)[4],gas chromatography[5],thin layer chromatography[6],micellar electrokinetic chromatography[7],spectrophotometry[8],spectrofluorimetry [9], mass spectroscopy [10], chemiluminescence[11],capillary electrophoresis[12]and colorimetry[13].Most of these techniques are time consuming,require complex equipment,or are costly.The development of electrochemical techniques can overcome some of these limitations because of their prompt response,simple equipment,higher sensitivity and selectivity as well as being cost-efficient,easily miniaturized with lower required power and usually no sample treatments.
Recent studies have extensively investigated the determination of PCT by electrochemical methods based on its oxidation reaction.The oxidation of PCT was first described in the works of Kissinger et al.,and Miner et al.[14,15],and later confirmed in other research works[16-19].This oxidation occurs via two-proton and twoelectron exchangeprocesstogiveN-acetyl-p-benzoquinoneimine(Scheme 1).Generally,the PCT oxidation at conventional working electrodes is irreversible and requires overpotentials,which decreases the sensitivity of detection[17].To resolve this problem,working electrodes are chemically modified with electrocatalysts to reduce the overpotential required and to enhance the electron transfer rate[18].Current evidence indicates that in the presence of chemical modifiers such as nanomaterials,the electrochemical oxidation of PCT becomes reversible or quasireversible due to the rapid electron transfer rate.
Chemically modified electrode(CME)is an electrode made of a conducting or semiconducting material that is coated with a selected monomolecular,multimolecular,ionic,or polymeric film of a chemical modifier[20].CME is coupled to a transducer,which converts its interaction with the analyte into a measurable electrochemical signal(Fig.1).One of the advantages of the CMEs is that they require the use of a minimum quantity of reagents for analysis.Another advantage is the reduction of interference which provides better selectivity of the analysis.

Scheme 1.Oxidation reaction of paracetamol to N-acetyl-p-benzoquinone imine.

Fig.1.Schematic representation of a chemically modified electrode,its interaction with the analyte,and the transduction of these interactions into measurable signals.
There are hundreds of research articles available in literature where CMEs have been used for the electrochemical detection of PCT.Recent papers have focused on the use of nanomaterials because of their unique properties for the modification of electrodes.Cernat et al.[21]reviewed the different configurations based on chemically modified carbon nanotubes(CNTs)and graphene(Gr)for PCT detection.Despite this great review article,the chemical modifiers used for the PCT detection are not limited only to this category.Furthermore,with trends in nanotechnology,different materials have recently been introduced.In the present review,we report the different modification materials used for the detection of PCT in pharmaceutical and biological samples with more or less combinations of few well-known materials such as CNTs,Grand its derivatives,fullerene,carbon quantum dots(CQDs),metal or metal oxides nanoparticles(NPs),metal organic frameworks(MOFs)andconductivepolymers(Fig.2).Wehave thoroughly discussed the properties of the modified electrode surfaces that enhance their electrocatalytic activity towards PCT.The detection of PCT in the presence of other interfering agents has also been focused.Information related to the performance of different approaches and their advantages/disadvantages for PCT determination is presented in tables.

Fig.2.Various sensing materials used in electrochemical detection of PCT.
2.Electrochemical sensing with carbon-based electrodes
Carbon-based electrodes,especially glassy carbon electrodes(GCEs),carbon paste electrodes(CPEs)and screen-printed carbon electrodes(SPCEs),are the most common working electrodes used for the electrochemical sensing of PCT.Their success is mainly due to the minimum necessary overpotential required for the estimation of PCTand the possibilities of mediator modification.The CPEs,initially reported by Adams[22],are widely utilized to determine PCT due to their low residual noise and current,wide anodic and cathodic potential ranges,lowcosts,ease of fabrication and prompt surface renewal.They have become one of the most popular electrode materials used for the electrochemical sensing applications[23].Generally,the sensitivity of unmodified CPEs is low and the limits of detection(LODs)are notadvantageous for routine analyses[22].Inspired by the work of Yamada and Sato[24],GCEs become very popular in the design of electrochemical sensors,due to their lowoxidation rate and high chemical inertness as well as extremely low pore sizes[25].It is pertinent to mention here that there are onlytwo reports available in literature[26,27],wherethe bareGCEs have been used for the determination of PCT.In contrary,the chemically modified GCEs are the most used in electrochemical sensing of PCT.
The further advancement in carbon-based electrodes has led to the development of SPCEs as a disposable electrode in various electroanalytical sensing applications.In contrast to other conventional electrodes,SPCEs have all the three electrodes system including reference,counter and working electrodes on a single platform[28].They have attracted more attention compared to other conventional electrode systems due to low cost,miniaturization of sensing process,disposability and portability.Besides,their microliter-level demand for sample and good repeatability makes them an ideal analytical platform for point-ofcare sensing.
The modification of electrochemical sensors with nanomaterials,from the oldest CPEs to GCEs and SPCEs,is carried out by different processes as schematized in Fig.3.Drop-cast electrodes have most frequently been used for PCT-sensing applications because of the simple modification process.Drop-casting modi fication consists deposition of a suspension of nanomaterial and suitable binders or solvents on the surface of the electrode toform a film on the surface of the electrode[29,30].Another approach to electrode modification is electrochemical deposition using an electrode/organic-phase/aqueous electrolyte three-phase junction.It is widely used for designing modified electrodes using various nanomaterials including CNTs,Gr,metallicNPs,conducting polymers as well as their composites[31,32].Electrochemical polymerization is also demonstrated to be a beneficial method that is used for the thin-film coating of polymers on the electrode surface.It consists the oxidation or reduction of a monomer in solution to an activated form that polymerizes to form a polymer film directly on the electrode surface[33].Paste mixing is another practical method for CPE modification which consists of simple mixing of the modifier with carbon paste using a suitable binder[23].The comparison of advantages and disadvantages of the various approaches is given in Table 1.It is important to select the best choice among different available techniques for the fabrication and characterization of modified electrodes to achieve better analysis results.The choice of an approach depends solely on the economical possibilities,analytical requirements,type of the electrode and nature of the modifier.

Fig.3.Common procedures used to modify carbon-based electrodes.
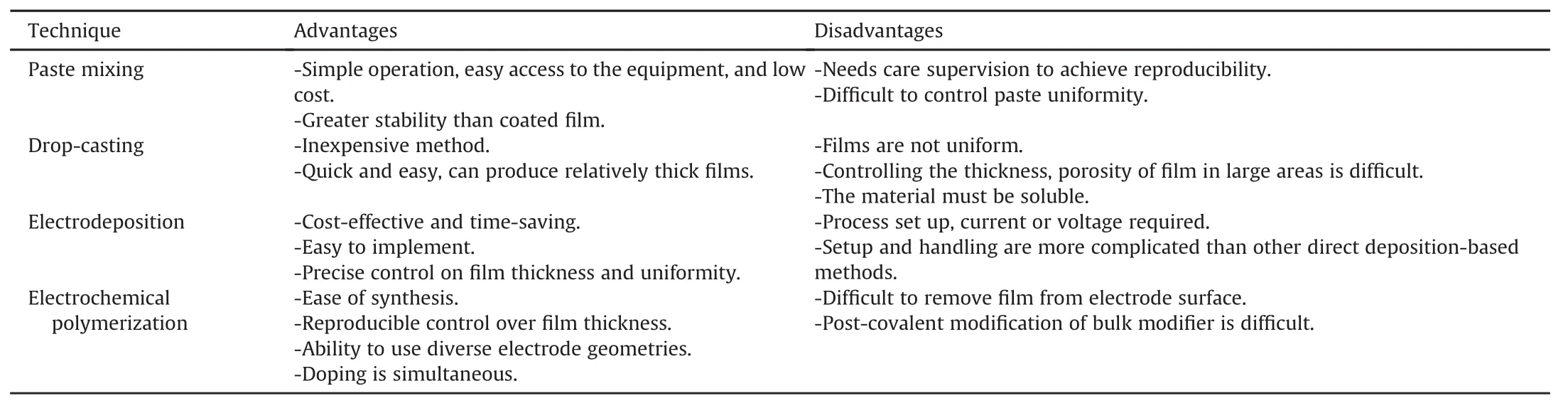
Table 1 Advantages and disadvantages of the commonly used modification procedures.
3.Carbon-based nanomaterials modified electrodes for PCT determination
Carbon-based nanomaterials are widely explored as electrode modifiers in sensors fabrication.They provide the opportunity to be combined with other nanomaterials for improved sensitivity and selectivity of the modified surfaces.This family is composed essentially of Gr,CNTs,CQDs and fullerene.Table 2[17,34-48]summarizes types of electrodes,modifiers,analytical figures of merits,and the advantages/disadvantages of each modifier used for PCT determination.

Table 2 Figure of merits of the carbon nanomaterials modified electrodes used in paracetamol analysis.
3.1.Gr,graphene oxide(GO)and reduced GO
Gr is a two-dimensional material consisting of sp2carbon atoms bonded in hexagonal configuration.Gr,the thinnest material known in the universe,is flexible and conducts electricity at room temperature more efficiently than any other materials[34].The oxidized derivative of Gr is GO,which is plentiful in carboxylic groups with many advantages in electrochemical field[49].GO modified GCE electrode shows good linearity of PCT concentration and very low LOD as documented by Alagarsamy et al.[35].However,GO-based materials have relatively low conductivity and catalytic activity compared to Gr.Different configurations have been chosen to introduce Gr on the electrodes to get altered effects.Typically,Gr is prepared by mechanical stripping,chemical vapor deposition or reduction of GO via a variety of routes such as chemical(CRGO),thermal(TRGO)and electrochemical(ERGO)[50].
In a study done byBahramipuret al.[34],CPE was modified with Gr by the addition of Gr into the carbon paste mixture for square wave voltammetry(SWV)detection of PCT.A quasi-reversible redox process at the modified electrode was obtained,and the over-potential of PCT decreased significantly compared with that of the bare CPE.The Gr/CPE has a linear range up to PCT concentration of 143 μM with a LOD of 0.6 μM.In another study,Kang et al.[36]deposited Gr suspension on the GCE surface for the determination of PCT in pharmaceutical tablets.Gr/GCE exhibited a low LOD of 0.032μM,good recoveries from 96.4% to 103.3% without interference from dopamine.Furthermore,Gr nanoflakes modified GCE was also used for the simultaneous determination of PCT and pentoxifylline by using amperometric method.The linear range was found to be 0.001-150μM and the LOD obtained was 0.00043μM.The electrode also provides high selectivity in the presence of an excess concentration of easily oxidizable interfering biological molecules and good recoveries in drug samples[37].
Gr used in electrochemical PCT sensors is commonly obtained by the chemical reduction of GO,and its deposition on surface of electrodes has typically been achieved via drop-casting.This procedure has intrinsic limitations such as a lack of control over film thickness and most importantly,toxic chemical reducing reagents in the preparation of Gr.On the other hand,the electrochemical approach is simple,green and fast for the reduction of GO to Gr.This method does not need chemical reducing reagents and more importantly,the reduced Gr could be directly deposited on the surface of the electrode,avoiding any additional deposition step that is commonly needed in the chemical reduced Gr.For example,Phong et al.[38]reported an ERGO modified GCE for the simultaneous determination of PCT,caffeine,and ascorbic acid.The ERGO/GCE was prepared from graphite to GO using the modified Hummers process and then electrochemical reduction of GO in suspension to Gr on GCE by cyclic voltammetry(CV)with a potential ranging from0 V to1.5 V,as illustrated in Fig.4.Using SWV method,the developed sensor exhibited a LOD of 0.25μM for PCT and average recoveries ranging from 95% to 106% in pharmaceutical samples.Another ERGO/GCE sensor was reported by Adhikari et al.[39]for the amperometric detection of PCT at 500 mV.The superb electrical conductivity,great surface area,and oxygen-related defects of ERGO make it a sensitive and rapid electrochemical sensing platform toward PCT detection.The ERGO/GCE showed good linearity in the range of 0.005-4μM with an excellent LOD of 0.0021μM.The sensor was employed for the determination of PCT in human serum without any interference.Despite its advantages,the electrochemical reduction of GO in suspension is limited by the mass transport process.Premature precipitation of partially reduced GO from electrode surface may occur to give product of mixed properties.
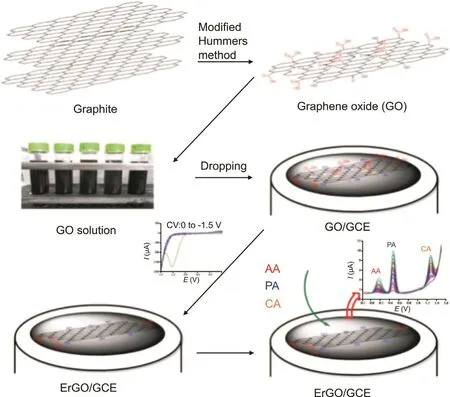
Fig.4.Schematic illustration of preparation procedures of electrochemically reduced graphene oxide-modified GCE.Reprinted with kind permission from Phong et al.[38].
In summary,because of the electronic transport properties of Gr and its derivatives,it is easy to provide electrons for the reduction process of PCT,which mayresult in a quasi-reversible process at the Gr-modified electrodes.The Gr nanoflakes were found to be better than others derivative in enhancing the oxidation peak currents and improving the reversibility of the oxidation.
3.2.CNTs
CNTs are nanometer-scale tube-like structures made of carbon,first discovered in 1991[51].These materials are divided into two categories:single-walled(SWCNTs)and multi-walled(MWCNTs).The modification of electrochemical electrodes by MWCNTs has attracted more interest because of the gain of high reversibility of the processes for increasing the velocity of electron transfer,and reduction of the overpotentials,thus achieving a higher selectivity and increased sensitivity.
The modification of CPEs with MWCNTs for PCT detection was first reported by Duarte et al.[40]in 2012.The experiments were performed in the presence of a cationic surfactant cetyl pyridinium bromide as an antifouling agent.The voltammetric results obtained showed clearly that the electrooxidation of PCT was significantly facilitated by synergic effect between CNTs and cationic surfactant.However,the sensor is not very useful for PCT as LOD is not satisfactory(2.1μM).Also,MWCNT/CPE allows simultaneous determination of PCT,tryptophan and ascorbic acid by SWV with a LOD of 0.8μM noted for PCT[17].Using CNTs modified GCE,the voltammetric determination of PCT was achieved in the presence of dopamine,ascorbic acid,and uric acid.The drop-casting modifi-cation produced significant improvements of the analytical signal resulting in a LOD of 0.00009μM[41],which was lower than those obtained when MWCNTs were used to modify CPEs.This result showed that immobilization of MWCNTs in the compacted structure of CPE has disadvantages towards the sensitivity of the obtained configuration.
Because of their nature,CNTs are inherently insoluble in most organic and aqueous solvents.The potential applications of CNTs need an extensive functionalization of the nanotubes by insertion of organic functional groups to make them processable and to tune their properties.For example,carboxyl functionalized MWCNT was used for SPCE[42]and GCE[43]modification and the electrodes were used for PCT determination using amperometry and differential pulse voltammetry(DPV)method,respectively.It was found that negative groups of MWCNT-COO-led to faster oxidation reaction through easy diffusion of PCT to the surface of electrode.The obtained LODs for modified SPCE and GCE were 0.1 and 0.6μM,respectively.
3.3.Fullerene
Fullerene is another carbon nanomaterial that has attracted much attention due to its remarkable electrochemical properties[52].The application of fullerene as an effective electrocatalyst for various chemical and biochemical reactions is in steady progression[53,54].However,the fabrication of electrochemical sensors based on fullerene is not fully investigated.Goyal and Singh[44]successfully applied C60-modified GCE for the voltammetric determination of PCT.The modification was achieved by casting of C60directly on the solid surface and then reduced in 1 M KOH solution in the potential range from 0.0 to-1.5 V.Sweep rate studies indicated that electrochemical reaction is followed by chemical hydrolysis.The C60-modified electrode showed a stable and reproducible response with outany influence of glucose,ascorbicacidandurea.However,paminophenol and caffeine do not interfere up to 4.0 mM,and at concentration>4.0 mM interferences occurred.Furthermore,the method does not require any sample pre-treatment.However,high LOD of 50μM is disadvantageous for the application of this sensor.Valentinietal.[45]used fullerene black for themodificationof SPCEs to determine PCT and guanine in urine and pharmaceutical formulations samples.The fullerene black was obtained under the form of carbon soot by arcing graphite in a helium atmosphere,and then coated on the surface of SPCE through drop-casting.The modified electrode displayed good linearity in the range of 1-300μM of PCT concentration with a LOD of 0.01μM.However,the lifetime of the film(one day)is a factor limiting its successful application.In a further study done by Mazloum-Ardakani et al.[46],an electrochemical sensor based on C60functionalized MWCNTs was elaborated.The sensor was prepared by casting the mixed solution of C60and MWCNTs on a GCE and thenwassubjected to potential scanning in acetonitrile solution containing 0.1 M tetrabutylammonium hexafluorophosphate as supporting electrolyte between 0.0 and-2.0 V until reversible multistep electron transfer reaction.The electrode showed low LOD of 0.035μM and good selectivity for the determination of PCT in the presence of levodopa.Nevertheless,the repeatability,selectivity and stability of the sensor were not determined.
3.4.CQDs
CQDs are zero-dimensional carbon nanomaterials that offer the opportunity to construct nanoscale electronic and optical devices.In electroanalytical chemistry,the CQDs have proved to be good choice to functionalize GCE electrode for enhanced response towards the analytes.Fu et al.[47]elaborated a GCE functionalized with nitrogen doped CQDs for the sensing of PCT and hydrogen peroxide.The modificationwas conducted byimmersing the GCE in the N-CQDs solution for 5 min,followed by a N2drying process.At optimal conditions,DPV displayed a linear dynamic range from 0.5 to 600 μM and a LOD of 0.157 μM with an excellent stability and reproducibility.Acceptable results were obtained in the analysis of pharmaceutical formulations with this electrode.However,the device was not tested for the analysis of biological fluid specimens.In a promising work realized by Ruiyi et al.[48],an ultra-high sensitivity for the detection of PCT(0.00038μM)was achieved by developing intimate electrical and chemical contact between Gr aerogel and octadecylamine-functionalized CQDs(GA@O-CQDs).This combination exhibits good stability,reproducibility and repeatability,and the method has been successfully applied for detection of PCT in pharmaceutical samples with the recoveries in the range of 96.4%-104.4%.Nevertheless,the practical application of this promising sensor is limited by its somewhat complicated and time-consuming preparation.
4.Non-carbon nanomaterials modified electrodes
The non-carbon nanomaterials used in the electrochemical detection of PCT regroup a wide variety of materials ranging from metals,metal oxides,ceramics and polymers.Table 3[18,55-73]lists the PCT sensors based on non-carbon nanostructure modified electrodes reported up to date.

Table 3 PCT sensors based on non-carbon nanomaterials modified electrodes.
4.1.Metal and metal-based nanomaterials
Metal and metal oxide NPs modified electrodes have attracted considerable attention in last decade because of their ability to improve charge transfer and catalytic activity.Recently,a high electroactive iron oxide(Fe2O3)-modified CPE was made by Vinay and Nayaka[55]for the voltammetric determination of PCT and dopamine.The advantage of this modified electrode is the simultaneous and individual determination of the analytes present in pharmaceutical samples with good recovery values.Despite simplicity,easy operation and low cost,the proposed sensor is not very useful due to unsatisfactory LOD of 1.16μM.Haghshenas et al.[56]employed magneto gold(AuNPs)to construct a CPE sensor for the detection of PCT.As illustrated in Fig.5,AuNPs were immobilized at the surface of Fe3O4,which acts as a sorbent for PCT molecules.After adding Au@Fe3O4to the PCT solution and stirring for 20 min,the Au@Fe3O4was gathered on the magneto electrode based on its magnetic field.This suggested electrode possesses manyadvantages such as lowLOD(0.045μM),good reproducibility,long-term stability and fast current response.Moreover,the sensor was successfully used to determine the concentration of PCT in pharmaceutical formulations and human serum samples.However,the higher requirements on electrode fabrication can be the factor limiting its successful application.Bismuth oxide(Bi2O2)nanorods were also prepared by Mahmoud et al.[57]for the modification of SPCE through drop-casting procedure for the simultaneous detection of PCT and isoniazid in pharmaceutical tablets,urine,human serum and saliva.The modified electrode presented excellent precision and reproducibility and good LOD(0.03μM)without any interference in optimum pH of 2,but at higher pH values,the oxidation peaks of PCT and isoniazid interfere together.The electrochemical studies revealed that the SPCE possesses much better performance than those of bismuth oxide modified GCE investigated by Zidan et al.[58].In that study the LOD obtained for PCT was only 0.2μM.Bismuth modified electrodes are more acceptable from green analytical chemistry,their figures of merits are fully compatible with the requirements for the determination of PCT,and their fabrication and pre-treatment are relatively simple.However,the shelf-life of the sensors had not been accomplished.In another study,cerium oxide(CeO2)NPs modified SPCE(CeO2/SPCE)was reported by Khairy et al.[59]for the simultaneous determination of PCT,codeine and caffeine.The SPCE coated with CeO2was prepared by drop-casting CeO2suspension on the SPCE surface,and then dried at 50°C.The modified electrode showed a good LOD(0.051μM)and exhibited good recoveries in human serum samples(97.7%-102%).Hassaninejad-Darzi et al.[18]fabricated a TiO2NPs and ZSM-5 nanozeolite modified CPE for the simultaneous determination of PCT,carbamazepine and pramipexole.The appearance of the reversible redox peaks indicates that modification of CPE by ZSM-5/TiO2can significantly accelerate the oxidation process.A LOD of 0.58μM was obtained for PCT associated with high selectivity in the presence of diverse interferents and good reproducibility,repeatability as well as high recovery values in human plasma.However,no pharmaceutical sample was tested by this sensor.

Fig.5.(A)Schematic illustration of the preparation of AuNPs@Fe3O4;(B)the stepwise procedure and electrochemical detection of PCT.Reproduced with permission from Ref.[56].
Mixed oxides were also investigated as modifiers.In a study done by Taei et al.[60],CPE was modified with Fe2O3@SnO2mixed oxides NPs and then tested for the simultaneous determination of PCT,tryptophan and epinephrine.PCT showed a well-defined oxidation peak with high current at the modified electrode.The high sensitivity and selectivity,submicro-molar LOD(0.2μM),high reproducibility,and ease of preparation and regeneration of the electrode surface make the electrode very suitable for the determination of PCT in tablets and human serum samples.Tavakkoli et al.[61]used zinc ferrite(ZnFe2O4)NPs modified carbon paste for the simultaneous determination of PCT,melatonin and epinephrine drugs with a LOD of 0.4μM for PCT.According tothis study,in acidic pH(pH≤4),the electro-oxidation of PCT on the ZnFe2O4/CPE involves two protons and two electrons,whereas at the higher pH(pH≥8),single proton and two electrons are transferred.
Compared to the above cited metal oxides and minerals,metal sulphides are not well investigated,except the study done recently by Mahanthappa et al.[62].In this study,zinc sulphide(ZnS)NPs modified CPE was used for the simultaneous determination of PCT,adenine and guanine.The ZnS were synthesized in solution phase by simple co-precipitation method and then were mixed with carbon paste.The obtained electrode exhibited a LOD of 0.041μM and good recovery values in pharmaceutical samples and herring sperm DNA.Layered double hydroxides(LDHs)are other promising materials to fabricate electrochemical sensors due to their special physical and chemical properties such as high adsorption capacity,low cost,tunable composition,catalytic activity and high chemical stability [74].The positively charged LDH layersofferan opportunity for the intercalation of numerous molecules to construct a sensor with better selectivity.In this context,hexacyanoferrate intercalated Ni/Al LDH(Ni-Al-HCF)was used for the modification of GCE through drop-casting procedure,as reported byAsadpour-Zeynali and Amini[63].The electrodewas used for the determination of PCT in tablets and spiked human urine samples with no interferences from glucose,L-tyrosine,vitamin B1 or ascorbic acid.This sensor exhibited a LOD of 0.8μM and good recoveries between 99.2% and 103%.In another study,Yin et al.[64]deposited organophilic LDH and AuNPs(AuNPs/SDS-LDH)film on the surface of GCE for the quantification of PCT,4-aminophenol and dopamine.The sensor was fabricated by drop-casting LDH solution onto the surface of GCE,and subsequently AuNPs were dropped onto LDH/GCE surface.The electrochemical oxidation of PCT at the electrode has quasi-reversible electrochemical behavior.The LOD of 0.13μM was obtained for PCT using DPV method.Furthermore,this developed sensor could be used for the detection of PCT in pharmaceutical tablets and spiked human serum samples with good recovery values in the range of 97.35%-104.21%.In summary,in spite of the fact that metal-based NPs have enhanced analytical performances,their future application can be limited by their toxic effects.
4.2.Nanostructured polymers
The nanostructured conductive polymers are among the most commonly used materials to modify electrode surfaces due to their important role in enhancing the sensitivity and electrocatalytic activity,protecting the surface of the electrode and reducing fouling.The modification of electrodes can be done either by direct electropolymerization of the monomer onto the working electrode or by simple adsorption of the polymer onto the surface of the electrode.Polyaniline(PANI)as one of the most extensively studied conducting polymers is an appropriate material for sensing applications because of its high conductivity and redox reversibility.Mazloum-Ardakani et al.[65]successfully applied PANI doped with tungstophosphoric acid(TPA)-modified CPE for the simultaneous determination of PCT,folic acid and norepinephrine in pharmaceutical and blood serum samples.The resulting DPV anodic oxidation currents exhibited a LOD of 0.2μM;however,reproducibility,stability and interference studies had not been accomplished.Another extensively studied conducting polymer is poly(3,4-ethylenedioxythiophene)(PEDOT).Electrochemical determination of PCT at PEDOT modified electrode prepared by electropolymerization on SPCE electrode was investigated by Su and Cheng[66]using CV,DPV and flow-injection amperometry(FIA).The LODs obtained were 3.71,1.39 and 0.16μM,respectively.At the same time,the developed sensor was successfully implemented in the detection of PCT in commercial tablet samples,with average recovery values in the range from 98% to 106.1%.In addition,Mehretie et al.[67]also used PEDOT modified GCE for the detection of PCT in pharmaceutical samples.The prepared electrode displayed remarkable electrocatalytic activity towards oxidation of PCT.However,the proposed sensor is not very useful for PCT due to unsatisfactory LOD of 1.13μM.Also,the reproducibility,repeatability,and shelf-life of the mentioned sensor were notdetermined.In a work done by Kannan and Sevvel[68],CV assisted electropolymerized poly-4-amino-6-hydroxy-2-mercaptopyrimidine(poly-AHMP)has been also investigated for GCE modification.In this study,PCT was detected down to LOD of 0.15μM with good recoveries(96%-102.75%)in tablet samples.
Polyamino acids have been also reported for the modification of electrodes such as cysteine(Cis)and histidine(His).A study done by Wang et al.[69]showed thatL-cysteine was successfully electropolymerized on GCE.The modified electrode presented relatively low LOD(0.05μM),high sensitivity and good stability in drug tablets and human urine.In another study,Li et al.[70]showed that GCE can be coated by poly-(L-histidine)/acetylene black for the determination of PCT.The obtained electrode presented relatively comparable LOD of 0.077μM and acceptable recoveries(97.8% and 105.2%)in the analysis of Ganmaoling granules samples.
Diglycolic acid(DA),a water-soluble indicator commonly used in complexometric titration,was also electropolymerized on GCE using CV.The study done by Xu et al.[71]indicated that the modified electrodepossessed excellentper for mance for the oxidation of PCT.The LOD was 0.0076μM associated with verygood stability,reproducibility and excellent recovery values(99.1%-101.8%)without any interference.Chitravathi and Munichandraiah[72]investigated poly(Nile blue)electro-polymerized on the surface of GCE to make a powerful electrochemical sensor for the determination of PCT,tramadol and caffeine in pharmaceutical dosages.The prepared electrode displayed remarkable electrocatalytic activity towards oxidation of PCT,caffeine and tramadol,which made it a suitable sensor for the simultaneous determination of the mentioned drug compounds.The electrode gave linear response in the range of 0.2-16.2 μM with a LOD of 0.08 μM and was also successfully applied for the determination of PCT in pharmaceutical dosages.Poly(chromium Schiff base complex)was also electro-polymerized on a GCE and implemented to electrochemically detect PCT and 4-aminophenol as documented by Kumar et al.[73].This sensor has demonstrated particularly good analytical performance with a very low LOD(0.0068μM).Moreover,the sensor was utilized to recover PCT only in pharmaceutical samples.However,no biological sample was tested by the sensor and furthermore,the shelf-life of the mentioned sensor was not determined.
5.Nanocomposites modifiers
Over the last few years,there has been a growing interest within the fabrication of nanocomposite materials for PCT sensing.Due to synergistic effects on the electrochemical performance of devices,nanocomposites can be useful to improve the resolution and the selectivity,especially when multi-analyte detection of close species is desirable.Table 4[19,25,75-123]shows the analytical response characteristics of PCT sensors based on nanocomposite modified electrodes.

Table 4 Analytical response characteristics of PCT sensors based on nanocomposite modified electrodes.

Table 4(continued)
5.1.Nanocomposites of Gr derivatives and metal-based NPs
The electrocatalytic effect of NPs on Gr sheets provides a pathway for the rapid transport of electrons,which contributes to an increase in the stability and the sensitivity of the sensor.Anuar et al.[75]prepared a platinum/nitrogen-doped Gr(NGr-Pt)nanocomposite which was coated on the surface of GCE through a simple drop-casting method for the determination of PCT.The deviceremarkablyreduced the over potential and displayed acceptable stability,very low LOD(0.008μM)and anti-interference properties in voltammetric detection of PCT in pharmaceutical samples.Wang et al.[76]developed a novel nanocomposite material of palladium-reduced GO and AuNPs modified GCE for the simultaneous determination of PCTand 4-aminophenol.The sensor was fabricated by drop-casting CRGO/Pd solution onto the surface of GCE,and subsequently AuNPs were electrodeposited onto the CRGO/Pd/GCE in HAuCl4solution.The developed sensor exhibited excellent electrochemical stripping characteristic for PCT with a LOD of 0.30μM.Yang et al.[77]fabricated a Gr-Cu2O nanocomposite by electro-deposition on GCE for the electro-catalytic oxidation of PCT.This sensor combined the excellent electrocatalytic activity of Cu2O and the electrical conductivity of Gr,with a LOD of 0.0067μM.The electrode was then subjected to PCT determination in pharmaceutical samples.Exhaustive interference experiments revealed that the sensor possessed high level of selectivity.PCT was also determined on RGO+Fe2O3[78]and RGO+NCeO2[79]modified electrodes by DPV with LOD of 0.0098 and 0.021μM,respectively.These modified electrodes were successfully used for PCT determination in real samples with good selectivity and sensitivity to interfering influence.Another example of NPs employed for the functionalization of Gr was represented by spinel NPs,which were used due to their good biocompatibility,low toxicity and strong super paramagnetic property.Combinations of spinel NPs with Gr can often result in more effective catalysts than individual metals or metal oxides.Two studies reported the detection of PCT using CPE modified with spinel NPs and Gr nanocomposite i.e.,NiFe2O4+Gr[80]and Gr+CoFe2O4[81]with LOD of 0.003 and 0.025μM,respectively.Both sensors were successfully applied for the determination of PCT in pharmaceutical tablets and also human body fluids.Detailed interference studies revealed that the modified electrodes possessed high level of selectivity.The stability of the both sensors was also great.The first sensor retained 95.8% of current response after one month and the second 94.3% of its initial value after 49 days.
ERGO has been also combined with metal-based NPs,e.g.,ERGO+ZrO2[82]and ERGO+Ni2O3-NiO[83];the linear ranges of PCT by means of such sensors were 9-237 and 0.04-100μM,respectively.The sensors showed excellent electrocatalytic activity toward the oxidation and reduction of PCT owing to the synergic effect of accessible reactive area and high catalytic activity.Despite neutral condition workability,good selectivity,repeatability and reproducibility,the sensor ERGO-ZrO2displayed relatively a linear range not satisfactory for PCT detection.
Similar to Gr,GO could be also functionalized by metal or metal oxide materials to enhance the performance of electrochemical sensors for PCT detection.For example,Li et al.[19]reported a GCE modified by drop-casting of palladium NPs/GO nanocomposite on its surface for PCT determination in commercial tablets and human urines.The device presented long-term stability(maintained about 90% of its initial value after three weeks),satisfactory reproducibility(RSD value of 3.9% for 9 independently fabricated modified electrodes),very low LOD(0.0022μM)and high selectivity in interference experiments.In addition to Pd,metal oxides have also been used for the decoration of GO-modified electrode.Yttrium oxide(Y2O3)and GO nanocomposite were mixed with graphite and paraffin oil with a ratio of 10:60:30(m/m/m)to achieve irregular electrode surface with large surface area.The platform showed satisfactory selectivityand repeatability.The LOD value obtained by this sensor was much higher(1.45μM)than that of GO-Pd/GCE device[84].GO nanosheets has also been combined with mixed oxide Fe3O4@SiO2which was casted on SPCE as documented by Beitollahi et al.[85].The advantage of this modified electrode is the simultaneous determination of PCT in the presence of tryptophan with a low LOD of 0.1μM.However,the reproducibility,repeatability,stability and interference studies were not determined.
5.2.Nanocomposites of Gr derivatives and polymers
Nafion(Nf),a copolymer of perfluorinated vinyl ether and tetrafluoroethylene,has been employed for the construction of sensors with great chemical,mechanical and thermal stability,as well as high conductivity and catalytic activity.The incorporation of Gr with Nf has also improved its dispersity in aqueous solution and prevented its aggregation.Kim et al.[86]reported activated porous Gr-Nf modified GCE for the determination of PCT and dopamine.Activated Gr was prepared from GO by thermal activation using KOH.The voltammetric measurements at the modified electrode showed an excellent electrocatalytic activity towards PCT with a LOD of 0.03μM.Acceptable results were obtained in the analysis of human urine samples with this electrode.However,no interference experiment was performed to determine the selectivity of the proposed sensor.In another work,Yiˇgit et al.[87]prepared a nonactivated Gr-Nf composite modified GCE for the determination of PCT,caffeine and aspirin individually,selectively and simultaneously.When the analytes were determined simultaneously,good linear current responses were obtained.The LOD of 0.0012μM was obtained for PCT,which was betterthan that of activated porous Gr-Nf modified GCE.ERGO-Nf modified GCE was also used for the sensingof PCT inpharmaceutical preparations and urine samples as documented by Filik et al.[88].The LOD was 0.025μM at this electrode.
Chitosan(Cs),polysaccharide natural biopolymer,has been also reported.It possesses many advantages,such as excellent strong film forming ability with high water permeability,good adhesion and high mechanical strength.In an investigation by Zheng et al.[89],a stable composite film of chitosan and Gr was made up on the surface of GCE.Under the optimized conditions,the PCT was quantified in the range from 1 to 100 μM with a LOD of 0.3 μM.In another study,Baccarin et al.[90]reported a sensor based on GCE modified with reduced GO and carbon black in a chitosan film for the simultaneous determination of PCT and dopamine.This modified electrode exhibited the linearity of 2.8-19μM,the reproducibility of 3.7% RSD and the LOD of 0.053μM for PCT.In another approach,Santos et al.[91]took advantages of GO,chitosan and nickel oxide nanocomposite modified GCE as a sensing element for the simultaneous determination of PCT and ciprofloxacin.The calibration curve of the electrode exhibited a linear response from 0.10 to 2.9μM with low LOD of 0.0067μM.
Gopal et al.[92]designed a poly(Valine)/GO(GO-poly(Val))modified CPE for the determination of PCT in pharmaceutical and human serum samples.L-Valine was electropolymerized onto the clear surface of CPE with the help of cyclic CV technique followed by the immobilization of GO on the surface of the electrode.The LOD of PCT was found to be 0.29μM.Even though the sensor was sensitive to PCT,potential interferences were not tested to provide better conclusion of its selectivity.TiO2-doped Gr/poly(methyl red)composite film modified GCE was proposed by Xu et al.[93].The sensor was fabricated by drop-casting TiO2-Gr solution onto the surface of GCE;subsequently the resultant electrode was scanned in an aqueous solution containing poly(methyl red)within the scan potential range of-0.8-1.8 V.The developed sensor exhibited excellent electrochemical stripping characteristic for PCT.Under the optimized conditions,the sensor showed several advantages,such as simple preparationprocedure,good reproducibilityand low LOD(0.025μM).The proposed method was applied to determine PCT in pharmaceutical samples with good recovery values in the range of 95.3%-104.2%.The modification of GCE by PEDOT polymer doped by Au@Gr core-shell was also documented by Li et al.[94].The modified electrode demonstrated a better electrocatalytic activity towards PCT determinationwith satisfactory selectivity in the presenceof dopamine and ascorbic acid.The LOD for PCT was found to be 0.041μM and the sensor was used for the determination of PCT in pharmaceutical formulations with good recoveries.
5.3.Nanocomposites of metal-based NPs and polymers
Oetal-based NPs can synergistically improve the characteristics of sensing matrices especially when combined with conducting polymers.Li et al.[25]reported a portable electrochemical sensor using the AuNPs-poly(caffeic acid)(AuNPs-PCA)composite modified GCE by simple electrochemical method for the determination of PCT.With high selectivity and excellent LOD(0.014μM),the electrode has been successfully applied to the determination PCT in blood,urine and pharmaceutical samples.Nanocomposites of AuNPs-Nf have been also used to modify CPE for selective determination of PCT and some neurotransmitters in pharmaceutical samples and biological fluids.The study done by Atta et al.[95]showed an excellent LOD of 0.0077μM and good recoveries in the range of 96.19%-103.59%.However,the selectivity of the sensor is unclear because of insufficient interference tests.Wong et al.[96]used the silver NPs,carbon black (CB),and poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS)modified GCE by drop-casting dispersion for the simultaneous detection of PCT and levofloxacin.The sensor showed good stability,low LOD(0.012μM)and no interference for the electrochemical determination of PCT in the presence of other compounds.The electrode was then successfully applied in synthetic urine with recoveries close to 100%.Sheikh-Mohseni and Pirsa[97]proposed another electrochemical sensor based on the combination of polypyrrole(PPy)and copper oxide NPs modified CPE for the simultaneous determination of PCTand dopamine.PPy,a cation selective polymer with good environmental stability as well as superior electric conductivity[124],is beneficial to electrons transfer,and the CuO NPs promote electrons to the substances at the electrode surface.Due tothe enhanced anodic peak currents of PCT,LOD of its determination was 0.025μM.However,the stability,repeatability and reproducibility of the sensor were not determined.In addition,no interference study was done by the researchers,and the selectivity of the proposed method was unidentified.Recently,Azab et al.[98]developed another modified electrode based on the electrodeposition of cobalt NPs on the surface of CPE modified with cellulose(Co-C/CPE)or starch(Co-S/CPE)for the simultaneous determination of PCT,caffeine and warfarin in commercial tablets.The modified electrode showed the best performance in terms of lowest LOD of 0.00099μM when starch was used for the modifi-cation.Moreover,the sensor exhibited attractive Specific capacitance and long life time because it retained 93% of current response after two weeks.
5.4.CNT-based nanocomposites
CNTs have been extensively combined with other nanostructures in electrochemical detection.For example,the platinum NPs decorated MWCNTs were used for the modification of CPE and the electrode was used for the simultaneous determination of PCT,phenylephrine and cetirizine in pharmaceutical,blood serum and urine samples.The nanocomposite showed proficient catalytic activity towards the oxidation of the analytes with low LOD of 0.0279μM for PCT.However,the presence of 4-aminophenol or ascorbic acid showed remarkable interfering effects on PCTanalysis[99].Pt/MWNCT was also utilized by D’Souza et al.[100]in combination with Triton X-100.This sensor exhibited low LOD of 0.0177μM for PCT,long-term stability,excellent reproducibilityand good recoveries in drugs and biological samples.In another work,CPEs were also modified by ZnO NPs and COOH-MWCNT and used for the determination of PCT,folic acid and dopamine with a LOD of 0.23μM for PCT[101].The modification of CPE with MWCNT,NiO and ethynylferrocene nanocomposite was also successfully elaborated for the detection of PCT with a LOD of 0.5μM and good recoveries in tablet and urines samples[102].
For PCT determination at modified GCE,different combinations were used.Madrakian et al.[103]modified GCE by using MWCNT-gold nanocomposite for the simultaneous determination of PCT,ascorbic acid and tyrosine.The LOD was found to be 0.03μM associated to recovery values in the range of 98%-102% when applied in blood serum and pharmaceutical samples.The obtained values for the repeatability and the reproducibility of the sensor was found to be 3.8% and 3.7%,respectively.The sensor was found to be susceptible to interference at high concentration(200μM)of uric acid and dopamine.Nevertheless,it is not problematic because un-metabolized form of these molecules does not surpass this concentration.GCE was also modified with MWCNT-cobalt NPs and was used for the simultaneous determination of PCTand dopamine.Good LOD of 0.001μM was obtained for PCT and the electrode was successfully applied for PCT determination in pharmaceuticals with high recoveries(≥99.7%)[104].Furthermore,the sensor demonstrated good stability and reproducibility in the detection of PCT.However,insufficient interference experiments were of weaknesses of this work.Another study carried out by de Holanda et al.[105]was based on the development of a sensor based on the modification of CPE with AuNPs,COOH-MWCNT and cobalt(II)phthalocyanine.The electrode was fabricated by electrodepositing AuNPs onto the GCE and subsequently COOH-MWCNT and cobalt(II)phthalocyanine solution were dropped onto the surface of the electrode.After optimizing the preparation and measurement conditions,thepreparedsensorshowed alinearrangeof 1.49-47.6μM,and a LOD of 0.135μM.The sensor was then applied for the analysis of PCT in various pharmaceutical formulations.Unfortunately,no biological specimen analysis or interference experiment was done in this work and moreover,the shelf-life stability of the sensor was not determined.MWCNT-Ni(OH)2nanocomposite modified GCE was also employed for the simultaneous detection of PCT andL-dopa with a low LOD(0.017μM)for PCT[106].The interference study of some species showed no significant interference with PCT andL-dopa in human urine.Baytak et al.[107]fabricated an electrochemical sensor based on GCE modified with MWCNTs and Y2O3,showing a linear responsetoPCT in the concentration range of 0.0001-0.018μM and a LOD of 0.00003μM.The LOD value obtained by this sensor was much better than those of other reported nanocomposite modified electrodes used for the detection of PCT.Moreover,the device presented long-term stability(only 5% decrease of current response after 15 days and 7%-8% after 30 days at 4°C).Another composite electrode was obtained by using MWCNT and disordered mesoporous silica(SiO2)thin film on GCE with important advantages,including enhanced physical rigidity,surface renewability by polishing and high sensitivity[108].The electrode was used for the simultaneous determination of PCT,uric acid and dopamine,with a LOD of 0.098μM for PCT.The use of MWCNT-alumina-coated silica nanocompositealso allowed the determination ofPCTas documented by Lu et al.[109].The electrodes displayed a LOD of 0.05μM using SWV and recovery values in pharmaceutical tables around 99%.However,the reproducibility,repeatability,stability and interference tests were not accomplished.
Association between MWCNTs and polymers was also used for the determination of PCT.Ghadimi et al.[110]fabricated nanocomposite of poly(4-vinylpyridine)and MWCNTs modified GCE by drop-casting for the selective determination of PCT in tablets and urine samples.The electrode exhibited a low LOD(0.00169μM),good stability and reproducibility towards PCT.Even though the sensor was insensitive to common physiological interferences,i.e.,ascorbic acid and uric acid,other potential interferents were not tested to provide better conclusion of its selectivity.Dalmasso et al.[111]used GCE modified with MWCNT-polyhistidine as a sensing layer for the highly selective quantification of PCTand ascorbic acid.The sensor exhibited low LOD of 0.032μM for PCT and good recovery values in commercial pharmaceutical formulations.However,the stability,selectivityand repeatabilityof the electrode were not determined.Narayana et al.[112]electrochemically detected PCT in the presence of folic acid and dopamine using a MWCNTspoly(glycine)composite modified GCE in a commercial tablet and human blood serum.The sensor was fabricated by electropolymerization of glycine in aqueous solution(pH 7.0),followed by drop-casting MWCNTs solution onto the surface of poly(glycine)/GCE.The redox system of PCT at the developed nanocomposite sensor was perfectly reversible with a LOD of 0.5μM.PCT was also determined on MWCNT-Chitosan[113]and fMWCNTs-Chitosancobalt ion[114]modified GCEs by DPV with LOD of 0.16 and 0.01μM,respectively.Moreover,both modified electrodes were successfully used for PCT determination in real samples with good recovery values.
The association of MWCNT with Gr and fullerene was also reported in literature.Arvand and Gholizadeh[115]fabricated a highperformance electrochemical sensor based on MWCNT and Gr for the simultaneous determination of PCT and tyrosine with a LOD of 0.1μM for PCT.In another study,Brahman et al.[116]reported nanocomposite film based on Cu NPs-fullerene-MWCNTs modified CPE for the determination of PCT.The modified electrode was prepared in a two-step process as illustrated in Fig.6.MWCNTs and C60suspension was directly casted on CPE;subsequently Cu NPs were electrodeposited onto the C60-MWCNTs/CPE from a CuSO4solution.The sensor exhibited a very low LOD(0.000073μM)and remarkable recovery values(99%-100%)in biological samples.The device presented long-term stability(retained 99.6% of current response after 50 days),satisfactory reproducibility and high selectivity.

Fig.6.Schematic representation of sensor fabrication.Reproduced with permission from Ref.[116].
SWCNTs have also been employed in combination with other nanostructures,e.g.,SWCNTs+Gr[117]and SWCNTs+Nd2O3[118].The LODs for PCT by means of such sensors were 0.038 and 0.05μM,respectively.In summary,SWCNTs-based sensors have shown performance similar to that of MWCNTs-based ones for PCT detection,and even MWCNTs are easier to prepare and lower in cost than SWCNTs.
5.5.MOF nanostructures
MOFs are a new subclass of coordination polymers consisting of organic bridging ligands and inorganic metals(or metal clusters),which form one-,two-,or three-dimensional structures.Given their excellent characteristics of large surface area,tunable pore size,attractive electrical,optical,and catalytic properties,MOF materials have attracted growing interest in electrochemical sensing applications.Minh et al.[119]synthesized the MOF-199 using domestic microwave at ambient temperature and pressure.The MOF-199 was simply deposited on the GCE by drop-casting,and then applied for the voltammetric simultaneous determination of PCT and caffeine in pharmaceutical formulations.The LOD for PCT was 1.3μM,which is not satisfactory for practical application.Chang et al.[120]prepared ferrocene-containing MOF modified CPE by a one-step procedure with very good reproducibility.The obtained thin film showed electrocatalytic activity for PCT oxidation.Using SWV,a low LOD was found for PCT(0.0064μM)and good recovery values in pharmaceutical and serum samples(98.7-104.3).
The electrocatalytic properties of metal NPs combined with the large surface area of the MOFs provide new electrode materials for various electrochemical sensors.Shi et al.[121]synthesized AuNPs and water-stable MOFs via hydrothermal and sonication methods,and used them for GCE modification.The modified electrode exhibited superior electrochemical performances for PCT detection in pharmaceutical samples,with an extremely low LOD of 0.0000011μM and awide linear range from 0.00001μM to 100μM.On the other hand,Li et al.[122]prepared a sensor based on AuNPs embedded porous carbon by a simple pyrolysis of UiO-66-NH2MOFs and HAuCl4precursors.Because of the unique structural properties and the synergetic catalytic effect,the modification of GCE offers an attractively enhanced electrocatalytic performance for the detection of PCT,which showed high activity and excellent analytical performance towards PCT,such as a wide linear range of 0.12-95.10μM,low LOD of 0.0494μM and anti-interferences ability.In another study,a copper-based MOF(terephthalate)combined with GO was proposed to modify GCE for the electrochemical sensing of PCT and dopamine.The electrode exhibited high stability due to hydrogen bonding,p-p stacking,and Cu-O coordination between MOFand GO.The LOD for PCT was estimated as 0.36μM associated to good anti-interference properties towards the determination of PCT in the presence of hydroquinone,glucose,tyrosine,ascorbic acid and L-cysteine.The practical utility of this sensor was further checked by quantifying the PCT in human serum and urine samples with recoveries of 98%-101%[123].
6.Other types of carbon-based electrodes for the determination of PCT
In addition to the above illustrated carbon-based electrodes,some research studies reported other carbon-based electrodes such as boron-doped diamond electrodes(BDDE)[125-128]and carbon-ceramic electrodes(CCE)[129-134]as shown in Table 5.Tyszczuk-Rotko et al.[125]successfully developed a bare(unmodified)boron-doped diamond electrode for the simultaneous determination of PCT and dopamine.This sensor was applied in blood and serum samples with a LOD of 0.0135μM.The same authors group developed another electroanalytical method for the simultaneous determination of PCT and ascorbic acid using a boron-doped diamond electrode modified with Nf and lead films[126].The electrode was tested in pharmaceutical samples with a LOD of 0.14μM.Sadok et al.[128]developed a bismuth particles/Nf covered BDDE for the simultaneous determination of PCT and caffeine using DPV.This sensor exhibited good LOD(0.0262μM)and applicability in tablet samples.Habibi et al.fabricated many carbon-ceramic electrodes modified CNTs[129-132].SWCNT modified carbon-ceramic electrode was used for simultaneous determination of PCT and caffeine[130].The electrode showed a linear range of 0.08-200 μM and a LOD of 0.05 μM without any interferences.A MWCNT modified CCE was employed for the simultaneous determination of PCTand ascorbic acid[131].A low LOD of 0.08μM was obtained for PCT associated to a very wide linear range of 0.1-6500μM.In addition,Majidi et al.[134]reported a voltammetric method for the simultaneous determination of dopamine and PCT using Gr nanoplatelets like structures formed on ionic liquid modified carbon-ceramic electrode.This electrode was successfully applied for the determination of PCT and dopamine in some pharmaceutical and urine samples with a LOD of 0.063μM for PCT.

Table 5 Modif i edboron-doped diamond electrodesand carbon-ceramicelectrodesfor paracetamol analysis.
7.Critical issues in PCT detection
This review offered an overview of the electrochemical sensors developed for the sensing of PCT during the last 10 years.Considerable progress was reported for the electrochemical determination of PCT with enhanced selectivity and sensitivity by utilizing CMEs.Carbon-based electrodes(CPE,GCE,SPCE,CCE,BBDE)and their modification configurations by CNTs,Gr and its derivatives,fullerene,CQDs,NPs,conducting polymer,nanocomposite and MOF nanostructure appeared to be appropriate for the elaboration of rational solution to determine PCT inpharmaceutical and biological samples.
A critical comparison between several research works has been provided in this review(Tables 2-5).A large number of carbon nanomaterials have been suggested for the modification purpose to detect PCT(Gr:6 studies;MWCNT:6 studies,C60:3 studies;CQDs:2 studies).As shown in Table 2,the LOD of few nanomolars needed for practical detection of PCT was achieved in only one study using GCE surface modified with MWCNT[41].Nevertheless,a great number of Scientific works achieving low concentration levels of PCT were obtained by using Gr modified electrodes.The use of CQDs as emergent material with Gr allowed the detection of PCTat trace levels(LOD of 0.00038μM)(see Table 2).
The electrode surfaces modified with non-carbon nanomaterials such as metal-based NPs(11 studies)exhibit excellent electrocatalytic properties towards PCT(Table 3).Metal-based NPs catalyze the electrochemical reaction and enhance electron transfer between PCT and electrode.However,the synthesis of metal-based NPs having a definite size and shape is not easy.There may be a difference in shape and size of NPs synthesized from one study to another,which leads to lack of reproducibility in results.On the other hand,nine studies report the detection of PCT using carbonbased electrode modified by conducting polymers.As summarized in Table 3,it is clear that the lowest value of LOD was declared by Kumar et al.[73]using poly(chromium Schiff base complex)which reaches 0.0068μM.Despite the advantages of polymers,their instability and mechanical weakness limit their application for electrodes modification.
The sensitivity and practical application of the electrochemical PCT sensors can be further improved by putting more focus on the fabrication of nanocomposites by means of appropriate combination of different advanced nanomaterials(52 studies).As shown in Table 4,the very low LOD of 0.0000011μM was obtained by combination between MOFs and AuNPs modified electrodes.In addition to the innovative methodologies developed,most of the other sensors cannot reach this level.Even,most of the nanocomposite approaches have utilized somewhat complex strategies such as the incorporation of two or three nanostructures which make the approach Difficult to be commercially available.
Chemical modification of carbon-based electrode had apparently resulted in an elimination of the interferences effects in pharmaceutical and biological samples.Many of the sensors developed were successfully applied to the determination of PCT in biological samples,such as human urine[17,34,44,57,69,78,82-87,90,91,96,99,104,106,108,110,113,116,123]and serum[18,35,39,43,55-61,63-65,97,99,100,103,112-117,120,123],enabling the use of PCT as a relevant biomarker of several diseases.Another common application of the fabricated sensors was the determination of the PCT in pharmaceutical samples [34,36,38,40-57,60-75,86,88,89,92-95,98-105,107,109-112,114,115,118-122].
The selection of the most suitable electrochemical technique is an important factor for the achievement of high selectivity and detecting trace levels of PCT. CV [25,58,67,72], DPV[18,19,40,43,52-57,59-62,64-68,70,72,73,76-79,83-85,87,89,92,93,95,97,98,101,103,106,108,110-115,119,121-123,125-132,134]and SWV[17,34,36,38,41,75,77,80,81,88,90,91,96,102,104,105,108,110,117,119,121]are the most common electroanalytical techniques employed for the determination of PCT.The DPV is the most useful technique to discriminate multiple compounds and improve sensitivity because it produces less background current,leading to lower LODs.Other electrochemical techniques including chronoamperometry[35,37,39,63,66,94,100,106],amperometricFIA hydrodynamic voltammetry[42,66,133],linear sweep voltammetry[69,82]and adsorptive stripping voltammetry[86,98]were also applied in the detection of PCT.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
 Journal of Pharmaceutical Analysis2021年2期
Journal of Pharmaceutical Analysis2021年2期
- Journal of Pharmaceutical Analysis的其它文章
- Current diagnostic and therapeutic strategies for COVID-19
- Development of chromatographic technologies for the quality control of Traditional Chinese Medicine in the Chinese Pharmacopoeia
- Design and preparation of a new multi-targeted drug delivery system using multifunctional nanoparticles for co-delivery of siRNA and paclitaxel
- The effective transfection of a low dose of negatively charged drugloaded DNA-nanocarriers into cancer cells via scavenger receptors
- A dual-signal sensor for the analysis of parathion-methyl using silver nanoparticles modified with graphitic carbon nitride
- Curcumin encapsulated dual cross linked sodium alginate/montmorillonite polymeric composite beads for controlled drug delivery
