Calcium-Sensing Receptor of lmmune Cells and Diseases
,MM,Yue Liu,MD,Jiaxing Sun,MD and Xinhua Yin,MD
1 Department of Cardiology,The First Aff iliated Hospital of Harbin Medical University,Harbin,150001 Heilongjiang,China
Abstract Calcium-sensing receptor (CaSR),which was initially found in the parathyroid gland,is ubiquitously expressed and exerts specif c functions in multiple cells,including immune cells.CaSR is functionally expressed on neutrophils,monocytes/macrophages,and T lymphocytes,but not B lymphocytes,and regulates cell functions,such as cytokine secretion,chemotaxis,phenotype switching,and ligand delivery.In these immune cells,CaSR is involved in the development of many diseases,such as sepsis,cryopyrin-associated periodic syndromes,rheumatism,myocardial infarction,diabetes,and peripheral artery disease.Since its discovery,it has been controversial whether CaSR is expressed and plays a role in immune cells.This article reviews current knowledge of the role of CaSR in immune cells.
Keywords:Calcium-sensing receptor;neutrophil;monocyte/macrophage;T lymphocyte;B lymphocyte
lntroduction
The extracellular calcium-sensing receptor (CaSR)is an approximately 120–160 kDa G protein–coupled receptor that was initially found in the parathyroid gland and is expressed in many other cell types and or gans,such as cardiomyocytes [1],f broblasts [2],aortic smooth muscle cells [3],and adipocytes [4].The human CaSR gene (CASR) is located on 3q13.3-21 [5].As a responder to changes in extracellular Ca2+concentrations,CaSR plays a primary role in regulating parathyroid hormone secretion and parathyroid hyperplasia.To date,activation of CaSR has been found to be involved in multiple different functions,including secretion [6],proliferation,differentiation,apoptosis,and chemotaxis [7],in various cell types [6,8].Even nearly three decades after its initial identif cation,new functions and mechanisms are still being attributed to CaSR beyond simply a response to physiological Ca 2 + concentrations.The expression and function of CaSR on immune cells have recently become a hot topic.However,since its discovery,it has been controversial whether CaSR is expressed and plays a role in immune cells.This article reviews cur -rent knowledge of CaSR,CaSR-mediated signaling pathways,the relationship between CaSR and immune cells,and the role of CaSR in disease.
Structure of CaSR
CaSR,which was f rst cloned in 1993,is a member of class C of the G protein–coupled receptors that responds to multiple extracellular cations,such as H+,Na +,Ca 2 +,and Mg2+[9].CaSR is lar gely expressed and dimerized on the cell membrane,and consists of three protein functional domains.Acting as a ligand-binding domain,the extracellular domain of CaSR binds to numerous physiological ligands,including Ca 2 + and other polyvalent species[10].The seven-transmembrane domain couples CaSR to activating or inhibitory G-proteins,which transduce intracellular signals [11].CaSR is linked to the cytoskeleton by an intracellular domain,which localizes the receptor to caveolae by binding f lamin A [12].
Gene Encoding CaSR
The human CaSR gene (CASR) is located on 3q13.3-21 [5].In contrast,CASR genes in rat,mouse,and bovine species are located on chromosomes 11,16,and 1,respectively [5,13].The human CASR gene contains eight exons,seven of which encode the extracellular domain and untranslated regions,while the seven-transmembrane domain and the carboxy terminus are both encoded by the seventh exon [5,14].CaSR contains 1078 amino acids,which are encoded by its fully processed mRNA.The extracellular domain,which is hydrophilic,is composed of 612 amino acids and forms the amino terminus to bind the ligand.The hydrophobic seven-transmembrane domain is composed of approximately 250 amino acids.The exceptionally long intracellular domain of CaSR is composed of 217 amino acids and forms the car -boxy terminus [15].Some human diseases,such as familial hypocalciuric hypercalcemia syndromes[16] and familial hyperparathyroidism [17],have been linked to mutations inCASR.
CaSR Signaling
CaSR signaling has been extensively reviewed by Hofer and Brown [18] and Ward [19].Brief y,many heterotrimeric G proteins,including G αq/11,G αi/0,G α12/13,and G αs,are involved in the activation of CaSR downstream signaling.When CaSR interacts with G αq/11,it activates phospholipase C(PLC) and phospholipase D.Inositol trisphosphate(IP3) and diacylglycerol are formed after the activation of PLC.Acting as second messengers,they consequently lead to an increase of intracellular C2a +concentrations via the activation of IP3 receptors and subsequent protein kinase C isoforms.Downstream of CaSR,PLC,which is activated by G αq/11,induces extracellular signal–regulated kinase 1 (ERK1)and ERK2 (also known as p42 and p44 mitogenactivated protein kinase (MAPK),respectively) [20,21].CaSR may also stimulate MAPK via β -arrestin proteins but not by a G protein–dependent mechanism [22].When interacting with G α12/13,CaSR can modulate cytoskeletal functions and smooth muscle cell contraction by activation of monomeric Rho GTPases [23].Cellular cyclic AMP,which is activated by adenylate cyclase and acts as a second messenger,is inhibited or activated when CaSR interacts with G αi/0or G αs,respectively.As a G protein that inhibits CaSR,G αi/0plays a role in reducing the open probability of Ca2+channels by inducing the opening of K + channels [24,25].
The expression of CaSR on the cell surface is determined by its signal transduction by an agonist-driven insertional signaling as well.Through this process,it increases the anterograde traff cking of newly synthesized CaSRs to the plasma membrane,and even in response to continual exposure to extracellular Ca2+,agonist-driven insertional signaling can prevent CaSR from undergoing functional desensitization [26,27](Figure1).CaSR-mediated signaling also occurs through the σ subunit of the heterotetrameric AP2 complex;its germ line mutations have been shown to impair intracellular Ca2+and MAPK signaling responses in CaSR-expressing cells and to cause hypercalcemia [28–31T h].rough the above-mentioned signaling,CaSR can modulate dif ferent physiological functions in calcitropic and noncalcitropic tissues [32].In calcitropic tissues,CaSR activity and/or signaling can regulate the level of intracellular Ca2+,while in noncalcitropic tissues,downregulation of CaSR activity and signaling is involved in disorders ranging from impaired wound healing to vascular calcif cation and colorectal carcinoma [33–35m]o;reover,upregulation of CaSR activity and signaling has been associated with brain injury or asthma [36,37],and further contributes to disease progression.However,the immune cell–specif c CaSR signaling pathway has not been identif ed,and further studies are needed.
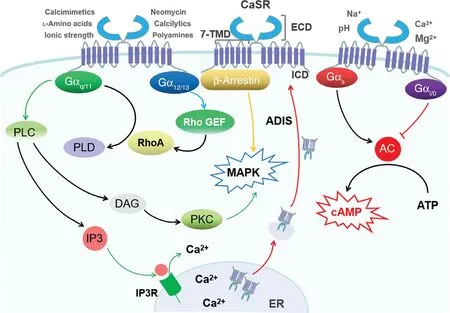
Figure1 Proposed Calcium-Sensing Receptor (CaSR)-Mediated Signal Transduction Pathway.
CaSR of lmmune Cells and Diseases
CaSR of Neutrophils and Diseases
During different infectious conditions,it is important for the immune system that diverse immune cells are mobilized to the sites of infection to promote immune defense.Neutrophils act as the earliest inf ltrating inf ammatory cells to defend against infection:the inf ammatory signals are sensed,prioritized,and integrated into a migratory response by these cells through phagocytosis and degranulation [38].The expression and function of CaSR in peripheral blood polymorphonuclear neutrophils (PMNs) had not been described until a study published by Zhai et al.[39] in 2017.They observed,for the f rst time,that CaSR was expressed in PMNs of rats [39],and not long after,our team detected the expression of CaSR in PMNs of humans [40].In the study of Zhai et al.,the activation of CaSR in PMNs decreased their apoptosis and production of reactive oxygen species and IL-10,and increased the secretion of IL-6 and myeloperoxidase [39].This revealed that CaSR can delay the apoptosis of PMNs so that they can combat the pathogen for a long time,and CaSR promoted release of proinf ammatory cytokines through nuclear factor κ B (NF- κ B) signaling to amplify the inf ammatory response.These f ndings suggested that CaSR could be used to regulate the immune function of PMNs to reduce inf ammatory damage,and CaSR may be a tar get for the prevention and treatment of inf ammatory diseases.However,the role of CaSR in PMNs in specif c diseases needs to be further determined in animal studies.
To date,we have investigated the role of CaSR in neutrophils only in models of myocardial infarction (MI).In humans and rats with MI,we observed the upregulation of CaSR and activation of NLRP3 inf ammasome-mediated inf ammatory factor IL-1β in PMNs,which was formed by the activation of NLRP3 inf ammasome–mediated caspase 1,in PMNs,and this upregulation peaked on day 1 and gradually decreased until day 7.This indicated the neutrophil CaSR was expressed early in both rat and human PMNs and rat myocardium in the MI wound healing.The role of CaSR-activated PMNs in myocardium was investigated by co-culture of PMNs in conditioned medium with cardiomyocytes and cardiac f broblasts.The results demonstrated that the CaSR-stimulated PMNs aggravated the apoptosis of cardiomyocytes by modulation of the proapoptotic or antiapoptotic proteins,and promoted the differentiation of cardiac f broblasts into myof broblasts and the upregulation of collagen secretion[40].These f ndings proved that the CaSR-activated PMNs specif cally promoted the reparative cardiac remodeling by IL-1 β/IL-1 receptor in the f rst few days of MI,and this may provide a basis for CaSR to be a therapeutic tar get for cardiac remodeling after MI.However in that study,we did not study the direct effects of PMNs on cardiomyocytes and cardiac f broblasts.Overall,there is little research on the function of CaSR in PMNs,and its role and mechanism in disease are poorly understood and should be further studied.
CaSR of Monocytes/Macrophages and Diseases
After the recruitment of neutrophils,monocytes differentiate into macrophages or dendritic cells and are recruited to sites of inf ammation to clear pathogens [41].The study of CaSR expression in monocytes began in 1997.Because of bone turnover,cells in the bone marrow niche are exposed to substantial changes in extracellular Ca2+concentration.House et al.[42] performed the f rst study to determine the expression of CaSR in bone mar -row cells.They found that CaSR is expressed in low-density mononuclear bone marrow cells and in several hematopoietic lineage cells.In the following year,Yamaguchi et al.[43] reported that human peripheral blood monocytes express CaSR,which could play a direct role in regulation of extracellular Ca2+concentration as previously described in the parathyroid gland.The expression of CaSR in monocytes/macrophages has been examined in many monocyte/macrophage cell lines,such as J774 cells [44],RAW 264.7 cells [45],and THP-1 cells[46],which were reported to express functional CaSR and to regulate cytokine secretion through the NF- κ B and PLC-IP3 pathways.In response to treatment with phorbol 12-myristate 13-acetate or 1,25-dihydroxyvitamin D3,human promyelocytic leukemia cells (HL-60),which express both CaSR protein and CaSR mRNA,could differentiate into a monocyte/macrophage phenotype in which process the expression of CaSR was increased at the level of translation [47].These studies suggest that CaSR is expressed on monocytes/macrophages and promotes cytokine secretion.These results provide a theoretical and experimental foundation for the functional study of CaSR on monocytes/macrophages.
As a pivotal component of innate immunity,macrophages play an important role in homeostasis and disease control.Macrophages are recruited to sites of cell death,which is a key process to induce an immunological response.Extracellular Ca2+produces a chemoattractant ef fect through the activation of CaSR via the phosphoinositide 3-kinase(PI3K)-Akt pathway [48].CaSR increases monocyte chemotaxis in a dose-dependent manner,and monocytes def cient in CaSR lack the normal chemotaxis to a Ca2+gradient in mice.CaSR-activated monocytes augment the transmigration response to monocyte chemotactic protein 1 (MCP-1),while MCP-1-stimulated monocytes reciprocally increase CaSR expression.The f ndings suggest CaSR and chemokines interact in a dual-enhancing manner in the recruitment of inf ammatory cells [49].
Macropinocytosis constitutively occurs in macrophages and supports the uptake of antigens for presentation.CaSR senses extracellular Ca2+and transduces signals to activate macropinocytosis through PI3K and PLC,and CaSR induces constitutive macropinocytosis to promote the sentinel function,which facilitates the e ffcient delivery of ligands to cytosolic pattern-recognition receptors of macrophages [50].CaSR participates in the switching of macrophage phenotypes in response to β -tricalcium phosphate extracts (a model biomaterial) and promotes material-stimulated osteogenesis [51].These results reveal that CaSR is involved in monocyte/macrophage chemotaxis,macropinocytosis,and phenotype switching.CaSR on monocytes/macrophages is associated with many diseases,such as peripheral artery disease,rheumatoid arthritis,cryopyrin-associated periodic syndromes,MI,obesity-related metabolic disor -ders,and osteoarthritis.A nearly 1.5-fold increased expression of CaSR in the monocytes of patients with peripheral artery disease and diabetes was observed during the posttranscription and was related to the concentrations of glucose and proinf ammatory cytokines and the severity of peripheral artery disease [52].Similarly to the role of CaSR on monocytes in peripheral artery disease patients,the expression of CaSR in monocytes is upregulated in rheumatoid arthritis patients with severe coronary artery calcif cation [53].Both studies show that CaSR expression on monocytes is associated with disease severity,so the detection of CaSR expression on human peripheral blood monocytes could be used as an ef fective method to monitor many diseases.However,whether there is temporal relationship between CaSR expression and atherosclerosis remains to be clarif ed.
In cryopyrin-associated periodic syndromes,which are induced by mutations of theNLRP3gene that lead to a series of autoinf ammatory diseases,CaSR activates the NLRP3 inf ammasome by increased intracellular Ca2+concentration and decreased cyclic AMP concentration,and this process promotes the secretion of proinf ammatory cytokines in bone marrow–derived macrophages and peripheral monocytes [54].In our own work,CaSR was found to be expressed on both M1 macrophages,and M2 macrophages,but only CaSR expressed on M1 macrophages induces IL-1 β secretion by NLRP3 inf ammasome activation via the PLC-IP3 pathway,and this process promotes car -diac remodeling by promoting the phenotypic transformation of cardiac f broblasts and modulation of collagen,matrix metalloproteinase 2 (MMP2),MMP9,and tissue inhibitor of metalloproteinases 2(TIMP-2),which was identif ed by co-culture of cardiac f broblasts with CaSR-activated or inhibited M1 macrophage supernatants [55].These f ndings indicate that CaSR-mediated macrophage-specif c promotion of reparative cardiac remodeling lasts as long as 14 days after MI.Therefore,it can be seen that macrophages are the dominant cells in the process of cardiac repair after MI,and CaSR intervention on macrophages may be one of the methods to regulate adverse ventricular remodeling after MI.
Obesity is a worldwide health problem,because it leads to obesity-related metabolic disorders,such as cardiovascular disease,type 2 diabetes,and cancer.So,it is very ur gent to explore the pathogenesis of obesity-related metabolic disorders.D ‘ Espessailles et al.[56] investigated the inf ammatory mechanism of these disorders.In that study,human LS14 preadipocytes were exposed to conditioned medium consisting of CaSR-activated or inhibited macrophages that were differentiated from THP-1 monocytes.It was found that LS14 preadipocytes could secrete proinf ammatory cytokines under the action of CaSR-stimulated macrophages,which resulted in increased secretion of inf ammatory markers and activation of the NLRP3 inf ammasome by CaSR activation on these macrophages themselves [56].These f ndings suggest that upregulation of CaSR on macrophages not only promotes the proinf ammatory features by positive feedback but also provokes the inf ammatory activity of adipocytes by paracrine signaling.Although these results need to be conf rmed in animal models,they suggest it may be possible to prevent obesity-related metabolic disorders by regulating CaSR function on macrophages in adipose tissue.
Compared with the other rheumatisms,CaSR expression in osteoarthritis patients was upregulated in monocytes extracted from peripheral blood and synovial f uids,with higher expression in synovial f uids that was associated with the inf ammatory nature of the synovial f uid [57].These f ndings demonstrate that in patients with rheumatism with synovial f uid,extraction of monocytes from the synovial f uid and detection of CaSR expression can not only provide important help for the diagnosis of osteoarthritis but can also ref ect the inf ammatory characteristics of the synovial f uid;moreover,monocytes may be a potential therapeutic target by the use of CaSR allosterizers.
CaSR of Lymphocytes and Diseases
In an inf ammatory microenvironment,homologous antigens carried by antigen-presenting cells(APCs) are followed or found by T lymphocytes by modifying their migration [58].Once the T lymphocytes recognize the antigens on the APC,the resulting binding synergistically activates the T lymphocytes [59].The experiments performed to determine CaSR expression in bone marrow cells by House et al.in the 1990s indicated that CaSR is expressed in low-density mononuclear bone marrow cells and in several hematopoietic lineage cells,while it was ambiguous whether CaSR is expressed in peripheral blood polymorphonuclear leukocytes [42].Fifteen years later,Li et al.[60] conf rmed the protein and mRNA expression of CaSR in human peripheral blood T lymphocytes and reported that activator of CaSR (Gd3+and Ca2+) promoted the secretion of proinf ammatory cytokines (IL-6 and lymphotoxin)in a concentration-dependent manner through the partial MAPK and NF- κ B pathways [60].These f ndings revealed that the immune regulation effect of CaSR on T cells may be realized by regulating intracellular signal and cytokine products,and fur -ther research is needed to determine whether regulation of CaSR function on T cells can be a method to treat the disease.
As inf ammatory cells,the role of T lymphocytes in some inf ammatory and sterile diseases,such as sepsis and MI,has been studied.In sepsis,upregulation of CaSR was observed in T lymphocytes of rats with sepsis.T lymphocytes obtained from rats with sepsis were exposed to activator and inhibitor of CaSR to study the function of CaSR in sepsis.It was found that increased CaSR expression could promote the secretion of proinf ammatory (TNF) and anti-inf ammatory(IL-4) factors and apoptosis of T lymphocytes through the partial MAPK and NF- κ B pathways[61].Although elevated CaSR expression promoted the expression of both proinf ammatory and anti-inf ammatory cytokines in sepsis,the analysis of the ratio of the two types suggests that proinf ammatory cytokines are predominant in CaSR-induced secretion of inf ammatory cytokines.In the same year,the same research team also found that activated CaSR on T lymphocytes could increase the expression of transient receptor potential channel 3 (TRPC3) 3 and TRPC6 through the PLC-IP3 pathway,thereby synergistically increasing the intracellular Ca2+concentration and promoting the apoptosis of T lymphocytes in sepsis [62].Therefore,inhibiting CaSR to reduce the apoptosis of T lymphocytes and the predominant secretion of proinf ammatory cytokines in sepsis may be an ef fective method to increase the survival rate of patients with sepsis.
MI is a sterile response in which lymphocytes play an important role [63].Zeng et al.[64] conducted the f rst study on the role of CaSR in MI.In acute MI and percutaneous coronary intervention (PCI) patients,increased CaSR expression,cytokine secretion,and apoptosis were observed in the T lymphocytes,and these results were probably associated with the NF- κ B pathway.In these patients,CaSR promotes both proinf ammatory and anti-inf ammatory cytokines in T lymphocytes,which is similar to the case in sepsis,and the levels of proinf ammatory cytokines decline more quickly than those of anti-inf ammatory cytokines.This means that CaSR on T lymphocytes plays a role in both the early injury stage and the late repair stage in MI.Moreover,the apoptosis ratio of T lymphocytes increased obviously at the onset of acute MI and on the f rst day of PCI,and it then returned to the baseline gradually on the third day of PCI.The expression of CaSR on T lymphocytes after MI is related to the stage of the disease and the state of the inf ammatory response;therefore,specif c regulation of CaSR can avoid unnecessary damage after MI.It was reported that expression of CaSR in cardiomyocytes was positively correlated with the sensitivity to MI in atherosclerotic rats [65].Zeng et al.[66]clarif ed the causal relationship between CaSR in T lymphocytes and MI.When hypoxic/reoxygenated mouse cardiomyocytes were co- cultured with human peripheral blood T lymphocytes or CaSR-silenced T lymphocytes,necrosis and apoptosis of cardiomyocytes,cytokine secretion,and the levels of MAPK pathway–related proteins of T lymphocytes were signif cantly increased or reversed.These f ndings demonstrate that CaSR on T lymphocytes promotes cardiomyocyte injury and damaged cardiomyocytes promote the activation of CaSR on the T lymphocytes and the secretion of cytokines to amplify the inf ammatory reaction.This reciprocal process is vital to MI development.
Ca 2 + in the environment plays an important role in the immune response of B lymphocytes.However,B lymphocytes can neither express CaSR nor be inhibited by CaSR-specif c inhibitors,so it is believed that the calcium receptor of B lymphocytes is not conventional CaSR [67].

Table1 Expression of,Functions of,and Diseases Related to Calcium-Sensing Receptor in Immune Cells.
These studies suggest that CaSR on T lymphocytes contributes to cytokine secretion and apoptosis,which play important roles in sepsis and MI.Although current studies have shown that CaSR on T lymphocytes regulates cytokine release and apoptosis of T cell subsets,whether it affects the phenotypic differentiation and changes in the proportions of T cell subsets and whether it plays a role in other diseases still require further study.
CaSR and lmmune Cell Migration
Immune cell migration is essential in the immune response and inflammation (see Table1).In addition to the functions of CaSR described above,CaSR is required for mineral trioxide aggregate (MT A)-induced cell migration in human acute T-cell leukemia cell Jurkat cells,THP-1 cells,human neutrophil-like HL-60 cells,human U937 monocytes,and mouse CD4+T cells.The CaSR-PI3K-CDC42 cascade and the CaSR–PLCγ–myosin light chain kinase cascade regulate MT A-induced cell chemotaxis and chemokinesis,respectively [68].Therefore,when tissue damage occurs,MT A can promote the recruitment of immune cells to the damaged site through CaSR on immune cells so as to promote the immune response and injury healing,and CaSR may become an important tar get for regulating the migration of immune cells in different diseases.
Conclusions and Future Perspectives
CaSR,which was initially identif ed in the parathyroid gland,is ubiquitously expressed and exerts specif c functions in multiple cells.The main role of CaSR is to regulate calcium homeostasis,but an increasing number of studies have also investigated its role in non-calcium-regulating cells.We have reviewed the expression and role of CaSR in some immune cells (Table1);however,the expression and role of CaSR have not been studied in natural killer cells,dendritic cells,eosinophils,basophils,and mast cells of the immune system.Current studies have shown that CaSR on immune cells is involved in a variety of disease processes.However,the roles and mechanisms of CaSR in other diseases need to be studied further to provide a basis for its use as an effective target for the treatment of these diseases.
AcknowledgmentsThis study was supported by the National Natural Science Foundation of China for Xinhua Yin and Wenxiu Liu (81370319 and 81700318),the China Postdoctoral Science Foundation for Wenxiu Liu(2018M631957),the Hei Long Jiang Postdoctoral Fund for Wenxiu Liu (LBH-Z17145),Doctor Funds of the First Aff liated Hospital of Harbin Medical University for Wenxiu Liu (201613007),and the Innovation and Entrepreneurship Training Program for College Students of Harbin Medical University for Wenxiu Liu (201910226157).
Conf ilcts of lnterest
The authors declare that they have no conf icts of interest.
 Cardiovascular Innovations and Applications2021年2期
Cardiovascular Innovations and Applications2021年2期
- Cardiovascular Innovations and Applications的其它文章
- Myocardial Fibrosis in the Pathogenesis,Diagnosis,and Treatment of Hypertrophic Cardiomyopathy
- Using Three-Dimensional Lorenz Scatter Plots to Detect Patients with Atrioventricular Node Double Path Caused by lnterpolated Ventricular Premature Systoles:A Case Study
- Mediastinal Tuberculoma Mimicking Malignant Cardiac Tumor:A Case Report
- A Case of Pediatric Heart Failure Caused by Anomalous Origin of the Left Coronary Artery from the Pulmonary Artery:Case Report and Literature Review
- Comparison of Diagnostic Effects of T2-Weighted lmaging,DWl,SWl,and DTl in Acute Cerebral lnfarction
- The Relationship between Abnormal Circadian Blood Pressure Rhythm and Risk of Readmission in Patients with Heart Failure with Preserved Ejection Fraction
