Uterine Expression of WNT7A and β-catenin After Induction of Oestrus in Sheep
Yuan Meng-yi, Chang Di, Xie Tong-tong, Sheng Xi-hui, Feng Zi-yan, Fu Bo-fan, Li Qian-ru, Liu Fang, Guo Yong, Ding Jin, Wang Xiang-guo*, and Ni He-min
1 College of Animal Science and Technology, Beijing University of Agriculture, Beijing 102206, China
2 College of Economics and Management, Beijing University of Agriculture, Beijing 102206, China
3 Beijing Xiongte Animal Husbandry Co., Ltd., Beijing 102206, China
Abstract: WNT7A and β-catenin localisations and roles in regulating periimplantation ovine conceptus development under natural estrous conditions have been elaborated. However, their locations and expression patterns have not been reported under induction of oestrus. The localisation, expression and function of WNT7A and β-catenin in the uterine tissues of the early pregnant and non-pregnant sheep on days 10, 12, 14, 16 and 18 following artificial induction of oestrus were investigated by means of in situ hybridisation, real-time RT-PCR, immuno-histochemistry and western blotting methods. WNT7A and β-catenin mRNA and protein were both restricted to the apical surfaces of the uterine luminal epithelium (LE) and glandular epithelium (GE). In pregnant sheep, protein localisation of WNT7A and β-catenin was observed both in the endometrial LE and GE. Their staining presented on day 10, increased between day 12 and day 16, and decreased on day 18. WNT7A and β-catenin mRNA and protein expression increased initially and then decreased from day 10 to day 18, peaking on day 16, and β-catenin reaching a peak on day 18 in the uterine tissues of pregnant sheep (p<0.05). By contrast, no significant changes in WNT7A and β-catenin mRNA and protein expression levels were observed from day 10 to day 18 of the oestrus cycle in the uterine tissues of non-pregnant sheep (p<0.05). Additionally, WNT7A and β-catenin mRNA and protein expression levels in the uterine tissues of the early pregnant sheep were significantly higher than those of non-pregnant sheep (p<0.05). Treatment of endometrial epithelial cells with WNT7A increased the mRNA expressions of β-catenin, c-myc and Cyclin D1. These results provided an underlying mechanism of periimplantation ovine conceptus development under induction of oestrus.
Key words: sheep, uterus, β-catenin, WNT7A, pregnant
Introduction
The key to the regulation of embryo implantation in mammals is a series of factors secreted by the maternal uterus. The successful establishment of a mammalian pregnancy is guaranteed by the spatial and temporal synchronization of an activated embryo and a receptive uterus. A large number of studies have confirmed that days 10-18 of the initial implantation process of the foetus are critical for successful pregnancy in ruminant animals (Spenceret al., 2004; Dorniaket al., 2013; Spenceret al., 2008; Pariaet al., 2002). During this period the expression of related genes, which include mucin glycoprotein (MUC1), glycosylated cell adhesion molecule 1 (GlyCAM1), interferon stimulated gene 15 (ISG15), integrin and osteopontin, largely determines the final fate of the foetus. Although researches on the activation of the animal embryo have made good progresses, the understanding of the key factors that regulate uterus capacity has lagged (Sistiet al., 2016). Researches on the regulation of uterine capacity changes in the early pregnancy have become a hot spot in the field of animal reproductive biology.
At present, researches on human and rats, mice, rabbits, guinea pigs, sheep, cattle, pigs and macaques have been widely studied at home and abroad (Spenceret al., 2008; Wang and Dey, 2006). The factors involved in the precise regulation of the early pregnancy in ruminant livestock such as sheep are quite different from those in these experimental animals. For sheep, except that progesterone (P4) and prostaglandin (PG) E2 differ greatly in their adjustment modes, other major regulatory factors include MUC-1, GlyCAM-1, interferon tau (IFN-τ), the highly conserved secretory WNT family of glycolproteins (e.g. WNT7A), ISG15, integrin, galectin-15 and osteopontin (Spenceret al., 2008; Igwebuikeet al., 2009; Spenceret al., 2007). The Wnt signalling pathway plays an important role in the attachment and placenta development of sheep embryos (Satterfieldet al., 2008). Previous studies found that the Wnt signalling pathway also plays an important role in the early development of cattle placenta (Luet al., 2013). Recent studies have confirmed that the regulatory effect of P4 on the Wnt signalling pathway in the endometrial epithelium is closely related to the development and growth of sheep blastocysts (Hayashiet al., 2007).WNT7Ais an important Wnt system gene that is induced by IFN-τ, which directly affects the development of the female reproductive system and the maintenance of normal uterine function in adult ovis (Hayashiet al., 2007). A large number of studies have confirmed that the hatching and embryo extension process in sheep happens from day 12 to day 15 after mating and requires maintenance of a high level of P4in vivo. An extension of the blastocyst, with secretion of the pregnancy recognition signal and trophoblast cell proliferation and differentiation, is a sign of sheep early pregnancy, and it is also the key to IFN-τ secretion and conceptus implantation (Guillomot, 1995). Related study confirmed that WNT7A regulates the proliferation of trophoblast cells by the msh homeobox 2 (MSX2) and myelocytomatosis oncogene (MYC) genes in ovis (Hayashiet al., 2007). As an important part of the classic Wnt signalling pathway,β-catenin plays a role in regulating cell growth, differentiation and apoptosis (Veemanet al., 2003). While there are studies in ruminants reported that bovine embryo-derived WNTs are dispensable for blastocyst formation, but participate in regulation of ICM proliferation (Tribuloet al., 2017).
Sheep are important economic animals, and making full use of its reproductive potential will ensure the healthy and rapid development of the national breeding and dairy industry. Sheep are seasonal oestrus animals, and the artificial induction of oestrus is a widely used technique to improve the efficiency of sheep breeding. It has been reported that WNT7A is a conserved regulator of conceptus-endometrial interactions in mammals and regulates periimplantation ovine conceptus development under natural oestrus-conditions (Hayashiet al., 2007). However, it may not be possible to mimic the synchronized expression of related regulatory genes in time and space that occurs during natural oestrus within the process of implantation after embryo transfer. In this study,in situhybridisation, semi-quantitative polymerase chain reaction (PCR), immuno-histochemistry and western blotting were used to detect the cellular localisation and expression of WNT7A andβ-catenin mRNA and protein in the early pregnant and non-pregnant sheep uterine tissues following artificial induction of oestrus and to explore the function of WNT7A on endometrial epithelial cells, with the aim of providing reference points related to embryo transfer, oestrus synchronisation and superovulation in this species.
Materials and Methods
All the experimental and surgical procedures involving animals were approved by the Beijing Laboratory Animal Management Committee.
Animals and tissue collection
Mature ewes (Ujumuq, 1-2-years of age) were purchased from Wardell Agri-tech Corporation (Beijing, China) and housed using routine breeding and reproductive approaches. Ewes (n=50) that exhibited at least two oestrous cycles of the normal duration (16-18 days) were randomly divided into two groups of 25 each: (1) artificial oestrus and nonpregnant; and (2) artificial oestrus and pregnant.
Ewes assigned to the pregnant status were bred with intact rams at oestrus and at 12 h post-oestrus, and the non-pregnant ewes were bred with vasectomized rams at the same time points. Artificial oestrus was induced with a controlled vaginal sustainedrelease drug device method (EAZI-BREED CIDR, Australia)+PG at 0.1 mg per sheep (Shanghai Institute of Planned Parenthood Research, Shanghai) for 12 days according to the instruction manuals. After that, the concentrations of progesterone in serum from day 10 to day 18 were assayed using the ELISA kits (BD Bioscience, San Jose, CA, USA), according to the manufacturer's recommendations. The optical densities at 450 nm of each well were determined using a micro-plate reader (Model 680, Bio-Rad, Hercules, CA, USA). The serum progesterone concentration was used to verify the reproductive status of the animals. When progesterone secretion met the requirements, sheep were killed followed by collection of their uterine tissues on days 10, 12, 14, 16 and 18 postoestrus, when the serum progesterone concentration was determined by Elecsys 2010 electroluminescent immunoassay and reached the indicated value listed in Table 1. Several 0.5-cm sections from the midportion of each uterine horn were immediately fixed and embedded in embedding medium or paraffin and used forin situhybridisation experiments and immunohistochemical staining, or frozen, stored and processed together for semi-quantitative PCR and western blotting analyses.

Table 1 Change of progesterone after estrus
Sheep endometrial epithelium cells (EEC) separation and identification
Uterus were obtained from slaughter house, endometrial epithelium cells (EEC) were separated and purified according to published procedures (Hamonicet al., 2018). The epithelial cell identities were monitored based on their morphologies. Immunofluorescence detection was used for purity identification of EECs. EECs at passage 3 were cultured in 24-well plates for 24 h. Immuno-fluorescent staining of keratin was performed. EECs were fixed in 4% paraformaldehyde for 30 min, and then permeabilized for 15 min with 0.1% Triton X-100 in PBS, subsequently blocked for 1 h with 5% BSA in PBS at room temperature, and co-incubated with anti-keratin antibody (Abcam, 1 : 500 dilution, ab111599) at 37℃for 2 h, respectively. After washing, followed by incubation with anti-rabbit secondary antibody (Invitrogen, A21206; 1 : 500 dilution) at 37℃ for 1 h,and the nuclei were stained with 4′, 6-diamidino-2-phenylindole (DAPI) for 3-5 min. The fluorescent signals were examined under a fluorescence microscope (Olympus, Japan).
Real-time reverse transcription-polymerase chain reaction (real-time RT-PCR) detection for WNT signaling-related genes
EECs were treated with 66 ng • mL-1humanrecombinant WNT7A (eBioscience Inc., San Diego, CA, USA) for 24 h. The concentration of WNT7A was chosen, because it was the upper limit of the range suggested forbiological activity of the product by the manufacturer (Tribuloet al., 2017). The total RNA of WNT7A treatment EECs was extracted using the Trizol(Invitrogen, Inc., Carlsbad, CA, USA), and cDNA was synthesized using the PrimeScriptTMRT Reagent Kit (TaKaRa Bio, Inc., Dalian, China), according to the manufacturer's protocols. Real-time PCR was subsequently performed using an ABI 7500 Sequencing Detection System and SYBR Premix ExTaqTM. The GenBank accession numbers and primer sequences ofβ-catenin, c-myc, Cyclin D1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were summarized in Table 2. All the reactions were performed at least three independent experiments and the calculated number of copies of target genes was normalized to the number of GAPDH mRNA copies in the same sample.

Table 2 Primer sequences used for qRT-PCR
In situ hybridisation
In situhybridisation with digoxigenin (DIG)-labelled RNA was performed in accordance with standard protocols (Ugajinet al., 2012) with slight modifications. Tissue sections were deparaffinized by placing each slide in three changes of xylene for 10 min each, hydrated through a graded ethanol series, treated with 0.2 mol • L-1HCl at room temperature for 10 min and then washed in phosphate-buffered saline (PBS; pH 7.4). The hydrated slides were treated with pre-digested proteinase K (10 μg • mL-1in 10 mmol • L-1Tris-HCl, 1 mmol • L-1EDTA, pH 8.0), fixed with 4%paraformaldehyde, and acetylated with acetic anhydride. After ethanol dehydration, the sections were air dried and the tissue sections on the slides were encircled with a PAP-PEN (Daido Sangyo Co., Japan). Hybridisation solution (a 30 μL aliquot of 50% formamide; 10 mmol • L-1Tris-HCl, pH 7.6; 200 μg • mL-1yeast tRNA; 1×Denhardt's solution; 10% dextran sulphate; 600 mmol • L-1NaCl; 0.25% SDS; and 1 mmol • L-1EDTA, pH 8.0) containing 0.5 ng • μL-1of the DIG-labelled RNA probe was placed on each section. The slides were placed without coverslips in an airtight, humidified chamber containing 50% formamide, and the tissue sections were completely covered in standard saline citrate (SSC; 2×). Hybridisation was conducted at 42℃ overnight. After hybridisation, the slides were washed twice in 2×SSC and twice in 0.2×SSC, for 15 min each time, at 37℃. Finally,in situhybridisation detection of the DIGlabelled RNA probes was performed in accordance with the BOSTER (Mannheim, FRG) instruction manual with the following modifications (Table 3). After washing briefly (5 min) in buffer I (100 mmol • L-1Tris-HCl, 150 mmol • L-1NaCl, pH 7.5), the slides were incubated in buffer I with 10% normal bovine serum (Sigma, USA) and 0.2% Tween 20 for 1 h at room temperature. After making a 1 : 700 dilution of the anti-DIG conjugate in buffer I containing 10% normal bovine serum, 50-100 μL of the antibody solution was applied to each tissue section. The slides were incubated in buffer I for 1 h at room temperature in a humidified chamber, washed in buffer I for 15 min, and in buffer II (100 mmol • L-1Tris-HCl, 100 mmol • L-1NaCl, pH 9.5) for 3 min. For colourisation, a solution equal to 50-100 μL (per mL: 4.2 μL 5-bromo-4-chloro-3-indolyl phosphate and 4.5 μL nitro blue tetrazolium) was applied to each section. The slides were incubated at room temperature in buffer II in the dark in a humidified chamber. After 18 h, the reactions were stopped by washing the slides for 3 min in 10 mmol • L-1Tris-HCl, 1 mmol • L-1EDTA, pH 8.0, at room temperature. Finally, the sec- tions were mounted in mounting medium (10 mmol • L-1Tris-HCl, 1 mmol • L-1EDTA, 50% glycerine, pH 7.0). The process was repeated three times for each section to determine the consistency of the observations.

Table 4 Sequences of oligonucleotide primers for real-time PCR

Table 3 mRNA sequence direct at sheep WNT7A and β-catenin
Semi-quantitative PCR analysis
The total RNA was isolated from sheep uterine tissues using TRIzol reagent (Invitrogen, Life Technologies, Grand Island, NY, USA). The RNA concentration and purity were measured using a method previously described (Linet al., 2015). The 1 µg cDNA was synthesized using a PrimeScript RT Reagent Kit (TaKaRa Bio, Inc., Dalian, China), following the manufacturer's instructions. RT-PCR was conducted using Eppendorf Mastercycler Nexus PCR instruments, according to standard RT-PCR protocols.β-actin was amplified in parallel with the target genes and used as a normalisation control. The PCR system reactions (25 µL) contained 12.5 µL 2×PCR Master Mix, 1 µL each primer (10 μmol • L-1each), 1 µL cDNA template and 9.5 μL ddH2O. The cycling conditions were 95℃ for 5 min, followed by 40 cycles of 95℃ for 15 s, 61℃ for 30 s, and 72℃ for 30 s. Gene expression levels were determined using 1% agarose gel electrophoresis of PCR products and photography of the gel imaging system.
The cDNA was subjected to RT-PCR using the primer pairs listed in Table 4.
Immuno-histochemistry
Uterine tissues were fixed in 4 % (v/v) paraformaldehyde (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) in PBS (pH 7.4) for 24 h, dehydrated through a graded ethanol series, and embedded in paraffin. The 5-mm thick sections were mounted onto glass slides precoated with poly-L-lysine solution (Sigma) and then incubated at 37℃ overnight. After dehydration, antigen retrieval was performed by heating the slides in citrate buffer (pH 6.0) in a 750 W microwave oven twice for 5 min each, after which the slides were washed in PBS. The sections were pre-treated with 0.3% (v/v) H2O2in methanol for 10 min to quench the endogenous peroxidase activity. After washing with PBS, the sections were incubated with 10% rabbit serum for 30 min at 37℃. After the blocking step, the sections were incubated with rabbit anti-sheep WNT7A IgG (Beijing Jiaxuan Biotech Co., China; 1 mg • mL-1, sequence: [C]QQSRARGSAEQQRF) andβ-catenin IgG (Abcam, 1 : 1 000 dilution) for 12 h at 4℃ and washed with PBS, followed by incubation with the biotinylated anti-rabbit IgG antibody (Beijing 4A Biotech Co., Ltd., Beijing, China) for 1 h at 37℃.
Sections were washed three times with PBS, and then incubated with horseradish peroxidase (HRP)-labelled streptavidin for 30 min at 37℃. Thereafter, positive reactions were visualised with diaminobenzidine-peroxidase substrate (Sigma) and counterstained with haematoxylin for 30 s. Negative control slide staining was conducted in parallel by omitting the primary antibody or substitution with an appropriate dilution of goat serum (data not shown). Photomicrographs of representative fields of the immuno-histochemistry slides were taken in brightfield using a Zeiss (New York, NY, USA) photomicroscope fitted with a CC10B colour compensating filter (Eastman Kodak, Rochester, NY, USA) for use with daylight film with a tungsten light source and Kodacolor Gold 100 (Eastman Kodak, Rochester, NY, USA) color film.
Protein extraction and western blotting
Proteins from uterine tissues were extracted using a total protein and mitochondrial protein extraction kit in accordance with the manufacturer's instructions (Nanjing KeyGen Biotech Co., Ltd., Nanjing, Jiangsu, China), and the total protein was quantified using a BCA protein assay kit (Applygen, Beijing, China) in accordance with the manufacturer's instructions. The total protein (20 μg) from each sample was separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) (Invitrogen, Life Technologies, Grand Island, NY, USA) followed by electrotransfer to nitrocellulose membranes (Pierce, Rockford, IL, USA). Kaleidoscope prestained SDSPAGE molecular weight standards (Bio-Rad) were loaded onto each gel. Blots were blocked overnight at 4℃ in t-butyldimethylsilyl+Tween (TBST) (20 mmol • L-1Tris, pH 7.5; 137 mmol • L-1NaCl, 0.05% Tween 20)containing 5% dried milk. The blots were then incubated with anti-WNT7A (Beijing Jiaxuan Biotech Co.,; 1 mg • mL-1, sequence: [C]QQSRARGSAEQQRF) andβ-catenin (Abcam, 1 : 1 000 dilution) or anti-Tubulin (Santa Cruz, 1 : 500 dilution) in TBST containing 5% dried milk with shaking overnight at 4℃. Blots were washed four times in TBST for 10 min each and placed in goat anti-rabbit IgG-HRP conjugate (1: 5 000 dilution of 1 mg • mL-1stock; KPL, Bethesda, MD, USA) for 1 h at room temperature with shaking. Blots were normalised using tubulin to correct for protein loading differences. The densitometric values of the immunoblot signals were obtained from the three separate experiments using the Image J image processing tool kit (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
For each group, the uterine tissues were collected from at least three individual animals for each collection time point. All the experiments were independently replicated at least three times, and the data are presented as the mean±standard deviation. The data were analyzed using an analysis of variance, followed by Fisher's Least Significant Different Test and Independent-SamplesTtest in SPSS software (Version 16.0; SPSS, Inc., Chicago, IL, USA). Results were considered statistically significant atp<0.05.
Results
Detection of WNT7A and β-catenin mRNA distribution in different uterine tissues in a time course following induction of oestrus
Aimed to determine whether differences existed for WNT7A andβ-catenin mRNA localisations in the uterine tissues of sheep at different time points following the artificial induction of oestrus. WNT7A andβ-catenin mRNA localisations in the uterine tissues of the early pregnant and non-pregnant sheep were detected usingin situhybridisation on days 10, 12, 14, 16 and 18 of the oestrous cycle. In pregnant and non-pregnant ewes alike, it was found that WNT7A andβ-catenin mRNA were both restricted to the apical surfaces of the uterine luminal epithelium (LE) and glandular epithelium (GE). Similarly, prominent staining for both WNT7A andβ-catenin mRNA was observed on day 10 and day 12 of the oestrus cycle in the uterine tissues of the non-pregnant sheep, and subsequently shallowed from day 14 to day 18. In contrast, prominent staining for both WNT7A andβ-catenin mRNA was observed on days 10, 12 and 14 in the uterine tissues of the pregnant sheep, which was followed by a significant, transient absent on day 16 of the oestrus cycle in these sheep. The degree of WNT7A andβ-catenin mRNA staining observed on day 10 was restored on day 18 of the oestrus cycle in the pregnant sheep. Overall, WNT7A andβ-catenin mRNA staining in the uterine tissues of the pregnant sheep was more intense than that of the non-pregnant sheep from day 10 to day 18 of the oestrus cycle (Figs. 1 and 2).

Fig. 1 Localisation of WNT7A mRNA in uterine tissues of early pregnant and non-pregnant sheep following artificial induction of oestrus as examined by in situ hybridisationLabels a-1, a-2, a-3, a-4 and a-5 represent uterine tissues from non-pregnant sheep on days 10, 12, 14, 16 and 18, respectively; b-1, b-2, b-3, b-4 and b-5 represent uterine tissues from pregnant sheep on days 10, 12, 14, 16 and 18, respectively. L, Luminal epithelium; G, Glandular epithelium; S, Stromal cells. Section, Transverse section of uterus (×200).

Fig. 2 Localisation of β-catenin mRNA in uterine tissues of early pregnant and non-pregnant sheep following artificial induction of oestrus as examined by in situ hybridisationLabels a-1, a-2, a-3, a-4 and a-5 represent uterine tissues from non-pregnant sheep on days 10, 12, 14, 16 and 18, respectively; b-1, b-2, b-3, b-4 and b-5 represent uterine tissues from pregnant sheep on days 10, 12, 14, 16 and 18, respectively. L, Luminal epithelium; G, Glandular epithelium; S, Stromal cells. Section, Transverse section of uterus (×200).
WNT7A and β-catenin mRNA expression in uterine tissues of early pregnant or nonpregnant sheep following induction of oestrus
Using RT-PCR, increased WNT7A mRNA expression levels from day 12 to day 16 of the oestrus cycle were observed, peaking on day 16 and declining from day 18, thereafter, in the uterine tissues of the pregnant sheep. By contrast, no significant changes in WNT7A mRNA expression levels were observed from day 10 to day 18 of the oestrus cycle in the uterine tissues of the non-pregnant sheep following the artificial induction of oestrus. The level of WNT7A mRNA expression in the uterine tissues of the pregnant sheep was higher than that of the non-pregnant sheep from day 10 to day 18 of the oestrus cycle (p<0.05; Fig. 3A and B).
Increased expression levels ofβ-catenin mRNA were observed from day 12 to day 16 of the oestrus cycle, peaking on day 16 and declining from day 18 thereafter, in the uterine tissues of the pregnant sheep. In the non-pregnant sheep,β-catenin mRNA expression levels were reduced on days 12, 14 and 16, restored on day 18. The level ofβ-catenin mRNA expression in the uterine tissues of the pregnant sheep was higher than that of the non-pregnant sheep from day 12 to day 16 of the oestrus cycle, but there was no significant difference between the groups on day 10 and day 18 (p<0.05; Fig. 3A, B and C).
WNT7A and β-catenin protein localisation in uterine tissues of early pregnant and nonpregnant sheep following induction of oestrus
WNT7A andβ-catenin localisation in the sheep uterus during different stages of the oestrus cycle was investigated. Tissue sections of the non-pregnant group were stained negative, and the back-ground was colourless or light blue, with no yellow or tan staining, indicating the immune response with the streptavidin-biotin complex immuno-histochemical method had specificity (Figs. 4 and 5). Expression of WNT7A andβ-catenin was detected by immunohistochemistry on days 10, 12, 14, 16 and 18 of the oestrus cycle.
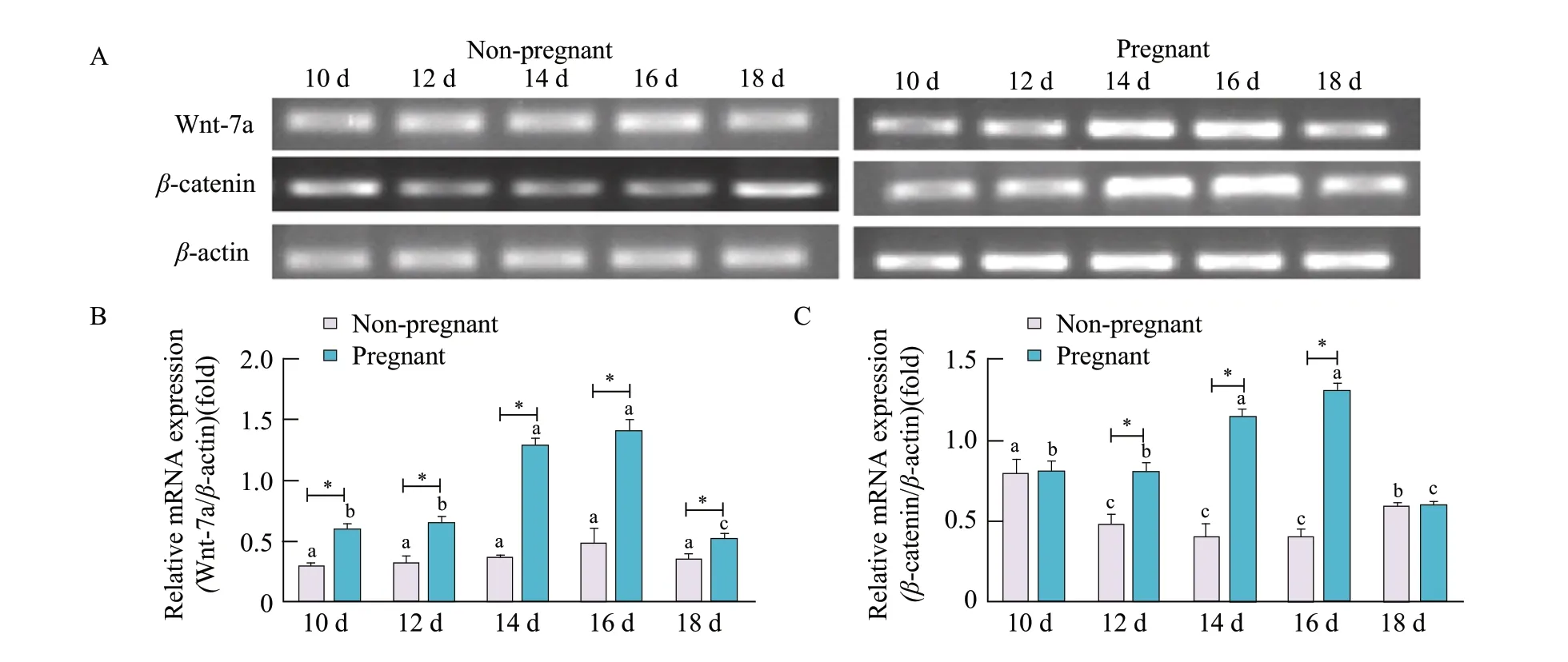
Fig. 3 WNT7A and β-catenin mRNA expression in uterine tissues of early pregnant or non-pregnant sheep after artificially-induced oestrus(A) RT-PCR to detect expression of WNT7A and β-catenin mRNA with β-actin shown as loading control. Densitometry determination of (B) WNT7A and (C) β-catenin mRNA PCR band intensity; data represent mean±standard deviations from three independent experiments (*p<0.05). *Significant difference (p<0.05) between pregnant and non-pregnant groups is from day 10 to day 18. Bars with different letters are significantly different in nonpregnant sheep or pregnant sheep on days 10, 12, 14, 16 and 18 (p<0.05).
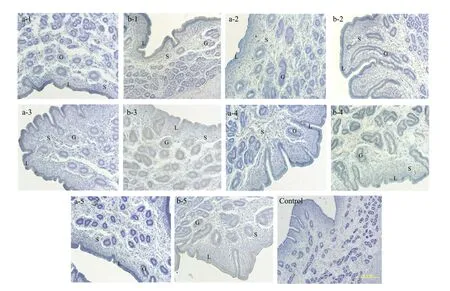
Fig. 4 Localisation of WNT7A protein in uterine tissues of early pregnant and non-pregnant sheep under artificial induction of oestrusLabels a-1, a-2, a-3, a-4 and a-5 represent uterine tissues of non-pregnant sheep on days 10, 12, 14, 16 and 18, respectively, after natural oestrus; b-1, b-2, b-3, b-4 and b-5 represent uterine tissues of pregnant sheep on days 10, 12, 14, 16 and 18, respectively, after natural oestrus. L, Luminal epithelium; G, Glandular epithelium; S, Stromal cells. Section, Transverse section of uterus (bars 1.0 mm).
In non-pregnant sheep, WNT7A localisation was restricted to the apical surface of the uterine LE only. In the uterine tissues of pregnant sheep; however, WNT7A localisation was observed on the apical surface of both LE and GE. The localisation of WNT7A was especially obvious in pregnant sheep on days 14 and 16 following oestrus (Fig. 4).
Most ofβ-catenin localisation was observed on the apical surface of the uterine LE, with slight expression on the apical surface of the uterine GE, in non-pregnant sheep. In the uterine tissues of pregnant sheep; however,β-catenin expression was observed on the apical surface of both LE and GE. The localisation ofβ-catenin was especially obvious in pregnant sheep on days 16 and 18 (Fig. 5).
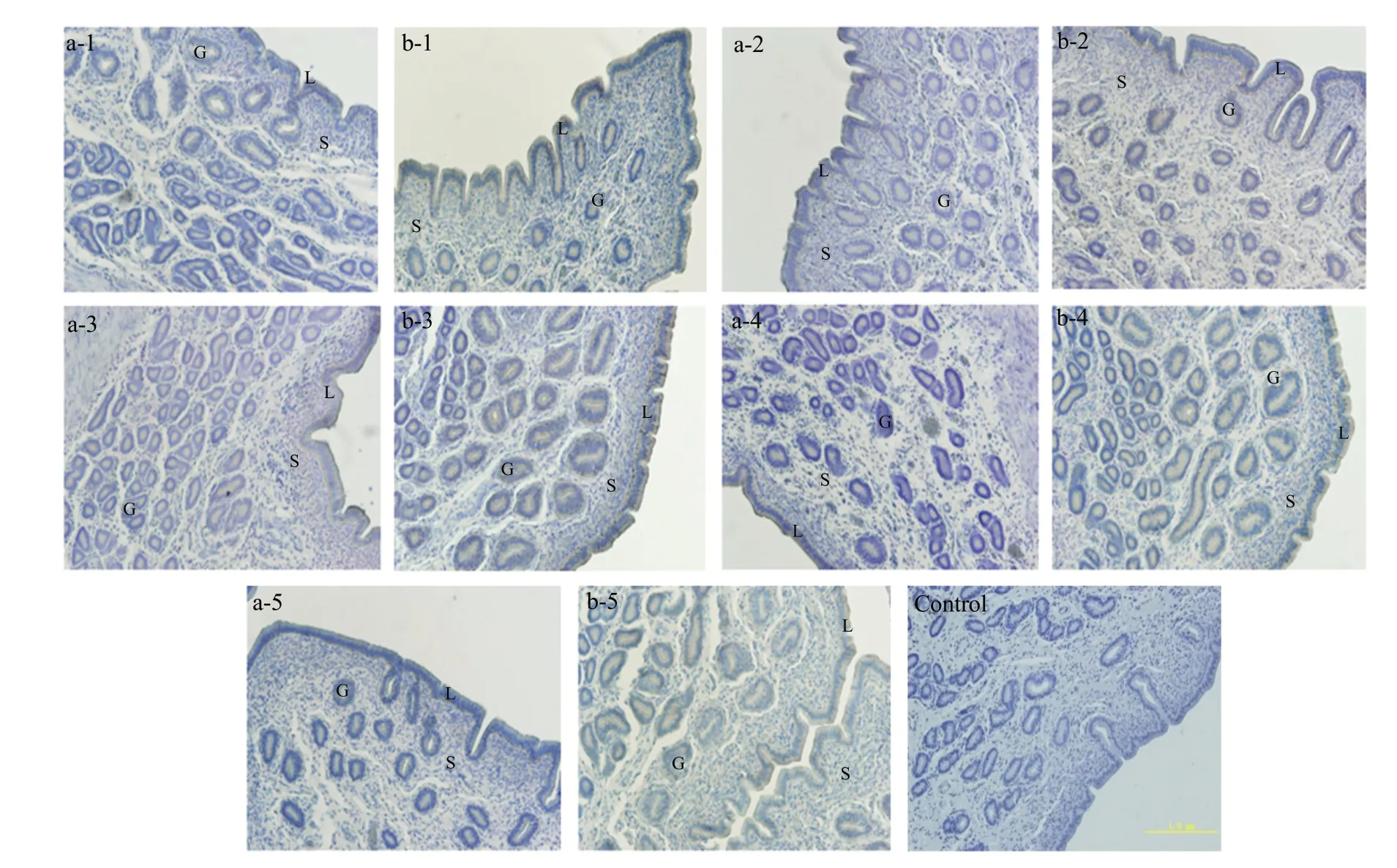
Fig. 5 Localisation of β-catenin protein in uterine tissues of early pregnant and non-pregnant sheep under artificial induction of oestrusLabels a-1, a-2, a-3, a-4 and a-5 represent uterine tissues of non-pregnant sheep on days 10, 12, 14, 16 and 18, respectively, after natural oestrus; b-1, b-2, b-3, b-4 and b-5 represent uterine tissues of pregnant sheep on days 10, 12, 14, 16 and 18, respectively, after natural oestrus. L, Luminal epithelium;G, Glandular epithelium; S, Stromal cells. Section, Transverse section of uterus (bars 1.0 mm).
WNT7A and β-catenin protein expression in uterine tissues of early pregnant and nonpregnant sheep following induction of oestrus
Changes in WNT7A andβ-catenin protein expression were measured using western blotting. In the uterine tissues of pregnant sheep, WNT7A expression increased and then decreased from day 10 to day 18, reaching the highest levels on day 16. In the uterine tissues of non-pregnant sheep; however, no significant changes in WNT7A protein expression were observed from day 10 to day 18 of the oestrus cycle. The expression level of WNT7A protein in pregnant sheep was higher than that in non-pregnant sheep from day 14 to day 16 of the oestrous cycle (p<0.05; Fig. 6A and B).
β-catenin expression decreased on day 12 and then increased from day 12 to day 18, reaching the highest levels on day 18 in the uterine tissues of pregnant sheep after natural oestrus. By contrast, there were no significant changes inβ-catenin protein expression levels on days 10-18 of the oestrous cycle in the uterine tissues of non-pregnant sheep. The expression ofβ-catenin protein in pregnant sheep was higher than that in non-pregnant sheep from day 16 to day 18 of the oestrus cycle and temporal lower than that on day 12 (p<0.05; Fig. 6A, B and C). Importantly, the results obtained from the western blotting experiments showed broadly similar trends to those obtained by RTPCR,in situhybridisation and immuno-histochemistry.
Activation of WNT signaling by exogenous WNT7A
Bovine endometrial epithelial cells (EEC) obtained by tissue mass culture at passage 3 showed a clear "paving stone" after pasting. To identify the purity of EEC, the cells were stained with the epithelial cell marker, keratin. In this study, all the EECs at passage 3 were specifically stained with keratin, demonstrating EECs were pure epithelial cells (Fig. 7A). Effects of human recombinant WNT7A on activation of WNT signaling in EECs were evaluated. Treatment of EECs with WNT7A increased the mRNA expression ofβ-catenin, c-myc and Cyclin D1 (p<0.01; Fig. 7B).
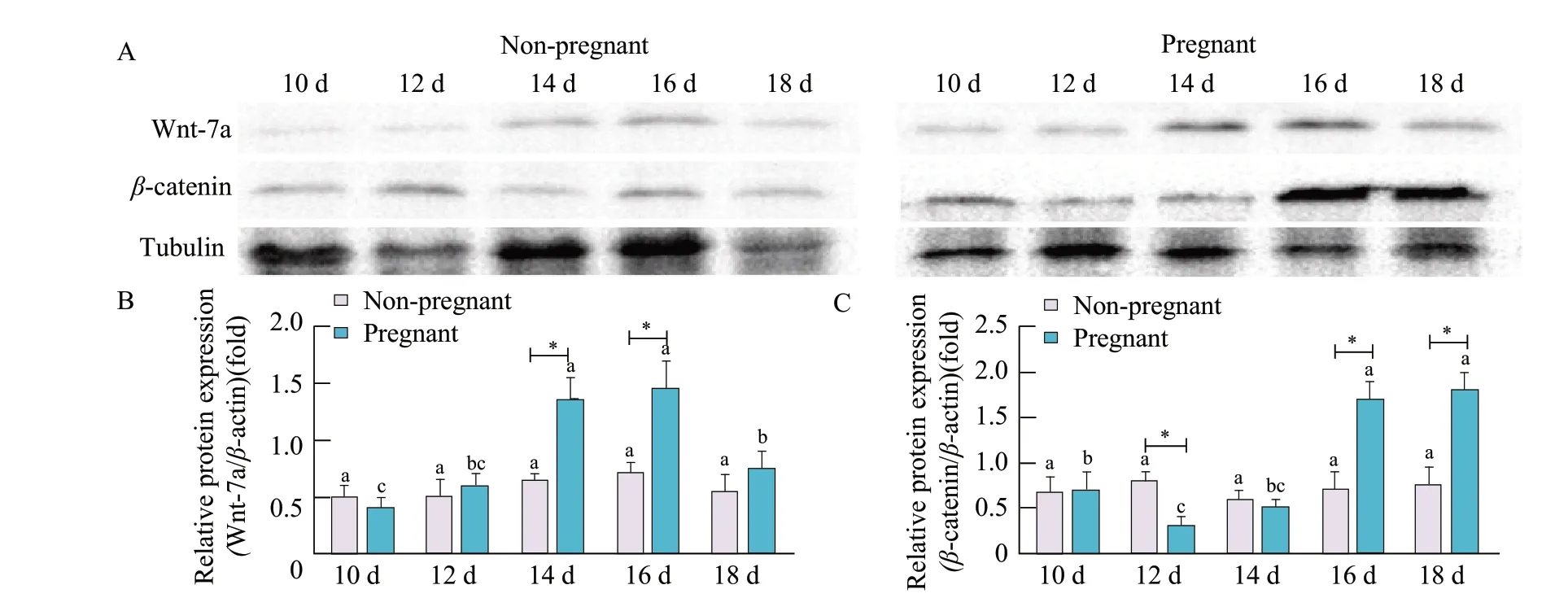
Fig. 6 Western blotting analysis of WNT7A and β-catenin expression in uterine tissues of early pregnant or non-pregnant sheep after artificial induction of oestrus(A) Western blotting of WNT7A and β-catenin with tubulin shown as loading control. Densitometric determinations of (B) WNT7A and (C) β-catenin protein band intensity; data represent mean±standard deviations from the three independent experiments (*p<0.05). *Significant difference (p<0.05) between pregnant and non-pregnant groups is from day 10 to day 18. Bars with different letters are significantly different in non-pregnant sheep or pregnant sheep on days 10, 12, 14, 16 and 18 (p<0.05).
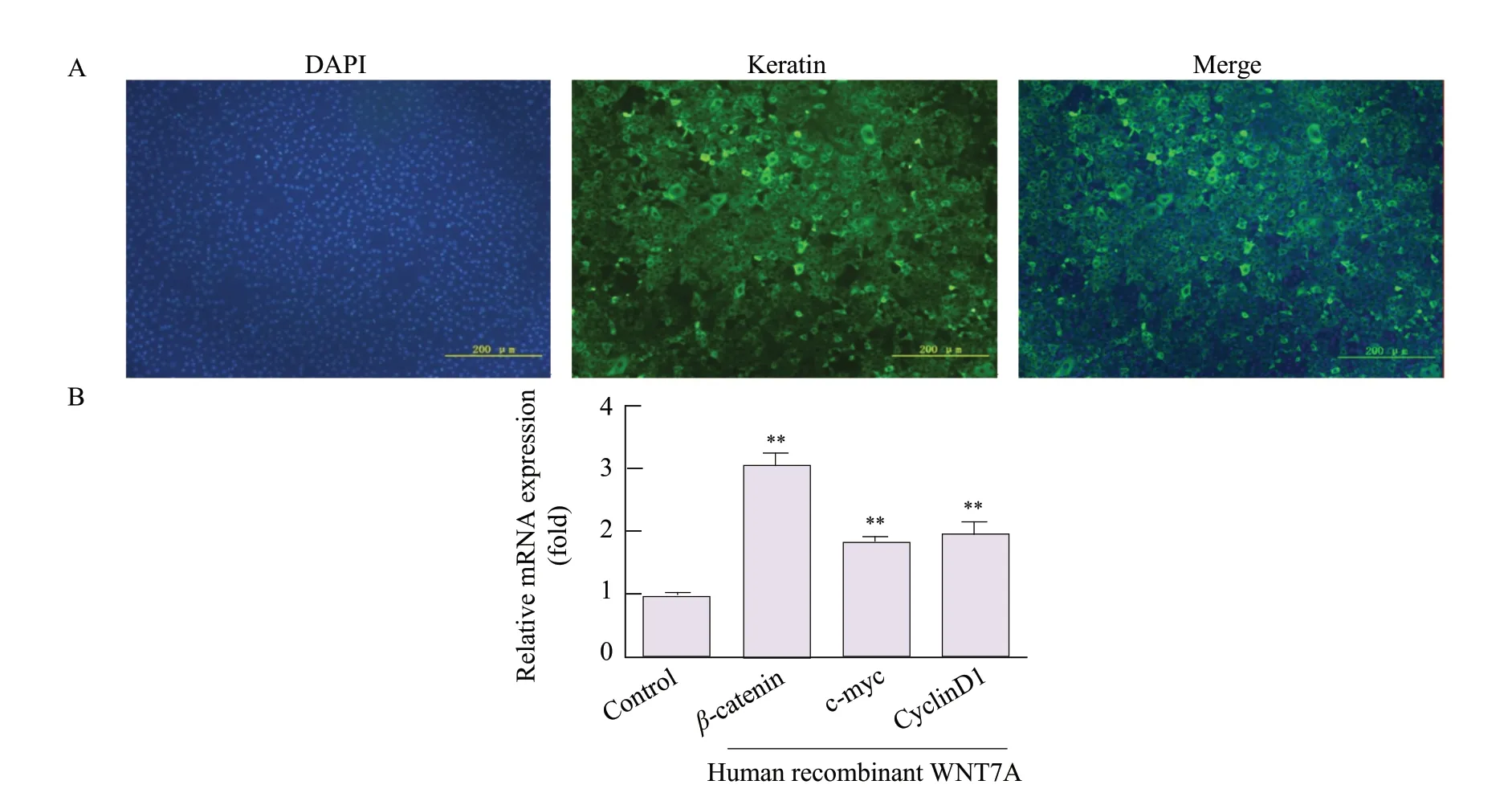
Fig. 7 Effects of human recombinant WNT7A on activation of WNT signaling(A) Immuno-fluorescence to detect expression of Keratin protein in EECs (B) qRT-PCR to detect expression of c-myc, Cyclin D1 and β-catenin mRNA in EECs treated with human recombinant WNT7A; data represent the mean±standard deviations from three independent experiments (**p<0.01).
Discussion
Techniques involving the artificial induction of oestrus are widely used to improve the efficiency of breeding in sheep, which are seasonal oestrus animals. However, the conception rate for sheep following artificially induced oestrus is significantly lower than that for sheep undergoing natural oestrus. This study focused evaluation on the cellular localisation and expression of WNT7A andβ-catenin mRNA and protein in the uterine tissues of the early pregnant and non-pregnant sheep after artificial induction of oestrus. WNT7A is a highly conserved signalling molecule that is widely distributed in various tissues of the body, interacting with the Wnt receptor through various secretion modes to regulate the expression of target genes. WNT7A also is a key regulator involved in cell fate, growth and differentiation and intercellular interactions (Nilsson,1958). Related studies reported that WNT7A mRNA presented in the luminal epithelium on day 10, was absent on day 12 and day 14, and increased between day 16 and day 20, and then increased to maximal abundance in LE/GE on day 20 in the uterus of natural estruspregnant sheep (Hayashiet al., 2007). In this study, WNT7A mainly distributed in the LE and GE of sheep uterus. However, WNT7A mRNA and protein increased between day 12 and day 16, and then increased to maximal abundance in LE/GE on day 16 in the uterus artificially induced estruspregnant sheep. The expression of WNT7A in the endometrium changed in response to the changes in hormone levels during the oestrus cycle, which was crucial for the development of the uterus, glandular formation and embryo implantation (Miller and Sassoon,1998). That was to say, the expression pattern of WNT7A was advanced under the action of inducing estrous hormone. In addition, no significant changes from day 10 to day 18 of the artificially induced oestrous cycle in the uterine tissues of non-pregnant sheep were observed. By contrast, in pregnant sheep an increased WNT7A expression level was observed from day 10 to day 18 of the artificially induced oestrus cycle, with a peak on day 16. The mRNA expression level of WNT7A in the uterine tissues of pregnant sheep was higher than that of nonpregnant sheep from day 10 to day 18 of the oestrus cycle. It had been speculated that WNT7A expression transformed the endometrium from an inhibitory state to an allowable implantation state, thus ensuring the successful implantation of the embryo after it entered the attachment stage.
As an adhesion factor,β-catenin played an important role in embryo implantation, which was essential to guarantee a successful pregnancy (Rowlandset al., 2000; Galbiatiet al., 2000).β-catenin was expressed in mammalian cartilage, uterus, breast and cancer cells, and a variety of cells. In one study,β-catenin was expressed in both endometrial epithelium and GE of pregnant mice, and the expression level decreased significantly during the implantation window (Liet al., 2005). In this study, there were significant changes in both the location and expression level ofβ-catenin in sheep uterus at different stages of pregnancy. Other studies had shown thatβ-catenin gene expression was consistent with the expression of progesterone receptor in sheep uterus from day 1 to day 15 after oestrus (Nilsson, 1958). It was well known that oestrous in mammals was modulated by different steroids, which included P4 and E2. The transition period from the follicular to the luteal phase involved a decrease in the E2 level to baseline, while the P4 level remained low. The duration of the normal oestrus cycle of sheep was 16-18 days, during which progesterone started to rise gradually on day 7, peaking on day 11, and gradually decreasing thereafter (Mohamedet al., 2005). The results of these studies on progesterone were consistent with this study, which showed that the mRNA expression ofβ-catenin appeared on day 10 and decreased on day 12, remaining at a lower level from day 12 to day 16. The expression ofβ-catenin mRNA was significantly increased from day 16 to day 18, which was consistent with a change in progesterone. Indeed, there was a peak of progesterone secretion at the end of oestrus cycle. The relative expression ofβ-catenin mRNA in the uterine tissues of pregnant sheep showed a trend of initially increasing and then decreasing from day 10 to day 18, peaking on day 16.β-catenin mRNA expression in the uterine tissues of pregnant sheep was higher than that in non-pregnant sheep on days 12, 14 and 18 of the oestrous cycle, and the changes were similar to those for WNT7A expression.
WNT7A was an important, well-established component of the Wnt signalling pathway and could activate the canonical Wnt pathway (Hartunget al., 2017). The expression ofβ-catenin, an important part of the classic Wnt signalling pathway, had been shown to increase in uterine tissues upon activation of this pathway (Hayashiet al., 2009). In this study,β-catenin mRNA expression reached a peak on day 16 in the uterine tissues of pregnant sheep, while related studies confirmed that progesterone expression reached a peak after 12 days of gestation in the late luteal phase. It had been speculated thatβ-catenin mRNA expression in the uterus of pregnant sheep was regulated by the Wnt family of signalling molecules. It was observed that the trends in WNT7A andβ-catenin protein expression paralleled those of WNT7A andβ-catenin mRNA expression. The peak inβ-catenin protein expression appeared after that of WNT7A protein expression, indicating the expression of these regulatory factors in time and space might differ during the process of embryo attachment. In order to confirm this hypothesis, effects of human recombinant WNT7A on activation of the Wnt signaling in EECs were evaluated. In this study, treatments of EECs with WNT7A increased the mRNA expressions ofβ-catenin,c-myc(a gene that promoted cell division and proliferation) andCyclin D1 (a regulatory gene of cyclin-dependent kinase CDKs). This finding confirmed the hypothesis that the Wnt/β-catenin signalling pathways in the blastocyst and uterus were initially suppressed during embryo implantation. The increase in WNT7A expression led to successful activation of the Wnt/β-catenin signalling pathway, relieving the inhibitory effect onβ-catenin protein and promoting the proliferation of EECs.
Conclusions
Overall, the cellular localisation and expression of WNT7A andβ-catenin mRNA and protein in the uterine tissues of the early pregnant and non-pregnant sheep was systematically compared under artificially induced oestrus. The findings offered reference points related to embryo transfer, oestrus synchronisation and superovulation.
 Journal of Northeast Agricultural University(English Edition)2021年1期
Journal of Northeast Agricultural University(English Edition)2021年1期
- Journal of Northeast Agricultural University(English Edition)的其它文章
- Identification of Co-dominant SSR Markers Associated with Genes Controlling α′- and α-subunit-null β-conglycinin Phenotypes in Soybean (Glycine max (L.) Merr.)
- Study on Marker-assisted Breeding of Soybean Vitamin E
- Effect of Drought Stress on Growth and Water Physiological Characteristics of Poa sibirica
- Biotransformation of Flavor Compositions During Fermentation of Litchi (Litchi Chinensis Sonn.) Fruits into Wine
- Effect of Endophytic Fungus on Pyrola calliantha H. Andr Responsed to Cold Stress
- Multi-objective Function Optimization for Environmental Control of a Greenhouse Based on a RBF and NSGA-II
