Identification of Co-dominant SSR Markers Associated with Genes Controlling α′- and α-subunit-null β-conglycinin Phenotypes in Soybean (Glycine max (L.) Merr.)
Pang Ze, Li Ming-xue, Zhou Jin-tao, Qiu Zhen-dong, Song Ying-ji, Song Yan-ru, Waqar Ahmed, Song Bo, and Liu Shan-shan
Key Laboratory of Soybean Biology in Chinese Ministry of Education, Northeast Agricultural University, Harbin 150030, China
Abstract: Studies have shown that the three subunits of β-conglycinin are the main potential allergens of soybean sensitive patients. And β-conglycinin has adverse effects on nutrition and food processing. So solation and production of lines with lower β-conglycinin content has been the focus of recent soybean breeding projects. Soybean lines with deficiency in one or all subunits of β-conglycinin have been obtained. An effective and rapid system to identify such mutations will facilitate genetic manipulation of the β-conglycinin subunit composition. Here, two segregating F2 populations were developed from crosses between Cgy-1/cgy-1 (CC), an α′-lacking line (Δα′), and DongNong 47 (DN47), a wild-type (Wt) Chinese soybean cultivar with normal globulin components, and Cgy-2/cgy-2 (CB), an α-lacking line (Δα), and DN47. These populations were used to estimate linkage among the cgy-1 (conferring α′-null) and cgy-2 (α-null) loci and simple sequence repeat (SSR) markers. Seven SSR markers (Sat_038, Satt243, Sat_307, Sat_109, Sat_231, Sat_108 and Sat_190) were determined to co-segregate with cgy-1, and six SSR markers (Satt650, Satt671, Sat_418, Sat_170, Satt292 and Sat_324) co-segregated with cgy-2. Linkage maps being composed of seven SSR markers and cgy-1 locus, and six SSR markers and the cgy-2 locus were then constructed. It assigned that the cgy-1 gene to chromosome 10 at a position between Sat_307 and Sat_231, and the cgy-2 gene to chromosome 20 at a position between Satt650 and Satt671. These markers should enable map-based cloning of the cgy-1 and cgy-2 genes. For different subunit-deficiency types [α′-null, α-null and (α′+α)-null types], the two sets of SSR markers could also detect of polymorphism between three normal cultivars and seven related mutant lines. The identification of these markers is great significance to the molecular marker-assisted breeding of soybean β-conglycinin subunits.
Key words: soybean (Glycine max), β-conglycinin, cgy-1 and cgy-2 loci, SSR marker, genetic linkage map
Introduction
The major components of soybean seed protein areβ-conglycinin and glycinin, which account for about 70% of the total seed proteins. From a nutritional viewpoint,β-conglycinin, which is composed ofα′-,α- andβ-subunits (Thanh and Shibasaki, 1976; 1978; Higgins, 1984), contains much fewer sulfurcontaining amino acids (methionine and cysteine), and thus exhibits poorer nutritional and food processing properties than that of glycinin (Koshiyama, 1968; Saioet al., 1969; Fukushima, 1991; Kohyama and Nishinari, 1993). In addition, during the processing of soy food, such as tofu,β-conglycinin has a detrimental effect on gel formation. A tofu-gel of crude glycinin is much harder than one of crudeβ-conglycinin (Saioet al., 1969). The breaking stress and breaking strain of fully gelled tofu ofβ-conglycinin is less than those of glycinin tofu (Kohyama and Nishinari, 1993). The degree of influence of eachβ-conglycinin subunit on gelation properties differs. When the amounts of glycinin are equal, soybean genotypes lacking theα′-subunit show higher gel hardness than all-subunitcontaining andα-subunit-lacking genotypes. Theβ-conglycininα-subunit has the least influence on gel hardness (Sallehet al., 2004). Furthermore, all the three sub-units ofβ-conglycinin are potential major allergens in soybean-sensitive patients (Krishinanet al., 2009). Because of the adverse impacts ofβ-conglycinin on aspects of nutrition, processing and allergies, it is highly desirable to produce soybean lines with reduced amounts ofβ-conglycinin (Krishinan, 2005).
It has now become possible to genetically manipulate the entire subunit compositions of both glycinin andβ-conglycinin. The modes of inheritance of theβ-conglycininα′- andα-subunits have been recognized. Genetic studies have been demonstrated that theα′- andα-subunits are controlled by respective single alleles (Kitamuraet al., 1984; Tsukadaet al., 1986; Takahashiet al., 1996). The presence or absence of the α′-subunit does not depend on the presence or absence of theα-subunit. The presence of theα′- or α-subunit is dominant to the absence of each. The gene symbolsCgy1/cgy1 andCgy2/cgy2 are assigned to the presence and absence of theα′- andα-subunits, respectively (Kitamuraet al., 1984; Tsukadaet al., 1986). Therefore, it is possible to breed soybean lines with variousβ-conglycinin subunit compositions. However, there are some results on the study of gene symbols. There are no clear results on the genes corresponding toα′- andα-subunits.
Genetic markers based on DNA polymorphism are handy tools to detect genetically modified organisms (Gachetet al., 1998) because of their high sensitivity and effectiveness. So far, several studies have been succeeded in mapping some of the loci responsible for soybeanβ-conglycinin deficiency traits, and in developing PCR-based molecular markers associated withβ-conglycinin deficiency. Aβ-conglycinin deficiency mutant, QT2, is identified from a wild soybean in Kumamoto Prefecture, and the phenotype has found to be controlled by a single dominant gene,Scg-1 (suppressor ofβ-conglycinin) (Hajikaet al., 1996; Hajikaet al., 1998). A restriction fragment length polymorphism (RFLP) marker associated with theScg-1 gene is first developed using theα-subunit gene as a probe (Teraishiet al., 2001), and then clarified thatScg-1 co-segregated with a region ofβ-conglycinin subunit genes in linkage group (LG) I/Gm20 and PCR-based markers forScg-1 are developed (Tsubokuraet al., 2006). Subsequently, Tsubokuraet al.(2012) proved that CGalpha-1 has placed in the same region as Scg-1 and suggested that this region corresponded to 'region A' reported by Haradaet al(1989). Anotherβ-conglycinin deficient mutant has been identified as being controlled by a single recessive gene,cgdef(Kitagawaet al., 1991). Thecgdefgene has mapped to LG/Gm 19 between the SSR markers Satt523 and Sat_388 (Hayashiet al., 2009). 'Keburi' is aCgy-1-null mutant variety (Kitamura and Kaizum, 1981; Ladinet al., 1984). By crossing with Keburi, the null allele ofCgy-1 has included in an elite soybean variety, Tachiyutaka, which has desirable alleles for other traits (Ishikawaet al., 2006). With these varieties, Ishikawaet al. (2006) developed a dominant PCR-based marker for detecting the presence or absence of theβ-conglycininα′-subunit, and then the deletion site forcgy-1 in the Keburi soybean variety has been determined and a set of PCR-based co-dominant markers comprising three allele-specific primers has been designed for multiplex PCR analysis (Kimet al., 2011). Although these PCR-based molecular markers can be used to screen rapidly for the absence of theβ-conglycininα′- orα-subunit alleles and effectively track theβ-conglycininα′- orα-subunit null phenotype during marker-assisted selection, SSR markers allowing to identify the allelic composition of genotypes in segregating populations for each of the threeβ-conglycinin subunits in soybeans have not yet been published.
In this study, two sets of SSR markers were reported, located on the flanks of thecgy-1 andcgy-2 loci on chromosomes 10 and 20, respectively. The applicability of the identified co-dominant markers to other soybean genotypes was further evaluated. These markers could be used both for marker-assisted selection in soybean breeding programs, and for seed purity tests in the food industry.
Materials and Methods
Plant materials
To transfer theα′- andα-subunit null mutant genescgy-1 andcgy-2, a high-oil soybean cultivar, DN47, bred in Heilongjiang Province, was used as the recurrent parent in a cross with a mutant collected from Japan, HS99B, which was used as the donor parent and carried theα′- andα-subunit null genescgy-1 andcgy-2. Two new soybean lines, anα′-lacking line (Δα′) designated CC and anα-lacking line (Δα) designated CB, were obtained in 2011. There were no significant differences in the stage of gemmation, growth and seed development in both CC and CB lines. Since 2001, the CC and CB lines were planted in four different locations: 'Tongliao' of Inner Mongolia, 'Lindian', 'Acheng' and 'Harbin' of Heilongjiang Provence in China, and theα′-null andα-null phenotypes could be inherited stable.
F2populations for this study were obtained by selfing F1plants derived from crosses between CC and DN47, and CB and DN47. This produced 157 plants belonging to thecgy-1 (α′-null) mutant type and 200 F2plants belonging to thecgy-2 (α-null) mutant type, respectively, for linkage analysis. The original varieties, DN47 and HS99B, were used as positive controls in this study. The Chinese soybean cultivars Heihe29 (normal), Hefeng41 (normal), Suinong10 (normal) and the original cross 'DN47×HS99B' produced four populations (BC1F7:8, BC2F4:5, BC2F5:6and BC3F3:4), and seven mutant lines with different subunit deletion types were screened out,α-null type of B4001, Cb15-4 and M1-5-4,α′-null type of C4809 and Cc3-9, andα′- andα-null types of Cd6-2 and D5006. They were used for polymorphism analysis.
Four populations were used DN47 as the recipient parent and HS99-B as the donor parent to prepare a hybrid combination, and DN47 was used as the recurrent parent for multiple consecutive backcross breeding. On the basis of the preliminary screening of the research group, they were sent to Hainan Province for self-addition to obtain a breeding population. After SDS-PAGE detection, individuals with different subunit deletion types were screened.
Bulked segregant analysis (BSA)
DNA from 15 dominant Wt plants, including both homozygous and heterozygous plants for theCgy1 andCgy2 alleles, and from 15 recessive plants homozygous for thecgy1 andcgy2 alleles in the mapping population, were pooled to construct Wt1, Wt2 and the correspondingcgy1 (α′-null),cgy2 (α-null) bulks, respectively. The pooled DNAs were used for bulked segregant analysis (Michelmoreet al., 1991) using SSR markers mapped on the integrated soybean linkage map (Songet al., 2004).
SSR marker analysis
Fresh leaves of the F2plants were collected, and DNA extraction for bulk segregant SSR analyses was conducted by the cetyltrimethylammonium bromide (CTAB) method (Murray and Thompson, 1980). SSR primers were designed based on sequence information available online at http://soybase.org/resources/ssr.php. The forward and reverse primer sequences used in the present study are given in Table 1. PCR reaction mixtures included 50 ng template DNA, 5 pmol of each primer, 10×Taqpolymerase buffer [500 mmol•L-1KCl, 100 mmol•L-1Tris-HCl (pH 8.5), and 1 mg•mL-1gelatin], 1.0 mmol•L-1of MgCl2, 0.5 mmol•L-1of dNTPs and 0.25 U ofTaqpolymerase in total volumes of 12.5 µL. PCR was performed in 96-well plates in a PTC-200 thermocycler. The reaction conditions were as the followings: 95℃ for 10 min, 30 cycles of 1 min at 95℃, 1 min at 55℃ and 1 min at 72℃, and a final extension step of 10 min at 72℃. The PCR products were analyzed on a 6% non-denaturing polyacrylamide gel by electrophoresis in 1×TBE at 150 V for 1 h. The amplified products were then visualized using the silver staining method.
Data analysis and linkage mapping for cgy-1 and cgy-2 genes
The markers used throughout this study were reported by Songet al(2004). Marker linkage analysis was done with MapMaker 3.0. The Kosambi function was used for calculating the map distance, and a logarithm of the odds score of 3.0 was used as the threshold for confirmation of linkage. The linkage map was constructed using the map chart software. Marker orders were assigned using the 'compare', 'try' and 'ripple' (minimum LOD score of 3.0) commands.
Screening of soybean β-conglycinin phenotype to confirm segregation analysis of α′-and α-subunits
Screening of the soybeanβ-conglycinin subunit composition phenotypes were performed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), using the distal portion of the seed of F2and F3generations. Five milligrams of cotyledon tissue was ground in 200 µL of sample buffer(2% SDS, 5% 2-mercaptoethanol, 10% glycerol, 5 mol • L-1urea, 62.5 mmol • L-1Tris amino methane), and was centrifuged at 5 000×g. Ten microliters of the supernatant was separated on 4.5% stacking and 12.5% separating polyacrylamide gels, and was stained with Coomassie Brilliant Blue R 250.
The genotypes of the F2individuals were identified by progeny testing. After SDS-PAGE analysis of F2individuals using the distal portion of the seed, the remaining proximal portions of the seed were grown in a greenhouse for 2 weeks, and 100 mg of young leaf tissue was used for DNA extraction. SSR analyses were then conducted as describing below. F3seed obtained from each individual F2plant was investigated by SDS-PAGE analysis as described above for the progeny test.
Results
ldentification of SSR markers co-segregating with cgy-1 or cgy-2 locus
With Wt1 and Wt2 as materials, the SSR markers cosegregated withcgy-1 orcgy-2 gene in Fig. 1 were identified by BSA. After screening 22 SSR markers, it could be found that seven SSR markers (Sat_038, Satt243, Sat_307, Sat_109, Sat_231, Sat_108 and Sat_190) located on linkage group O (renamed chromosome 10), LG O/Gm 10 of the map of Song (Songet al., 2004), co-segregated with thecgy-1 gene (Fig. 1a), and six SSR markers (Satt650, Satt671, Sat_418, Sat_170, Satt292 and Sat_324) co-segregated withcgy-2 on LG I/Gm 20 (Fig. 1b).
To confirm these results, to perform segregant analysis of individuals comprising the DNA pools of these SSR markers. Part of the segregation analysis with the two sets of SSR markers for theα′-andα-subunits ofβ-conglycinin observed in this study is shown in Fig. 2. The Satt243 and Sat_418 markers clearly co-segregated withcgy-1 andcgy-2, respectively(Fig. 2). The fragment product with the Satt243 primers in the dominant homozygous genotype (Cgy-1Cgy-1) for theCgy-1 gene was the 'A' band (Fig. 2a), which indicated the presence of theα′-subunit protein, while the 'a' band fragment was amplified in the recessive homozygous genotype (cgy-1cgy-1) and thus indicated the absence of theα′-subunit protein. Heterozygous (Cgy-1cgy-1) plants had both the'A' and 'a' fragments (Fig. 2a). For theα-subunit, Sat_418 amplified the 'B' band DNA fragment in the homozygous dominant genotype (Cgy-2Cgy-2) and the 'b' band fragment in the recessive homozygous genotype (cgy-2cgy-2). Heterozygous (Cgy-2 andcgy-2) plants had both the 'B' and 'b' fragments (Fig. 2b).

Fig. 1 BSA on pools wild-, α′-null and α- null type F2 genotypesa, Seven portions of BSA profiles generated with seven SSR markers (Sat_038, Satt243, Sat_307, Sat_109, Sat_231, Sat_108 and Sat_190) locate on chromosome 10. b, Six portions of BSA profiles generated with six SSR markers (Satt650, Satt671, Sat_418, Sat_170, Satt292 and Sat_324) locate on chromosome 20. c, Linkage group O (renamed chromosome 10) LGO/Gm10 of integrated soybean linkage map quoted from Song et al (2004). d, Linkage group I (renamed chromosome 20) LGI/Gm20 of integrated soybean linkage map quoted from Song et al (2004).D=DN 47 (recurrent parent); Wt1=DNA pools for α′-normal; Wt2=DNA pools for α-normal; Δα′ bulk= DNA pools for α′-null; Δα bulk=DNA pools for α-null.
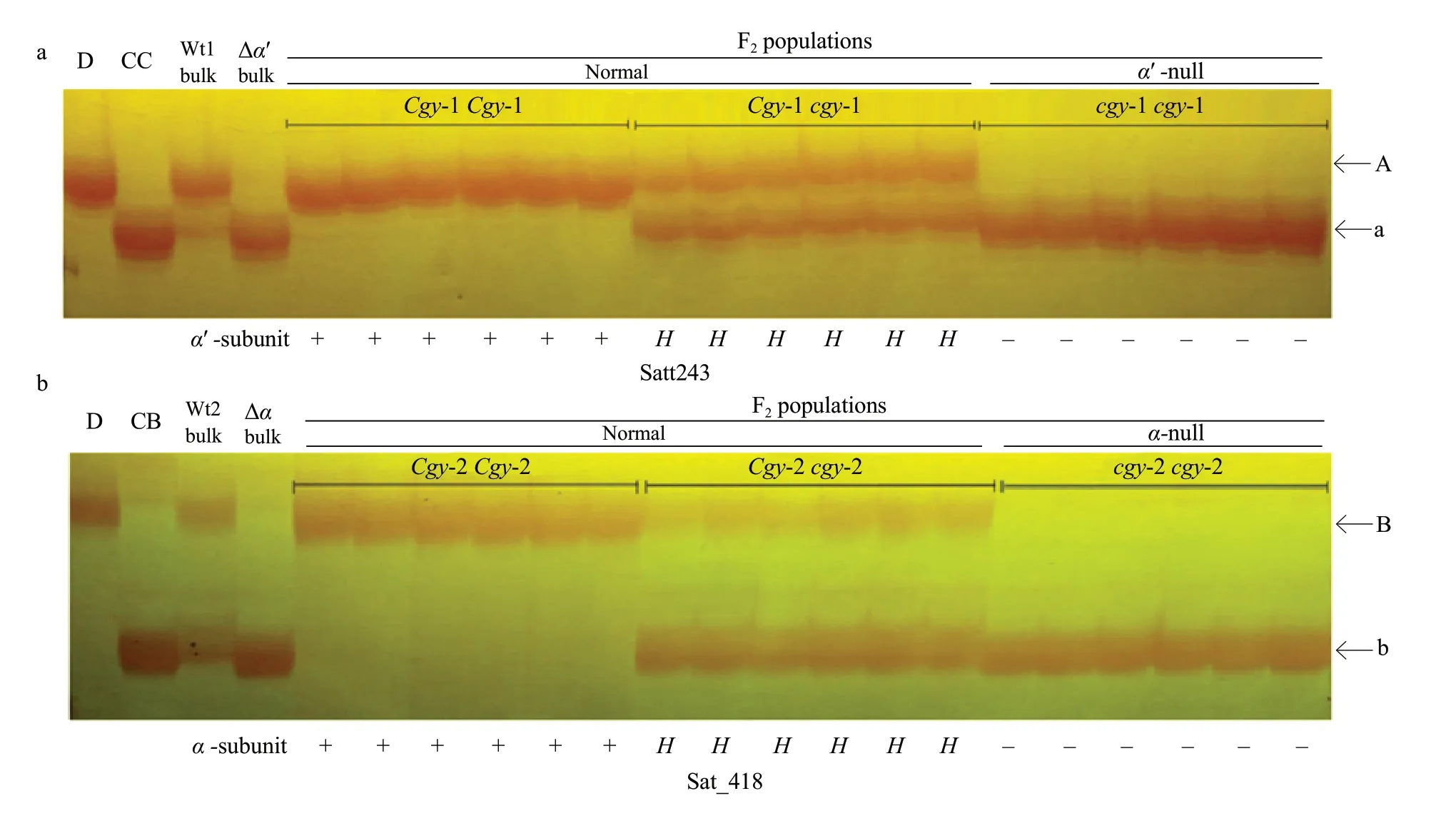
Fig. 2 A part of segregation analysis with SSR markers of Satt243 and Sat_418 for (a) α′-subunit and (b) α-subunit of β-conglycinin in their corresponding BC4F2 populationsAlphabets on side of electrophelogram indicate band types. Genotypes of individuals are identified by progeny test and are shown at top of figure. Normal or deficiency (±) of α′- and α-subunit is shown at bottom of figure. D=DN 47 (recurrent parent); CC=α′-null type parent; CB=α-null type parent; A, Band linked to the Cgy-1 locus; a, Band linked to cgy-1 locus. B, Band linked to Cgy-2 locus; b, Band linked to cgy-2 locus. +, Wild-type allel;-, Null allel; H, Heterozygote. Wt1, DNA pools for α′-normal; Wt2, DNA pools for α-normal; Δα′ bulk, DNA pools for α′-null Δα bulk, DNA pools for α-null.
These two sets of markers for Table 1, which were co-dominant, enabled accurate construction of local maps around thecgy-1 andcgy-2 loci and estimation of genetic distances. To assess linkage among thecgy-1 andcgy-2 loci and the two sets of SSR markers, to perform segregant analysis on individuals derived from (1) the cross 'CC'×'DN47', which produced 157 F2plants belonging to thecgy-1 (α′-null) mutant type; (2) the cross 'CB'×'DN47', which produced 200 F2plants belonging to thecgy-2 (α-null) mutant type. Linkage maps for these two groups were constructed based on multiple linkage analysis in the Mapmaker 3.0 computer program is shown as Fig. 3. Two results were confirmed. The first seven SSR markers, Sat_038, Satt243, Sat_307, Sat_231, Sat_108, Sat_109 and Sat_190, were mapped in the 'CC'×'DN47' population (Fig. 3a). Analysis of the data showed that the seven SSR markers were linked to thecgy-1 with distances of 19.5, 10.7, 6.1, 10.2, 14.6, 19.0 and 26.1 centimorgans(cM), respectively. Thecgy-1 locus was defined by the marker loci Sat_307 and Sat_231. Sat_307 was the closest marker, at a genetic distance of 6.1 cM from thecgy-1 locus. Furthermore, in this study, Sat_109 did not map between Sat_307 and Sat_231 as reported in the integrated linkage map of Songet al.(2004) (Fig. 1c), but was instead mapped between Sat_108 and Sat_190 (Fig. 3b). For (2), six SSR markers and thecgy-2 locus were mapped in the order of Satt650,cgy-2, Satt671, Sat_418, Sat_170, Satt292 and Sat_324 with distances of 3.6, 4.1, 6.5, 8.5, 21.2 and 24.1 cM, respectively (Fig. 3b). The six SSR markers lay within a genomic region of approximately 27.7 cM (Fig. 3b). These six markers mapped in the same order as those in Songet al(2004) (Fig. 1d). Thecgy-2 locus was defined by the marker loci Satt650 and Satt671(Fig. 3b). Satt650 was the closest marker, at a distance of 3.6 cM from thecgy-2 locus. The identification of these markers probable usefully for marker-assisted selection in the breeding of soybean lines with various subunit compositions ofβ-conglycinin.
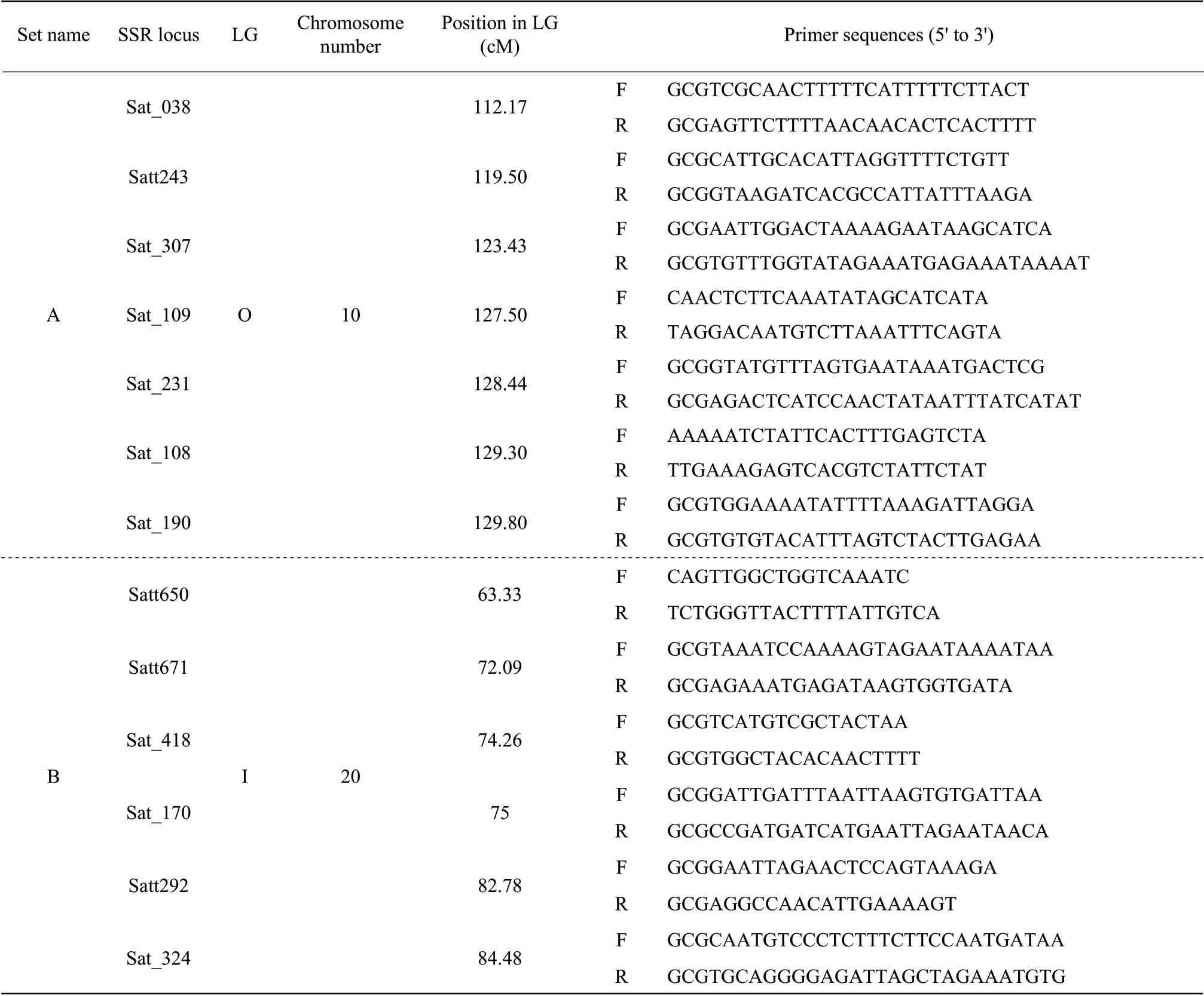
Table 1 Forward and reverse primer sequences for two sets of SSR loci, located on soybean linkage groups O and I
Evaluation of co-dominant markers for cgy-1 and cgy-2
To evaluate the co-dominant markers forcgy-1 andcgy-2, 10 other soybean genotypes including three normal cultivars and seven related mutant lines of different subunit-deficiency types [α′-null,α-null and (α′+α)-null type], along with the recurrent parent DN47 and the corresponding donor parent HS99B, were tested with Satt243 and Sat_418 at Fig. 4. The polymorphism among SSR markers and thecgy-1 orcgy-2 locus was evident by comparing the banding patterns. With Satt243, which was specific for thecgy-1 locus, three normal cultivars, Heihe29, Hefeng41 and Suinong10 (Fig. 4, Lanes 1-3), and threeα-null type lines (Fig. 4, Lanes 4-6) showed 'A' band amplification of the same size as the wild-type DN47 genotype; twoα′-null type (Fig. 4, Lanes 7 and 8) and two (α′+α)-null (Fig. 4, Lanes 9 and 10) lines produced the 'a' band as seen in the donor parent HS99B. With Sat_418, which was specific for thecgy-2 locus, twoα′-null type (Fig. 4, Lanes 7 and 8) lines showed 'B' band amplification of the same size as the wild-type DN47 genotype; threeα-null type lines (Fig. 4, Lanes 4-6) and two (α′+α)-null (Fig. 4, Lanes 9 and 10) lines produced 'b' bands as seen in the donor parent HS99B. However, analyses of the three normal cultivars, Heihe29, Hefeng41 and Suinong 10, revealed three different SSR alleles at the Sat_418 locus (Fig. 4, Lanes 1-3). These results further verified that these two SSR markers were feasible and accurate. Therefore, SSR markers identified in this study could become a practical tool to introducecgy-1 andcgy-2 into many Chinese cultivars.
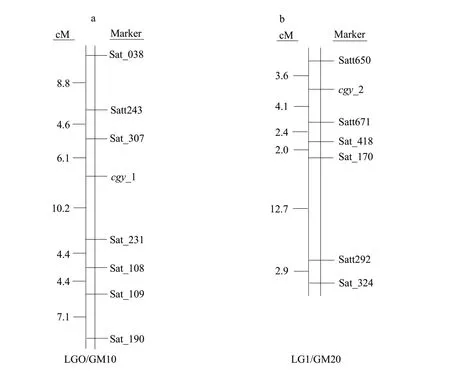
Fig. 3 SSR markers on soybean linkage groups associated with α′-null and α-null phenotype, respectivelya, Location of seven SSR markers (Sat_038, Satt243, Sat_307, Sat_231, Sat_108, Sat_109 and Sat_190) flanking the cgy-1 locus on linkage group O. Genetic distance in centimorgans (cM) between adjacent loci are estimated based on the analysis of 157 BC4F2 plants; b, Location of six SSR markers (Satt650, Satt671, Sat_418, Sat_170, Satt292 and Sat_324) flanking cgy-2 locus on linkage group I. Genetic distance in centimorgans at adjacent loci is estimated based on analysis of 200 BC4F2 plants.
The practicality of the co-dominant markers forcgy-1 andcgy-2 in heterozygotes was also assessed using 15 F1hybrids derived from different subunitdeficiency types [α-null,α′-null and (α′+α-null) type] lines (Fig. 4, Lanes 11-25).
The F1plants showed two distinct DNA bands that were inherited from each of the parental genotypes. The Δα-type hybrids had both 'B' and 'b'bands (Fig. 4, Lanes 11-15), the Δα′-type hybrids had both 'A' and 'a' bands (Fig. 4, Lanes 16-20), while Δ(α′+α)-type hybrids (Fig. 4, Lanes 21-25) had 'A', 'a', 'B' and 'b' bands. These results revealed that the co-dominant markers for thecgy-1 andcgy-2 genes allowed us to select heterozygous individuals into which the null alleles ofcgy-1 andcgy-2 had retrogressed.
Genotypic assay with SSR markers
Based on the data above, both Satt243 and Sat_418 were used to assay lines derived from HS99B (cgy-1cgy-1/cgy-2cgy-2)×DN47 (Cgy-1Cgy-1/Cgy-2Cgy-2), and the data was proven to be highly effective in identifying lines carryingα′- andα-subunit-null traits at theCgy-1 andCgy-2 loci. An example of genotypic assay of the SSR markers from this study is shown in Fig. 5. Using SDS-PAGE analysis, to sereen the initialβ-conglycinin subunit phenotypes of each F2seed harvested in 2011; part of the SDS-PAGE pattern of the F2seed is shown in Fig. 5a. Since SDS-PAGE analysis could not distinguish the heterozygotes, only four band types for theα′-andα-subunits were observed in the F2seeds: normal types in which theα′- andα-subunits presented (Fig. 5a, Lanes 1-4), two types lacking one subunit-theα′- (Fig. 5a, Lanes 5 and 6) orα-subunit (Fig. 5a, Lanes 7 and 8), and one type lacking bothα′- andα-subunits (Fig. 5a, Lane 9).

Fig. 4 Analysis of PCR products using Satt243 (lower bands) and Sat_418 (upper bands) in three soybean cultivars, seven related mutant line and 15 F1 individuals of different subunit-deficiency types [α′-null, α-null and (α′+α)-null type]D=DN47 (recurrent parent); R=HS99B (donor parent); A, Band linked to Cgy-1 locus; a, Band linked to cgy-1 locus. B, Band linked to Cgy-2 locus; b, Band linked to cgy-2 locus.
SSR markers were co-dominant markers that could identify the presence of heterozygous alleles. To detect heterozygosity at theCgy-1 andCgy-2 loci, samples of the two markers, Satt243 and Sat_418, for each F2:3plant derived from the F2seeds in which the subunit phenotype was identified with SDS-PAGE (Fig. 5a), were run on the same lane at 15-minute intervals in Fig. 5b. Nine band types, AB, AaB, ABb, AaBb, aB, aBb, Ab, Aab, and ab were detected(Fig. 5b, Lanes 1-9); variations in band type with Satt243 and Sat_418 were used to predict the genotypes of F2seeds as the followings: AB=Cgy-1Cgy-1/Cgy-2Cgy-2, AaB=Cgy-1cgy-1/Cgy-2Cgy-2, ABb=Cgy-1Cgy-1/Cgy-2 cgy-2, AaBb=Cgy-1cgy-1/Cgy-2cgy-2, aB=cgy-1cgy-1/Cgy-2Cgy-2, aBb=cgy-1cgy-1/Cgy-2cgy-2, Ab=Cgy-1Cgy-1/cgy-2cgy-2, Aab=Cgy-1cgy-1/cgy-2cgy-2, and ab=cgy-1cgy-1/cgy-2cgy-2. All the nine genotypes could be distinguished clearly with Satt243 and Sat_418.
Seed of F3families from each F2individual whose genotype was estimated as homozygous, single heterozygous or double heterozygous was subsequently analyzed using SDS-PAGE to confirm the genotype predicted by the SSR markers (Fig. 5c). At least 40 F3seeds obtained from each individual F2plant were investigated. Three types of results were obtained(Fig. 5c, I, II and III): (1) only one phenotype that was the same as in the F2seeds was identified in the F3seeds from the F2plants whose genotypes were estimated as homozygous:Cgy-1Cgy-1/Cgy-2Cgy-2,cgy-1cgy-1/Cgy-2 Cgy-2, Cgy-1 Cgy-1/cgy-2cgy-2 andcgy-1cgy-1/cgy-2cgy-2, whose corresponding SSR band types were 'AB', 'aB', 'Ab' and 'ab', respectively (Fig. 5c, I); these results confirmed that there was no segregation in the progeny F3seed populations. (2) Two different phenotypes could be identified in the F3seed from the F2plants whose genotypes was estimated as single heterozygous:Cgy-1cgy-1/Cgy-2Cgy-2,Cgy-1Cgy-1/Cgy-2cgy-2,cgy-1cgy-1/Cgy-2cgy-2 andCgy-1cgy-1/cgy-2cgy-2, whose corresponding SSR band types were 'AaB', 'ABb', 'aBb' and 'Aab', respectively (Fig. 5c, II). (3) Four different phenotypes were identified in the F3seed from F2plants whose genotypes were estimated as double heterozygous:Cgy-1cgy-1/Cgy-2cgy-2, whose corresponding SSR band types was 'AaBb' (Fig. 5c, III). The phenotypic results of SDS-PAGE analysis were completely consistent with the molecular identification using the SSR markers, and the above results suggested that the heterozygosity of F2plants at theCgy-1 andCgy-2 loci could be ascertained clearly using the co-dominant SSR markers Satt243 and Sat_418. The data from the two markers, either individually or jointly, predicted the absence ofα′- andα-subunits of all the plants examined in this study correctly (Figs. 2 and 5).
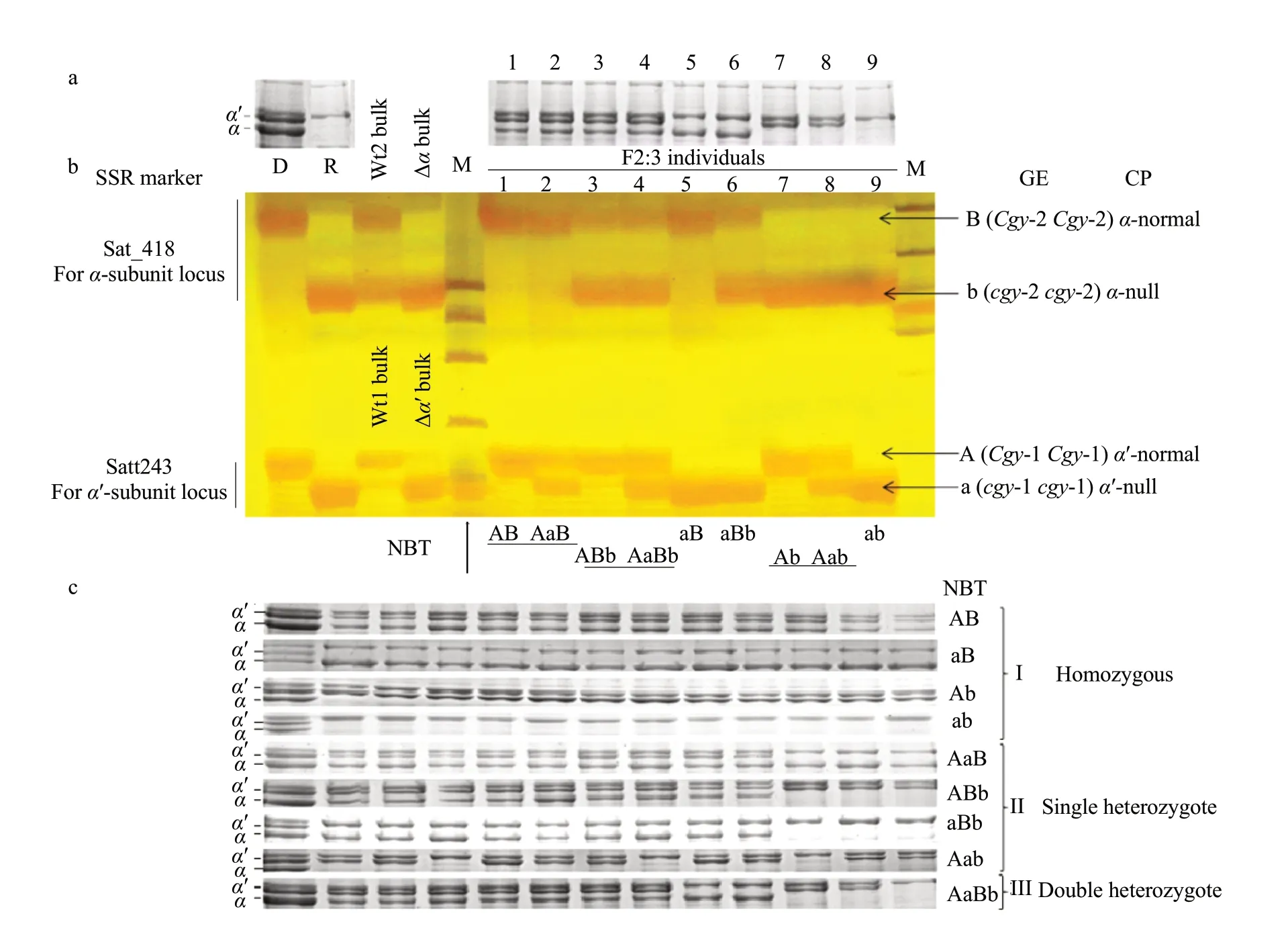
Fig. 5 Genotypic analysis of α′- and α-subunits of β-conglycinin by SDS-PAGE and SSR markersa, Segregation patterns of F2 seed derived from a cross between HS99B (cgy-1cgy-1/cgy-2 cgy-2) and DN47 (Cgy-1Cgy-1/Cgy-2 Cgy-2). Left two lanes show banding pattern of recurrent parent DN47 (with normal globulin components) and donor parent HS99B (lacking both α′- and α-subunits). Lane 1 to Lane 9, F2 individuals. b, Segregation pattern of Satt243 and Sat_418 among F2:3 plants. Samples are loaded onto gel in two separated groups each at 15-minute intervals. Lower band is about cgy-1 allele (α′-null) and upper band is cgy-2 allele (α-null). c, Seed of F3 families from each F2 individual whose genotypes are estimated as homozygous, single heterozygous and double heterozygous are subsequently analyzed using SDSPAGE for confirming the genotype predicted by SSR markers. D=DN47 (recurrent parent); R=HS99B (donor parent); Wt1=DNA pools for α′-normal; Wt2=DNA pools for α-normal; Δα′ bulk=DNA pools for α′-null; Δα bulk=DNA pools for α-null; Lane 1 to Lane 9: individuals of F2:3 plants derived from HS99B×DN47 (all the nine genotypes can be distinguished). M, A molecular-size standard; A, Band linked to the Cgy-1 locus; a, Band linked to cgy-1 locus. B, band linked to Cgy-2 locus; b, Band linked to cgy-2 locus. NBT=Nine band types are detected by progeny test. GE, Genotype estimated; CP, Corresponding phenotype.
Discussion
Mianet al.(1999) reported that selection of SSR markers from the soybean LGs with known disease resistance gene clusters reduced the number of SSR markers to be screened by 80% compared with the number of markers needed for a random genomewide search. This strategy of screening SSR markers was proven to be an efficient and effective one for us. The research took advantage of the integrated linkage map of soybean constructed by Songet al. (2004), and DNA marker information that was readily available online at http://soybase.org/resources/ssr.php. TheCgy-1 gene was found to be located on chromosome (Chr) 10 (LGO) through a BLAST search of its cDNA sequence against the soybean reference sequence. Furthermore, an RFLP marker for theα-subunit gene (Cgy-2) was mapped to the same locus as theScg-1 (Suppressor ofβ-conglycinin) gene that controlled the deficiency ofβ-conglycinin in QT2 (Hajikaet al., 1996; 1998) in linkage group I (Tsubokuraet al., 2006). Although some PCR-based markers forcgy-1 andcgy-2 had been reported (Hayashiet al., 2000; Tsubokuraet al., 2006), their positions in the soybean linkage map had not been fully elucidated. This work was to select two sets of SSR markers near these previously knownβ-conglycinin-subunit-correlation gene clusters and tried to map thecgy-1 andcgy-2 genes with SSR markers. The results showed that:(1) 10 SSR markers located around theCgy-1 gene. Sat_274 generated polymorphism between parents, but not polymorphisms between the bulk of normal orα′-null plants (data not shown). Satt153 generated no polymorphisms between the two parents and bulk of normal orα′-null plants (data not shown), while Sat_038, Satt243, Sat_307, Sat_109, Sat_231, Sat_108 and Sat_190 all showed polymorphism between the two parents and two bulks. (2) Eleven SSR markers were located near theScg-1 locus. Out of the 11 SSR markers identified for thecgy-2 locus in the F2population from a CB×DN47 cross, three SSR markers (Sat_104, Satt330 and Sat_421) generated no polymorphisms among the two parents' and bulks of normal orα-null plants (data not shown). Two SSR markers, Sat_268 and Satt049, generated polymorphism between the two parents but no polymorphisms between bulks; DNA from bulks of normal orα-null plants in the F2:3individuals all exhibited the same banding as the recurrent parent DN47 (data not shown). Other six SSR markers, Satt650, Satt671, Sat_418, Sat_170, Satt292 and Sat_324, all showed polymorphism between the two parents and two bulks. In summary, mapped (1) thecgy-1 locus for theα′-null phenotype in soybean to the end of the long arm of chromosome 10, and (2) thecgy-2 locus for theα-null phenotype to a cluster ofβ-conglycinin subunit genes corresponding to 'region A' reported by Haradaet al(1989). Tsubokuraet al. (2006; 2012) also classified theβ-conglycinindeficiency gene,Scg-1, as located near this cluster on LG I. The availability of SSR markers forcgy-1 andcgy-2 was a prerequisite for the isolation of theβ-conglycinin allergic-subunit-null genesviamapbased cloning. Studies were under way to clone thecgy-1 andcgy-2 genes, based on their chromosomal locations.
Modifying seed protein constituents in soybean breeding was important for both health management and food processing. Many studies had been conducted on the isolation and production of lines with a lower content of soybeanβ-conglycinin. Conventional phenotypic assays for the presence or absence of theβ-conglycininα′- andα-subunits commonly involved SDS-PAGE analysis and antibody tests, such as the enzyme-linked immunosorbent assay (ELISA) technique. These assays were laborious, time-consuming (Gachetet al., 1999) and required seed destruction to extract crude protein, which made it difficult to select early generation lines for use in breeding programs, and heterozygotes couldn't be distinguished by SDS-PAGE analysis. As an alternative to these laborious and time-consuming methods, SSR markers associated with genes controlling theα′- andα-subunit-nullβ-conglycinin phenotypes in soybean were sought. Marker-assisted selection (MAS) had been advocated as a highly efficient breeding method as it had possible rapid and precise selection of the target gene (Tanksleyet al., 1989). SSR markers were ideal genetic markers that they (1) were highly abundant, (2) appeared to be evenly distributed throughout the genome (Weber, 1990), (3) were highly polymorphic (Tautz, 1989), (4) could be typed rapidlyviaPCR, and (5) were disseminated easily among laboratories by publishing primer sequences. As an important tool for MAS, SSR markers had been used in breeding programs for various crop species (Wuet al., 2011; Kwonet al., 2005; Jeong and Maroof, 2004). This study identified two sets of SSR markers that enabled allelic discrimination by the sizes of PCR products. These two sets of SSR markers were capable of detecting the presence or absence of theβ-conglycininα′- andα-subunits. Using these markers to assess the presence or absence of thecgy-1 andcgy-2 genes in segregating lines of BC1F7:8, BC2F4:5, BC2F5:6, and BC3F3:4populations, the phenotypic results were completely consistent with the molecular identification using the SSR markers. These results indicated that selection of this genomic region with SSR markers was as accurate as SDS-PAGE assays in predictingα′- andα-null traits, and was much more efficient in terms of time and labor than SDS-PAGE assays.
Near-isogenic lines (NILs) were unique biological materials for characterizing single genes. The development ofα′- andα-null NILs would help us to study the effect of each subunit locus of soybeanβ-conglycinin on nutritional and processing quality. And to summarize two new ways to developα′- andα-null NILs on the basis of conventional breeding methods as the followings: (1) individual plant back crossing assisted by SDS-PAGE. This method used self-pollination and background marker-assisted selection to choose target subunit-deficienciesviaSDS-PAGE. (2) A hybrid genealogy technique assisted by SDS-PAGE. This method used regular pedigree breeding in the field to choose lines with the heterozygous genotype in each advanced generation until F7, from which high quality NILs could be easily developed. The second method provided a rapid, technically simple alternative for hybridizing each generation to transfer thecgy-1 orcgy-2 genes. The only prerequisite was the existence of a population resulting from a cross that segregates for thecgy-1 andcgy-2 genes, and the success of the approach would depend on the selection of heterozygotes. Therefore, distinguishing between homozygous and heterozygous lines was a challenging task, particularly in the early stages of selective plant breeding programs. The present study identified two SSR markers, Satt243 and Sat_418, which co-segregated withα′- subunit-deficiency (cgy-1) andα-subunit-deficiency (cgy-2), respectively. Because of their co-dominant nature, these two markers allowed us to differentiate clearly among nine classes of genotypes. Thus, these two markers could be used to monitor the heterozygous genotype forcgy-1 orcgy-2 alleles from the donor parent. In addition, since samples of the two markers could be run on the same lane, lacking ofα′- andα-subunits ofβ-conglycinin could be detected simultaneously using these two SSR markers. With these markers, studies were underway to construct NILs for thecgy-1 andcgy-2 genes. The best SSR marker was to be used for selection ofcgy-1 orcgy-2 was Sat_307 forcgy-1 and Satt650 forcgy-2. The reason was on the basis of analysis of Sat_307 and Satt650, and they were more closely linked to thecgy-1 andcgy-2 loci, respectively. Such molecular markers would provide plant breeders with a powerful tool for (1) screening for the absence of theβ-conglycininα′- andα-subunits within advanced soybean breeding populations easily and quickly, (2) clipping transfer of theα′- andα-subunit-null traits to elite soybean cultivars and (3) pyramiding multipleβ-conglycinin-subunit-deficiency genes.
Conclusions
This study identified two sets of SSR markers, both of them could detect the presence ofβ-conglycinin,α'-andα-subunits. And thecgy-1 locus of the soybeanα′-null phenotype was located at the end of the long arm of chromosome 10, and thecgy-2 locus for theα-null phenotype to a cluster ofβ-conglycinin subunit genes corresponding to 'region A'. Identified two SSR markers, Satt243 co-segregated withα′- subunitdeficiency (cgy-1) and Sat_418 co-segregated withα-subunit-deficiency (cgy-2), respectively. Because of their co-dominant nature, these two markers allowed us to differentiate clearly among nine classes of genotypes. The best SSR marker was to be used for selection ofcgy-1 orcgy-2 was Sat_307 forcgy-1 and Satt650 forcgy-2. The reason was on the basis of the analysis of Sat_307 and Satt650 and they were more closely linked to thecgy-1 andcgy-2 loci, respectively. These molecular markers could provide a powerful tool for the rapid screening ofβ-conglycininα′- andα-subunit deletion varieties in soybean breeding populations, and the breeding of high-qualityα′ andα-subunit deletion soybeans. This study summarized two new construction methods ofα′-andα-null NILs. The two sets of SSR markers also enabled detection of polymorphism between three normal cultivars and seven related mutant lines of different subunitdeficiency types [α′-null,α-null and (α′+α)-null types]. The identification of these markers might be useful for molecular marker-assisted breeding programs targeting modifications in soybeanβ-conglycinin subunit composition.
Acknowledgements
Li Ming-xue and Pang Ze contributed equally to this work.
 Journal of Northeast Agricultural University(English Edition)2021年1期
Journal of Northeast Agricultural University(English Edition)2021年1期
- Journal of Northeast Agricultural University(English Edition)的其它文章
- Study on Marker-assisted Breeding of Soybean Vitamin E
- Effect of Drought Stress on Growth and Water Physiological Characteristics of Poa sibirica
- Biotransformation of Flavor Compositions During Fermentation of Litchi (Litchi Chinensis Sonn.) Fruits into Wine
- Effect of Endophytic Fungus on Pyrola calliantha H. Andr Responsed to Cold Stress
- Uterine Expression of WNT7A and β-catenin After Induction of Oestrus in Sheep
- Multi-objective Function Optimization for Environmental Control of a Greenhouse Based on a RBF and NSGA-II
