Biotransformation of Flavor Compositions During Fermentation of Litchi (Litchi Chinensis Sonn.) Fruits into Wine
Lu Yan, Zeng Xia, Cao Jian-ping, and Luo Lan-ping
1 Department of Biological Engineering, Guangdong University of Petrochemical Technology, Maoming 525000, Guangdong, China
2 Analysis and Testing Center, Guangdong University of Petrochemical Technology, Maoming 525000, Guangdong, China
Abstract: The aim was to examine the biotransformation of chemical compounds during the fermentation of litchi wine. S. bayanus BV818 was inoculated to litchi juice (Heiye) to initiate the fermentation. Acetic acid decreased dramatically, succinic acid and DLmalic acid increased sharply. Saturated free fatty acid increased, especially the concentration of the free fatty acid with long carbon chain (more than 10 carbons) increased significantly. The unique flavor compounds of fresh litchi including linalool, α-terpineol, β-citronellol and other terpenoids remained in the litchi wine were transformed to other aroma constituents, by which the primary litchi flavor was retained. The wine had a fruity flavor and delicate bouquet and had harmonious sourness and sweetness. The litchi 'Heiye' was suitable for being fermented into litchi wine.
Key words: litchi wine, flavor, fermentation, biotransformation
Introduction
Litchi (Litchi ChinensisSonn.) is a kind of fruit cultivated in the tropical and subtropical areas, which has been cultivated for more than 2000 years in the south of China (Li, 2008). The fresh litchi is always well received by consumers and known as the 'queen' of fruits for its sweet flesh, best flavor and high nutritional value (Kumar, 2016). Litchi is rich in polyphenols and can be expected to be selected as a natural source of antioxidants for functional foods (Septembre-Malaterreet al., 2016; Aluko, 2012). However, there are problems influencing the income increase and nitiative of litchi growers. Pericarp browning and fruit rot affect the product quality and reduce the shelf life seriously (Ahmad and Siddiqui, 2015; Kumaret al., 2012). Therefore, export of litchi fruit is limited by these factors. Much attention has been paid to resolve the problem of pericarp browning, and varieties of methods have been tried to delay browning and extend the shelf life of litchi (Aliet al., 2016; Barmanet al., 2014; Jianget al., 2004; Martínez-Castellanoset al., 2011; Zhanget al., 2015). But so far, there is no suitable preservation method afforded. On the other hand, researchers proposed that processing the litchi fruit into wine can prevent pericarp browning of litchi and retain the nutrition of litchi (Chen and Liu, 2016b). After fermentation, the original fragrance and healthcare function of litchi is retained, and the special components in wine are produced, which is its superiority to the traditional grain liquor (Moreno-Arribas and Polo, 2009). The higher added value will be gained. Meanwhile, litchi is suitable for wine making for its high juice yield, moderate sugar content and acidity. The properties of litchi varieties differ widely with each other (Pareek, 2016). So correspondingly, the compositions and taste of wines from different litchi cultivars vary widely. However, it is difficult to make accurate comparisons about transformation of chemical constituents of litchi wines from different litchi cultivars, because the production technologies used are different, such as yeast, fermentation temperature and additives. After fermentation of 'Nuomi Ci' to wine with co-inoculation ofO. oeniand yeast, L-malic acid is transformed into L-lactic acid with concomitant formation of ethyl lactate, and citric acid is converted to lactic acid and acetic acid, while higher retention of key litchi aroma-impact compounds (linalool, geraniol andcis-rose oxide) is got (Chen and Liu, 2016b). It is found that the production of pyruvic and succinic acid significantly reduces and the amounts of 2-phenylethyl alcohol, 2-phenylethyl acetate, 2-phenylethyl isobutyrate and 2-phenylethyl hexanoate increases obviously owing to the addition of L-phenylalanine, when the effect of amino acid addition on non-volatile and volatile compositions in litchi wine fermented from 'Nuomi Ci' and withS. cerevisiaeMERIT. ferm is studied (Chenet al., 2014).
Guangdong is a main litchi production province, especially in Maoming City, litchi is an important commercial fruit crop. 'Heiye' is widely cultivated in Maoming City for its low requirements on growth environment. Its high yield and short picking time make it cheaper than other varieties. Moreover, 'Heiye' tastes poorer than other varieties, as it is sour. However, wine production from 'Heiye' fruits can mitigate some of these problems and add its economic value.
Some active ingredients in the litchi juice are retained in the wine, while others are catalyzed into new substances by the enzymes during fermentation. The fragrance, taste, nutrition and health care function of litchi wine come from all these compositions, which are influenced by the fermentation process. Therefore, the transformation of compositions from litchi juice of 'Heiye' cultivar to litchi wine was investigated. The research on the law of transformation of compounds would contribute to find out a practical and operational process for litchi wine making, and to discuss about the possibility of processing 'Heiye' to wine and increase the economic value of 'Heiye'.
Materials and Methods
Litchi
The ripened litchi fruits (Heiye) were picked from Jintang Town in Maoming City, Guangdong Province, China. Then, they were brought to the laboratory and processed immediately.
Wine yeast
S. bayanusBV818 (Angel Yeast Co., Ltd.) was used in the fermentation process. Before fermentation, yeast was activated by using 5% glucose.
Fermentation process
Litchi fruits were washed by tap water, skin and seed were removed by hand. The pulp was crushed into juice by using a mixer/grinder, and the juice was collected by filtration through sterile cotton cloth. Potassium metabisulfite (100 mg • L-1) was added into the juice to inhibit the growth of undesirable microorganisms, and pectinase (80 mg • L-1) was added to clarify the juice. The total soluble solid (TSS) was adjusted to 20 °Brix with sugar (initial TSS of litchi juice °Brix 13.0) and pH was adjusted to 4 with citric acid (initial pH 5.55). The activated yeast was inoculated into the juice with the concentration of 1% (v/v). Then, the pretreated litchi juice was adjusted separated into sterile Erlenmeyer flasks. Fermentation was carried out at 20℃ for 14 days. After fermentation, the wine was stored at 4℃ in the refrigerator overnight to be clarified, the supernate was taken out for further analyses.
Analysis of organic acids by high perfomance liquid chromatograpy (HPLC)
Organic acids were measured using HPLC (Shimadzu HPLC, LC-20A, Japan) equipped with UV detector (Luet al., 2016). The 5 mL litchi wine or juice was diluted to 25 mL with 0.01 mol • L-1NH4H2PO4. Separation of organic acids was performed with the activated C18SUPELCO column (SUPELCO, USA) by 5 mL diluted samples. Then, analysis of organic acids in samples was carried out with a Thermo C18reversedphase column (4.6 mm×250 mm, 5 μm; Thermo, USA). 0.01 mol • L-1NH4H2PO4was used as mobile phase with the flow rate of 1.2 mL • min-1. The column temperature was set at 45℃ and measurement wavelength was 210 nm. Identification and quantification of compounds were carried out using retention time and standard curves of pure organic acids (Sigma-Aldrich, Fluka, Merck).
Analysis of saturated free fatty acid by gas chromatography (GC)
Saturated free fatty acid was analyzed by GC as described by Wanget al(2018). The samples were pretreated by methylation before GC analysis.
Methylation procedure
The 10 mL litchi juice or litchi wine was transferred into a test tube, 30 μL of 1.0 mg • mL-1C17 : 0 in n-hexane was added to each tube as internal standard. pH was adjusted higher than 12 with 10% NaOH. Then, 6 mL of mixture of n-hexane and methylene chloride (2 : 1) was added to extract for three times. Aqueous phase was adjusted pH to lower than 2 and 6 mL of mixture of n-hexane and methylene chloride (2 : 1) was added to extract for three times, and then organic phase was combined. The 5 mL deionized water was added to the organic phase and the top layer was dried with anhydrous sodium sulfate, and finally was concentrated to 5 mL. The sample was esterified with 2 mL of methanol and zeolite at 80℃ for 40 min. The 2 mL of deionized water was added, then 5 mL of n-hexane was added to extract twice, the organic phase was dried with anhydrous sodium sulfate and was concentrated to 0.5 mL by nitrogen blowing, which was finally transferred to a vial. Vials were stored at 4℃ until analysis by gas chromatography.
GC analysis
Fatty acid methyl esters from free fatty acid in litchi juice and litchi wine were determined on an Agilent gas chromatography unit (model 7890B, USA) equipped with a flame ionization detector (FID). The column HP-5 (30 m×0.32 mm×0.25 μm, Agilent, USA) was used. Nitrogen was used as carrier gas at a flow rate of 1 mL • min-1. The injector temperature was set at 280℃,and detector temperature at 310℃. The temperature was programed from 50℃ (2 min) to 200℃ at a rate of 10℃ • min-1and then to 290℃ at a rate of 5℃ • min-1.
Identification of fatty acid methyl esters was performed by comparing the retention time with the fatty acid standard and the analysis with GC/mass spectrometry (MS) (GCMS-QP2010 plus, Shimadzu, Kyoto,Japan) which the conditions were the same as those of GC analysis. The procedure of methylation and GC analysis of standards was the same as that of samples. The concentrations of free fatty acid in litchi juice and litchi wine were calculated by the fatty acid standard.
Analysis of volatiles by SPME (solid-phase micro-extraction)-GC/MS
A Shimadzu 2010 (Shimadzu, Kyoto, Japan) GC equipped with a MS-QP 2010 (Shimadzu, Kyoto,Japan) series mass selective detector was applied to separate and identify the volatile compounds. A Rxi®-5Sil MS capillary column (30 m×0.25 mm×0.25 μm,RESTEK, USA) was used. The 2.5 mL litchi wine or juice and 1 g NaCl were transferred into a screwcapped head space vial, and subjected to extraction at 40℃ for 30 min, under 250 r • min-1agitation using a 85 μm CAR/PDMS SPME fiber (SUPELCO, USA).Afterward, the fiber was inserted into the GC splitless injector and analytes were desorbed at 250℃ for 3 min. Before each sampling procedure, fiber was reconditioned for 15 min in the injection port of gas chromatograph at 250℃. Helium was used as the carrier gas at a flow rate of 1 mL • min-1. The column was maintained at 40℃ for 2 min after injection,and then programmed at a rate of 8℃ • min-1to 230℃,which was maintained for 10 min. And ion-source temperature was set at 200℃. All the mass spectra were acquired in electron-impact (EI) mode and the ionization voltage was 70 eV and the mass range was 50-350 m • z-1.
The peaks were identified by comparison of the obtained mass spectra of the relevant chromatographic peaks with spectra of the NIST 05 library. Semiquantification was carried out based on peak area normalization method.
Statistical analysis
Mean values and standard deviations were calculated from data obtained from triplicate fermentations.
Results
°Brix and ethanol
The °Brix decreased to 7% sharply during the first 4 days, and then remained at 6%-7%. Correspondingly, the alcohol content increased to 18% sharply during the first 4 days, and then kept stable (Fig. 1). It indicated that the strainS. bayanusBV818 possessed good fermentative capability and alcohol producing capability.
pH, organic acid and saturated free fatty acid
pH was adjusted to 4.0 with citric acid, before fermentation and declined about 0.3 during the first 4 fermentation days, and then kept balance (Fig. 2). Acetic acid, citric acid and lactic acid were the main organic acids in litchi juice and litchi wine. The concentration of acetic acid in juice was the highest, which accorded with the taste of sour. After fermentation, acetic acid decreased sharply from 11.66 to 4.09 g • L-1. Citric acid in the wine was partially introduced from pH adjustment. Lactic acid decreased after fermentation as it was closely related with tricarboxylic acid (TCA) cycle. Other two main organic acids, succinic acid and DL-malic acid, increased obviously. The concentration ofα-ketoglutaric acid changed a little and pyruvic acid also increased. The fatty acid with long chain (more than 10 carbons) increased much more than that with short chain after fermentation, especially palmitic acid and stearic acid (Table 1).

Fig. 1 Changes of °Brix and alcohol content during litchi wine fermentation
Volatiles
A total of 51 and 36 volatiles were determined by SPME-GC/MS with CAR/PDMS SPME fiber in the litchi juice and litchi wine, respectively. The major volatiles are listed in Table 2.

Fig. 2 Change of pH during litchi wine fermentation
The esters accounted for 20.99% in litchi juice and 41.04% in litchi wine with their relative areas. Isoamyl acetate and 2-phenethyl acetate were the main acetate esters.
The unique flavor of litchi wine came from another category of volatiles terpenoids. Linalool,α-terpineol andβ-citronellol accounted for the highest relative peak area (up to 47.11%) in litchi juice and still retained a lot (about 30%) in litchi wine after fermentation. Most terpenoids dropped after fermentation.β-myrcene, p-menta-1, 3, 8-triene, D-limonene, nerol oxide, citral (genanial),α-terpineol, trans-carveol,β-citronellol andcis-isogeraniol remained partly in the litchi wine. Cosmene,β-cis-ocimene,cis-linalool oxide,cis-rose oxide, (R)-lavandulol,cis-carveol,γ-terpineol, geraniol and (R)-(+)-citronellic acid were undetectable in litchi wine.
The compound 2-methoxymethyl-2, 4, 5-trimethyl-1, 3-dioxolane was confirmed in litchi wine, while not in litchi juice.

Table 1 Concentrations of organic acids and saturated free fatty acids of litchi juice (day 0) and litchi wine (day 14)

Table 2 Volatile compounds and their percentage relative areas (PRA %) in litchi juice (day 0) and litchi wine (day 14)
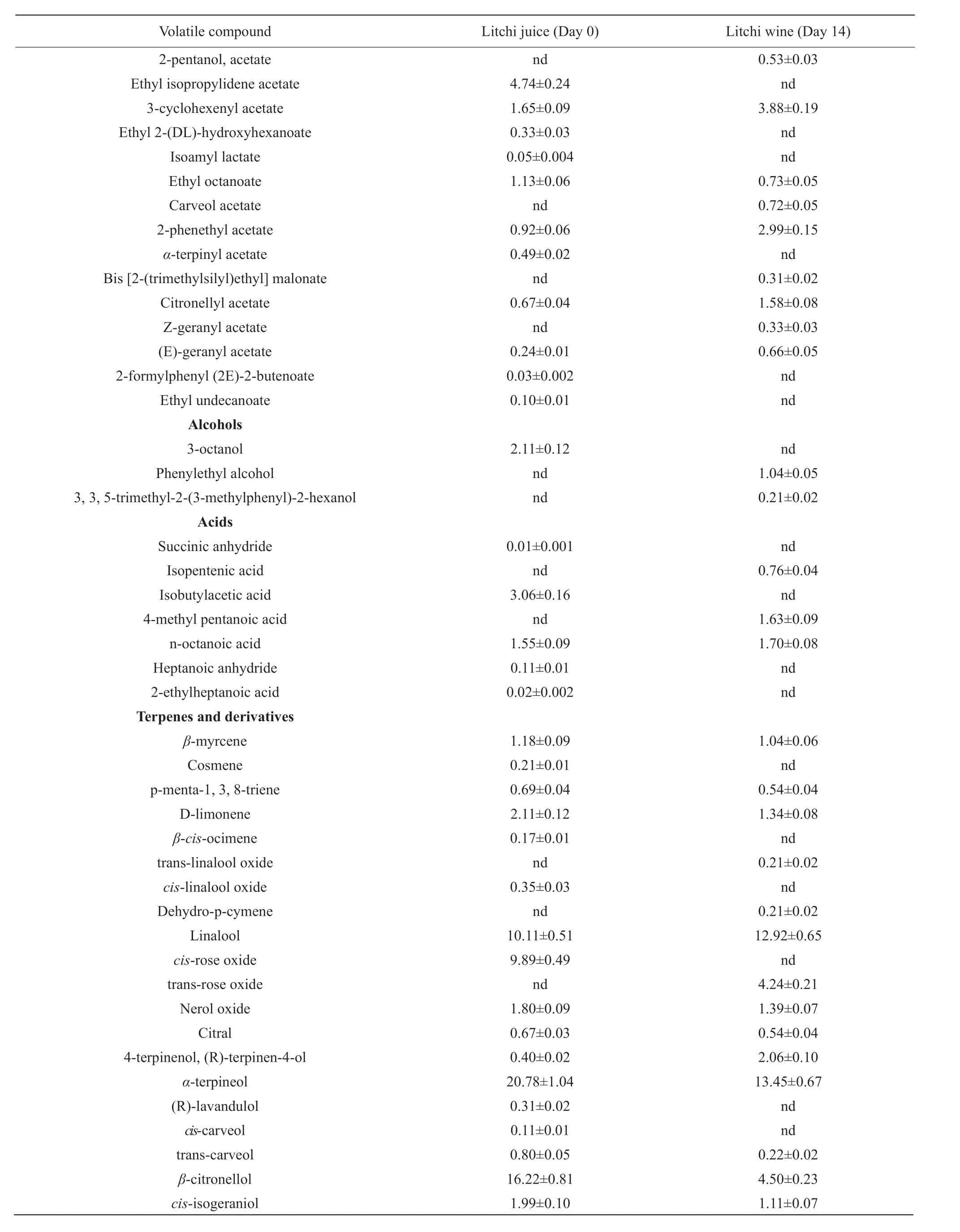
Continued
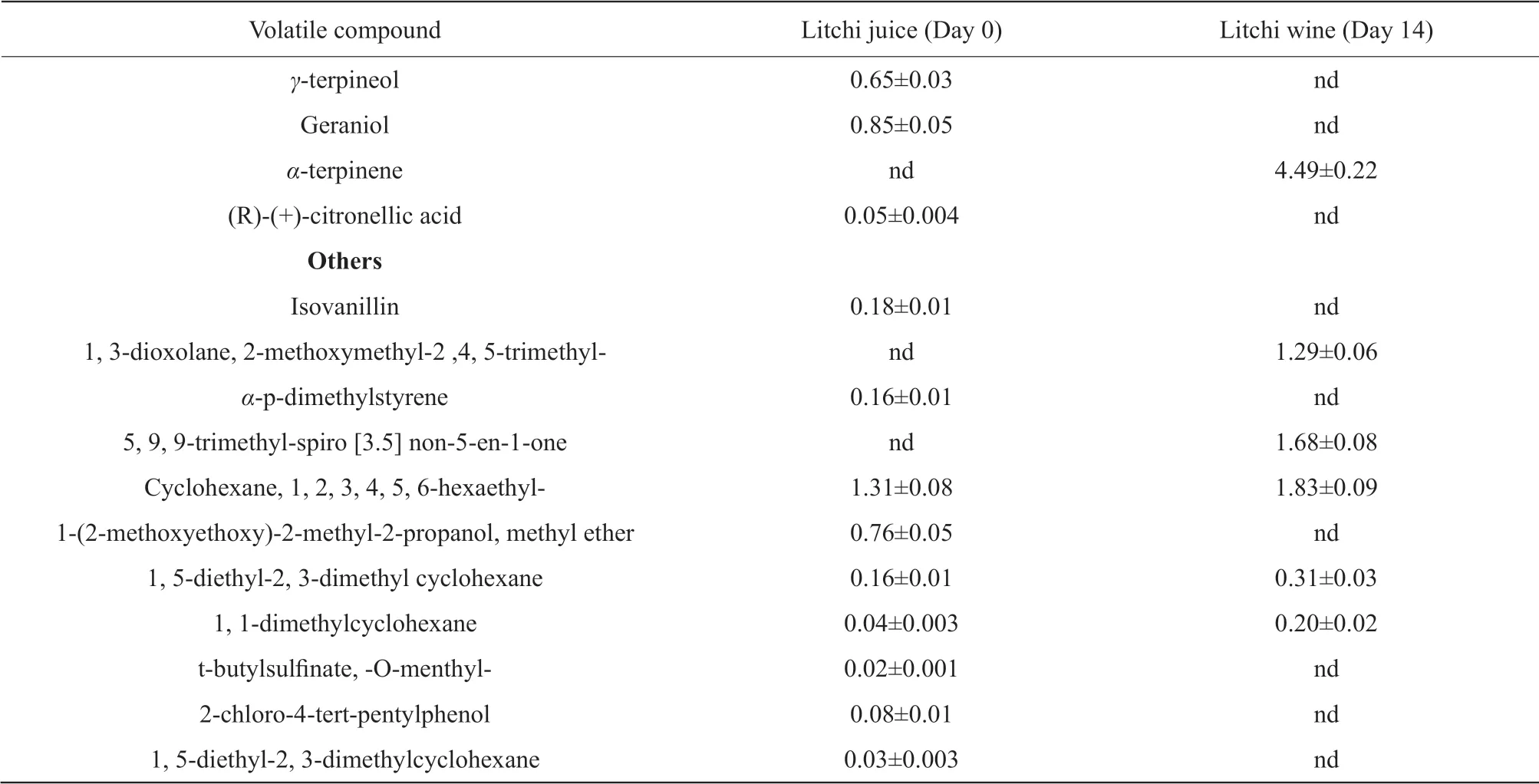
Continued
Discussion
The increase rate of alcohol content and the decrease rate of °Brix were concerted during alcoholic fermentation byS. bayanusBV818 which finished quickly. Ethanol was producedviaglycolysis. Sucrose, glucose and fructose were consumed and transformed to ethanol by alcoholic fermentation.
The concentration of acetic acid dropped sharply as acetic acid could be activated to acetyl CoA which could enter TCA cycle and produce fatty acid or react with alcohol to generate acetate esters (Chen and Liu, 2016a). The concentration of acetic acid in wine was still high after fermentation. Liuet al. (2011) found that the concentration of acetic acid decreased from about 11 g • L-1to 3 g • L-1after fermentation, then decreased to about 1.3 g • L-1after 2 months' storage. But the vinegary taste of wine was pleasant. Succinic acid and DL-malic acid changed obviously as they were the products of TCA cycle, coming from yeast metabolism. The concentration of pyruvic acid varied as it was producedviaglycolysis, and then was partially catabolized to generate ethanol and partially entered into TCA cycle, partially transformed to acetyl CoA which was the carbon source of fatty acid.
Palmitic acid and stearic acid increased most significantly after fermentation. Fatty acid was synthesized from acetyl-CoA which might come from sugar and acetic acid in the mitochondria. And citric acid with high concentrations could strengthen the anabolism of fatty acid as citric acid helped acetyl-CoA transfer out of mitochondria and urged the formation of active polymer of acetyl-CoA carboxylase.
Esters were the important byproducts of alcoholic fermentation produced by yeast and gave fruity flavor to litchi wine. Most esters were produced during fermentation. Acetate esters were the important esters from the esterification of alcohol and acetic acid in the litchi wine. The volatiles were analyzed only after fermentation, the ethyl esters would increase after storage, and the ethanol would decrease.
The terpenoid was another kind of significant volatility in the litchi juice and played the key role in the unique flavor of fresh litchi (Chenet al., 2015; Wuet al., 2009). Citronellol was very important for litchi wine making, because its metabolism could produce lots of aroma components (Alveset al., 2010; Demyttenaereet al., 2004). Most terpenoids decreased after fermentation, which might be related to metabolism, biotransformation, or volatility, or entry into the cell membrane (King and Dickinson, 2000).Cis-linalool oxide might be transformed to translinalool oxide, andcis-rose oxide transformed to transrose oxide, which was owing to isomerization. Some terpenols reduced with yeast metabolism, which might be esterified (Wuet al., 2011). Carveol,β-citronellol, terpineol and geraniol were partly converted into acetate esters correspondingly, and these esters increased indeed during the process of fermentation. Maintaining the terpenoids to a proper level was useful to retain the characteristic flavor of the litchi wine. The compound 2-methoxymethyl-2, 4, 5-trimethyl-1, 3-dioxolane in litchi wine was identified, while 2, 4, 5-trimethyl-1, 3-dioxolane was confirmed by Alveset al. (2010) in the four litchi fermented beverages.
Conclusions
The biotransformation of components including organic acid, saturated free fatty acid and volatile compounds was evaluated during the fermentation process of litchi wine making. The change trend of organic acid was different owing to the complicated metabolic pathways. And most free fatty acid increased after fermentation. Most characteristic aroma components retained or were transformed to other flavor compounds during fermentation. In general, the litchi wine had a fruity flavor and delicate bouquet and had harmonious sourness and sweetness. Fermentation alleviated the sour of 'Heiye' effectively. The litchi 'Heiye' had the potential of being fermented to litchi wine.
 Journal of Northeast Agricultural University(English Edition)2021年1期
Journal of Northeast Agricultural University(English Edition)2021年1期
- Journal of Northeast Agricultural University(English Edition)的其它文章
- Identification of Co-dominant SSR Markers Associated with Genes Controlling α′- and α-subunit-null β-conglycinin Phenotypes in Soybean (Glycine max (L.) Merr.)
- Study on Marker-assisted Breeding of Soybean Vitamin E
- Effect of Drought Stress on Growth and Water Physiological Characteristics of Poa sibirica
- Effect of Endophytic Fungus on Pyrola calliantha H. Andr Responsed to Cold Stress
- Uterine Expression of WNT7A and β-catenin After Induction of Oestrus in Sheep
- Multi-objective Function Optimization for Environmental Control of a Greenhouse Based on a RBF and NSGA-II
