Effect of Endophytic Fungus on Pyrola calliantha H. Andr Responsed to Cold Stress
Li Ai-yu, Wen You-wei, Liu Dan, and Wang Li-juan
College of Life Sciences, Northeast Agricultural University, Harbin 150000, China
Abstract: This study measured the antioxidant enzyme activities of Pyrola under cold and endophytic fungal infection conditions [i.e., peroxidase (POD), catalase (CAT) and superoxide dismutase (SOD)], and the contents of malondialdehyde (MDA), soluble sugar and soluble protein. In addition, the changes in the anatomical structure of Pyrola vegetable organ during cold stress upon inoculation with a synergistic endophytic fungus were also analyzed. It was found that the soluble sugar and soluble protein contents and POD, SOD and CAT activies of Pyrola all increased, when the plants were exposed to temperature of -5℃. In particular, the MDA contents and the POD activies significantly increased compared with those of the control. In addition, the soluble sugar and soluble protein contents increased during the treatment, which involved in both inoculation with an endophytic fungus and low temperature; however, with a reduction in the concentration of endophytic fungus, those two indicators decreased. The optimal concentration of endophytic fungus was 5×107 cfu • mL-1. From the anatomical structure of Pyrola root, endophytic fungi were found to improve the health of Pyrola in cold weather. These results showed that endophytic fungi played a vital role in the growth of Pyrola, which was an evergreen plant that could survive exposure to low temperature.
Key words: Pyrola calliantha H. Andr, endophytic fungus, low temperature
Introduction
Pyrola callianthaH. Andr is evergreen and perennial (Genget al., 2014).Pyrolais a herbaceous plant widespread in China. It is a small herb of the genusPyrolaof the family of Pyrolaceae, which is mainly distributed in the northeast of China. It is an indicator plant of the mixed and mixed forest of the temperate and temperate regions. In the Da Hinggan Mountains, where the winter temperature is -50℃, the green body under snow can pass through the winter, and a cold-adapted mechanism has been formed in the high-cold area. Therefore, it has great potential for application in the fields of gardening, greening and ornamental.Pyroalis not only widely used in landscaping, but also prominent in medicine. Traditional Chinese medicine uses it as a Chinese herbal medicine. It has been used for the treatment for neuralgia, gastric hemorrhage, pulmonary hemorrhage, and arthritic diseases (Wanget al., 2014).Pyrolahas also been used as tea for daily consumption. In recent years, it has been widely studied for its characteristics of resisting cold weather (Liuet al., 2007). Previous studies are performed on its distribution and classification, active ingredients, pharmacological effects, cultivation, production and domestication (Yaoet al., 2013; Zhanget al., 2013). At present, there are few reports about the successful cultivation ofPyrola. The problems of low success rate of introduction and domestication and difficult controls of cultivation conditions are important. One of the reasons is that the genus must be symbiotic with endophytic fungi to grow well (Vincenotet al., 2008). Plant endophytic fungus is a type of normal fungus that grows inside healthy plant tissues and organs and does not cause obvious symptoms, including mycorrhizal fungus and saprophytic fungus growing on the surface of plant tissues. They can be isolated from the surface of plants after disinfection. This kind of microorganisms can promote the growth of the host, improve the host's resistance to disease, stress and other abilities. Endophytic fungus can change the physiological characteristics of plants by affecting the material metabolism of the host plants and producing physiologically active substances, improve the resistance of plants, and stimulate plant growth (Arnold, 2007). Researches have shown that an endophytic fungus can play a vital role in the plant's ability to survive cold weather. The objective was to discover the reason why an endophytic fungus could helpPyrolaresist low temperature and to explore the effect of the endophytic fungus. In this study,Pyrolawas exposed to low temperature (-5℃) and endophytic fungus at different concentrations. The plants were analyzed from two aspects. The investigated physical indexes included peroxidase (POD), catalase (CAT) and superoxide dismutase (SOD) activities, as well as the contents of malondialdehyde (MDA), soluble sugar and soluble protein (Zouet al., 2000; Liet al., 2000; Haoet al., 2004). To further study the mechanism of the plant's adaption to cold damage, this study also focused on the anatomical structure of its roots, stems and leaves.
Materials and Methods
Plant materials and growth conditions
Pyrola, including a root ball of soil, was collected from Yichun Cold Nature Reserve, Heilongjiang Province (N 47°11′, E 330°53′). The plant was cultivated in a pot and illuminated for a daily photoperiod of approximately 16 h before the experiments. The culture was maintained at (23±2)℃ (Chenet al., 2013; Sunet al., 2011). The endophytic fungus was derived from our lab.
To determine the physical indexes of different concentrations of endophytic fungus, the endophytic fungus was inoculated on modified Melin-Norkrans Medium with the following formula: fresh potato juice 100 mL, glucose 20 g, beef extract 10 g, CaCl2• 2H2O 0.066 g, KH2PO40.5 g, NaCl 0.025 g, (NH4)2HPO40.25 g, MgSO4• 7H2O 0.15 g, Fe2(SO4)30.015 g, VB10.001 g, and agar 15 g. Then, distilled water was supplied to bring the volume to 1 000 mL. pH was adjusted to 5.3-5.7. Finally, the mixture was autoclaved for 20 min to achieve sterilization.
The endophytic fungus solution was divided into four concentrations: S1 (5×108cfu • mL-1), S2 (5×107cfu • mL-1), S3 (5×106cfu • mL-1) and S4 (5×105cfu • mL-1). The culturedPyrolawas divided into six different treatments. Each treatment contained three replicates. The roots ofPyrolain all the treatments were cultured with an equal amount of nutrient solution. Experimental groups were cultivated with nutrient solution, which was added with different concentrations of the endophytic fungus solution, while the control group was cultivated with equivalent nutrient solution only. The control group included six replicates, three of them were used as blank controls, and others were treated with no endophytic fungus solution. After 20 days, all the samples were stored at a refrigerator at -5℃ for 24 h. Then, the physical indexes were measured. Antioxidant enzyme activities (SOD, POD and CAT), MDA, soluble sugar and soluble protein contents were all measured with a kit produced by Keming Biotechnology. During those 20 days, the nutrient solution was renewed every 2 days and supplemented with 2 mL of 1 mol • L-1penicillin and 10 mL of different concentrations of endophytic fungus solution every 4 days. The endophytic fungus colonization rates were also determined. A small part ofPyrolaroots, which was treated with different concentrations of endophytic fungus listed above, was taken, washed carefully, cut into 1 cm section, and fixed with FAA. Then, it was soaked in a 10% KOH solution in 90℃ for 20 min, immersed in 2% HCl solution for 5 min and dyed with 0.01% acid magenta solution in 90℃ for 20-60 min. The method of grid line interception was used to observe and calculate the colonization rates (Chenet al., 2013).
The physical indexes ofPyrolaon endophytic fungus were determined at different low temperatures.
According to the results from the experiments above, it was found that the optimum concentration of endophytic fungus was 5×107cfu • mL-1, which was used to culturePyrola. Unlike previous studies, in this study all the treatments were stored at refrigerators set to 0℃, -5℃, -10℃, -15℃, -20℃ and -25℃ for 24 h. Then, these samples were used to determine the physical indexes.
Anatomical observation of Pyrola
Further researches on the treatments were conducted for the second experiment. Using paraffin section, observed the anatomical structures of the samples, which were also used as parts of the second experiment (Liet al., 1987). The paraffin section of the test referenced toThe Plant Physiology Test Guideedited by Zou Qi.
Statistical analysis
All the experimental data were analyzed using GraphPad Prism 5 and SPSS17.0.
Results
Determination of physical indexes in different concentrations of endophytic fungus
The results of the colonization rates are shown in Fig. 1.It could be inferred that there were already trace amounts of endophytic fungus in the wildPyrola(control treatment). The colonization rate was 23.25%. However, for thosePyrola, which were infected by endophytic fungus, the colonization rates decreased with the decrease in the concentration of endophytic fungus. Among the test group, the highest colonization rate was 57.25%, achieved by S2 with concentration of 5×107cfu • mL-1; the second highest colonization rate was 43.25%, achieved by S3 with concentration of 5×106cfu • mL-1; and the third was 35.13%, achieved by S4 with concentration of 5×105cfu • mL-1. However, the colonization rate in S1 was only 22.25%. The results of this study indicated that infection by endophytic fungus had a high capability to improve the amount of endophytic fungus inPyrola, which could be an evidence for endophytic fungus andPyrolasymbiosis.
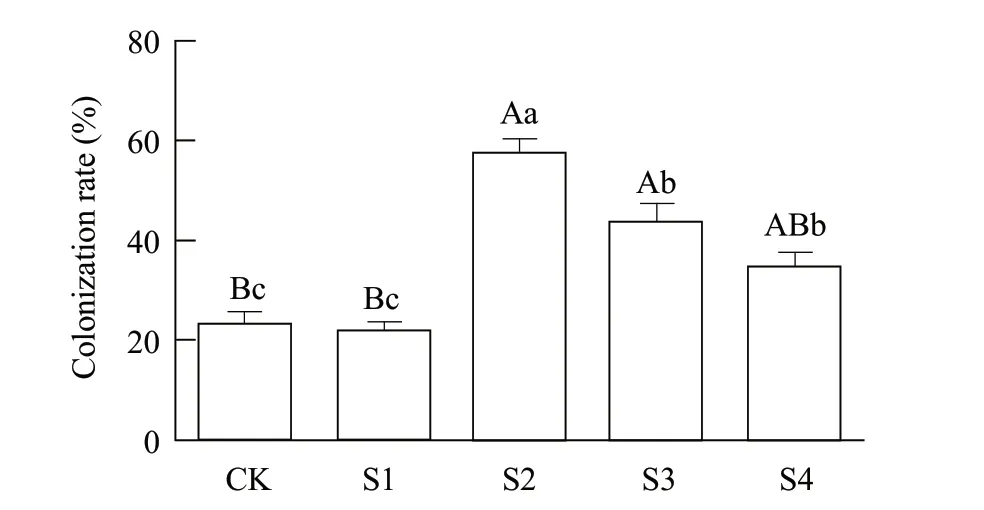
Fig. 1 Colonization rate of endophyte fungus in Pyrola calliantha H. Andr rootsControl represents wild Pyrola, S1-S4 represent different fungus treatments of Pyrola seedlings with concentrations of 5×108, 5×107,5×106 and 5×105 cfu • mL-1. Vertical bars represent standard deviations; different capital letters represent extremely significant differences among physiological indexes with different concentrations of infection (p≤0.01); different lower-case letters represent significant differences among physiological indexes with different concentrations of infection(p≤0.05).
This test analyzed the soluble sugar, soluble protein and MDA contents, and SOD and POD activities. The results are shown in Fig. 2A-E. From the results, it could be seen that these five physiological indexes all increased when the temperature was set at -5℃, especially MDA content and POD activity, which increased at highly significant levels. However, for thosePyrolathat suffered both infection and cold stress, the soluble sugar and soluble protein contents increased (Fig. 2A and B). However, the concentration of endophytic fungus was lower than 5×107cfu • mL-1, those two indexes decreased with the decrease in the endophytic fungus concentrations. Concurrently, MDA content decreased remarkably, especially in group S2 (Fig. 2C), which indicated thatPyrolahad a high ability to resist cold when the concentration was 5×107cfu • mL-1. The reasons for this effect were the reduction in membrane lipid peroxidation and the enhancement of biological membrane permeability (Songet al., 2016). Different concentrations of endophytic fungus didn't show any significant effects on SOD. However, the largest value of SOD activity occurred with S2 treatment was 106.73 U • g-1FW(Fig. 2D). However, POD activity significantly decreased with the decrease in endophytic fungus concentrations. The maximum value also appeared in S2,25.68 U • g-1FW • min-1(Fig. 2E). These results demonstrated that endophytic fungus played an important role in resisting POD activity, but did not have a significant effect on SOD activity. This experiment found that the optimum concentration was 5×107cfu • mL-1. Subsequent experiments on endophytic fungus and plant joint training would adopt this concentration.

Fig. 2 Changes in physiological indexes in -5°C treatment of Pyrola with different concentrationsControl represents normal wild Pyrola, 0 represents -5℃ processing of wild Pyrola, S1-S4 indicate treatment of Pyrola seedlings at -5℃ with different concentrations of fungus co-culture (5×108, 5×107, 5×106 and 5×105 cfu • mL-1). Vertical bars represent standard deviations.
Determination of physical indexes of Pyrola infected with endophytic fungi under different low temperatures
The physical indexes ofPyrolainfected with endophytic fungi under different low temperatures are shown in Fig. 3. The soluble sugar and soluble protein contents showed significant changes, initially increasing and then decreasing (Fig. 3A and B). Until the temperature dropped to -10℃, the soluble sugar and soluble protein contents appeared significant increase compared to those at -5℃. The maximum values of soluble sugar and soluble protein contents occurred when the temperature was -15℃, and the control group also appeared to have the maximum values at this temperature, which were 12.99 and 8.26 mg • g-1, 17.20 and 14.58 mg • g-1, respectively. These significant changes indicated that plant cells had been suffered severe damage under cold stress (Songet al., 2016; JaIali-e-Emamet al., 2011). The specific performance of serious damage could be due to the impediment of soluble sugar and soluble protein sources, although the mechanism of plant response to cold stress had previously started, and then the soluble sugar and soluble protein contents increased to maximum levels to protect the plant and maintain osmotic balance (JaIali-e-Emamet al., 2011;Wanget al., 2011). In addition, endophytic fungus could produce certain metabolites that might include soluble sugar and soluble protein (Birgitet al., 2013), so from Fig. 3, it could be seen that the soluble sugar and soluble protein contents apparently increased compared with those of the control treatment, especially when the temperature was -15℃. The results demonstrated thatPyrolacould resist the cold stress by increasing the soluble sugar and soluble protein contents. Moreover, the existence of endophytic fungus could increase osmotic regulation of substances to reduce the freezing point, further improving the cold resistance (Yooyongwechet al., 2014; Suravootet al., 2016).
The content of MDA increased significantly with decreasing temperature, indicating a negative correlation between MDA content and temperature (Fig. 3C). Until the temperature dropped to -10℃, MDA content increased significantly compared with that at -5℃. However, MDA content of the treatment group (S2) was obviously smaller than that of the control group (CK). In addition, the numerical difference between the treatment group and the control group was relatively obvious. It was worth noting when the temperature was -25℃, MDA content was 0.36 µmol • L-1,the amount of decrease reached 127% compared with the control group. This result demonstrated that MDA content was an important indicator, when a plant encountered cold stress (Wanget al., 2011). When the temperature was low, the plant cells were damaged, MDA content increased at the same time. Moreover, the existence of endophytic fungus could increase osmotic regulation of substances to reduce damage, further improving the cold resistance of the plants (Birgitet al., 2013).
SOD and POD activities were changed significantly, increasing initially and then decreasing (Fig. 3D and E). The maximum values of SOD activity occurred when the temperature was -10℃ and the control group also exhibited a maximum value at -10℃, 107.5 U • g-1FW and 110.3 U • g-1FW, respectively. When the temperature decreased, there were no significant changes among the groups. The endophytic fungus could increase SOD activity of this plant, but not significantly, as the temperature decreased. The maximum value of POD activity occurred when the temperature was -5℃, and the control group also appeared to have a maximum value at -5℃, 25.68 U • g-1FW • min-1and 35.96 U • g-1FW • min-1, respectively. Then, coinciding with the decrease in temperature, POD activity decreased, unlike SOD activity, there were differences among the groups. Furthermore, POD activity value of the treatment group was smaller than that of the control group. As important antioxidant enzymes in plants, SOD and POD could remove ROS; therefore, they could reduce the damage of plants caused by ROS (Liet al., 2012). Therefore, the improvement of SOD and POD activities could directly reflectPyrolas' resistance to cold stress.
Anatomical observation of Pyrola
Changes in Pyrola roots with cold stress and inoculation with synergistic endophytic fungus
Most ofPyrolaroots was fibrous, however, the real roots didn't develop. From Fig. 4 A-D, it could be seen that the roots consisted of epidermis, cortex and vascular cylinders (Bertet al., 2013; Fabioet al., 2016; Eswaranpillaiet al., 2015). Fig. 4A showed a cross cutting of wildPyrolaroots. The epidermal cells were composed of a small amount of hartig net, but no root hair. In addition, endophytic fungus formed hyphae in cortex parenchyma cells (Salmaet al., 2015; Hanset al., 2013; Gaoet al., 2010), and hyphae was not observed in vascular cylinder cells. Fig. 4B showed thatPyrolaroots were infected by endophytic fungi. Compared with Fig. 4A, the results suggested that the volume of cortex parenchyma cells increased, the cells became empty, and the hypha content significantly increased. Fig. 4C showed a cross cutting ofPyrolaroots subjected to -5℃ treatment. Compared with Fig. 4A, the results showed that the volume of cortex parenchyma cells decreased, and the cell content decreased.
Fig. 4D showed that a cross cutting ofPyrolaroots was infected by endophytic fungi under -5℃ treatment. Compared with Fig. 4A, Fig. 4D showed that the structure ofPyrolaroots returned to normal levels. Moreover, the hartig net of epidermal cells significantly increased. All these results demonstrated that the roots were parts of the endophytic fungal colonization, which existed in the cortex cells. The structure ofPyrolaroots changed at low temperature. This result demonstrated thatPyrolawas damaged under low temperature; however, endophytic fungus had the ability to help the plant resist the low temperature damage, which could contribute to endophytic fungus forming abundant hartig nets around the roots, which could enhance the capacity ofPyrolaroots to resist cold stress.
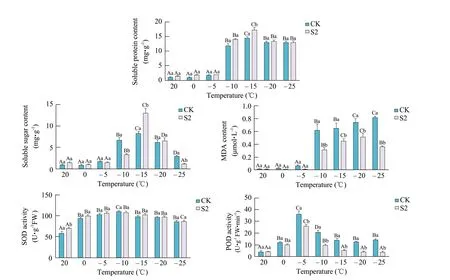
Fig. 3 Changes in physiological indexes in S2 (5×107 cfu • mL-1) treatment of Pyrola at different temperaturesVertical bars represent standard deviations.
Changes in Pyrola rhizome with cold stress and inoculation with synergistic endophytic fungus
Pyrolahad white and tan rhizomes (Huet al., 1990). From Fig. 4E and F, it could be seen that the rhizome also consisted of epidermis, cortex and vascular cylinder. These two pictures showed the wildPyrola. From Fig. 4E and F, it could be found that 1-3 layers of epidermal cells were arranged near the cortex, and numerous hyphae were distributed in the intercellular matrix. The difference in vessel bore diameter was not obvious. Fig. 4 G showed the wildPyrolarhizome rip cutting, it could be observed that the primary vascular was annular. Fig. 4H showed aPyrolarhizome rip cutting with endophytic fungal infection, while Fig. 4I showed aPyrolarhizome rip cutting under -5℃ treatment. Fig. 4J showed the rip cutting ofPyrolarhizome infected by endophytic fungus under -5℃ treatment.
From Fig. 4H, I and J, it could be observed that the vessel bore diameter ofPyrola rhizomedecreased; however, this problem could be alleviated by endophytic fungus infection. These results demonstrated that low temperature resulted in a decrease in the vessel bore diameter ofPyrolarhizomes; for this reason, the water transportation was affected. However, the existence of endophytic fungus could alleviate the phenomenon to some extent. It provided favorable conditions for leaf photosynthesis, thereby enhancing the ability of resistance to low temperature forPyrola.
Changes of Pyrola leaves with cold stress and inoculated with endophytic fungus
The cuticle ofPyrolaleaves developed, and there was no trichome on it, below the cuticle was collenchyma. Fig. 4K and L showed the wildPyrolaleaf cross cuttings, it was found that the leaf were isolateral and there was no difference between palisade tissues and spongy tissues. Fig. 4M showed a cross cutting ofPyrolaleaves under -5℃ treatment. Fig. 4N showed a cross cutting ofPyrolaleaves infected by endophytic fungus under -5℃ treatment. Compared with the wildPyrolaleaf cross cuttings, from Fig. 4M and N, it could be seen that endophytic fungus could increase the leaf thickness, which had a positive effect onPyrolaagainst low temperature.

Fig. 4 Observation of anatomical structures of different processing Pyrola organs by optical microscopeScale bar 100 µm. X, Xylem; Ph, Phloem; Vs, Catheter; Pa, Cortex parenchyma; E, Exodermis; Co, Cortex; Ma, Marrow; Ue, Upper epidermis; Le, Lower epidermis; Sc, Sclerenchyma; Vac, Vascular cylinder. A, Wild Pyrola root cross cutting, 100×; B, Endophytic fungus-infected Pyrola root cross cutting, 100×; C, -5℃ treatment of Pyrola root cross cutting, 100×; D, -5℃ treatment of endophytic fungus infected Pyrola root cross cutting, 100×; E, Wild Pyrola rhizome cross cutting, 40×; F, Wild Pyrola rhizome cross, 100×; G, Wild Pyrola rhizome rip cutting, 100×; H, Endophytic fungal reinfection of Pyrola rhizome rip cutting, 100×; I, -5℃ treatment of Pyrola rhizome rip cutting, 100×; J, Within -5℃ treatment endophyte reinfection of Pyrola rhizome rip cutting, 100×; K, Wild Pyrola leaf cross cutting, 40×; L, Wild Pyrola leaf cross cutting, 100×; M, -5℃ treatment of Pyrola leaf cross cutting, 40×; N, -5℃ treatment of endophytic fungus-infected leaves of Pyrola cross cutting, 40×.
Discussion
Determination of physical indexes in different concentrations of endophytic fungus
Pyrolaand endophytic fungus were in the symbiotic system, so the colonization of endophytic fungus inPyrolacould be observed, and the colonization rate was higher. When the plant was re-infected with different concentrations of endophytic fungus, the colonization rate of the fungus decreased with the decrease of concentration, and the optimum concentration was 5×107cfu • mL-1. While too high concentrations of endophytic fungus would inhibit themselves' growth. The regulation was conducted in the present study in a such way as to correspond to studies on the colonization ofP. fluorescensroot (Wanget al., 1996). In addition, the colonization of endophytic fungus in the roots of this study might also be affected by factors such as the sampling period. From the physiological indexes, it could be found that endophytic fungus could improve the content of the osmotic regulation substance such as soluble sugar and protein contents, and reduce the level of membrane lipid, that was, the reduction of MDA content could help plants get the ability to resist stress. A possible explanation for these results was that those endophytic fungi could synthesize large amounts of the secondary metabolites during their growth and enhance the ability of tolerance in host through complex regulatory approaches. The results also showed that endophytic fungus had no effect on SOD activity, while it could inhibit POD activity. In this study, POD activity was abnormal, because endophytic fungus stimulated the activities of other protective substances or its metabolites directly inhibited POD activity, and it needed to be further explored.
Determination of physical indexes of Pyrola infected with endophytic fungus under different low temperatures
Through the study of the determination of physical indexes ofPyrolainfected with endophytic fungus under different low temperatures, it could be found that the contents of soluble sugar, soluble protein, MDA, SOD and POD activities in the leaves ofPyrolaall increased first and then decreased with the decrease of temperature, and the similar results were reported by Sunet al(2011). This might be due to the hindrance of photosynthesis, which caused the plant body to be damaged under low temperature conditions.
In addition, the study had several interesting discoveries as the followings. From these results, it was found that -5℃ was the critical value of the response ofPyrolato the cold stress. Moreover, the maximum content of soluble sugar and soluble protein appeared at -15℃ and decreased with the decrease of temperature, because endophytic fungus needed a certain temperature range to survive, when the temperature was lower than -15℃, the growth rate slowed down, the synthesis of the secondary metabolites reduced, and the ability of the host to cope with low temperature weakened. Therefore, it was speculated that -15℃ might be the lethal temperature ofPyrola, while this speculation needed to be further confirmed.
Anatomical observation of Pyrola
Through the anatomical study on the rhizome and leaves ofPyrola, it was found that the leaves ofPyrolawere composed of upper and lower epidermis, mesophyll and vascular bundles. In addition, the stratum corneum ofPyroladeveloped well (Zhaoet al., 2005). What's more, there were studies showed thatPyrolawas a C4 plant. This also made it possible to help plants to resist the cold stress.
Conclusions
Pyrolawas infected by endophytic fungus at different concentrations. It could be concluded that the optimum concentration was 5×107cfu • mL-1. After this, the colonization rate decreased with the decrease in the concentration. With this concentration of endophytic fungus,Pyrolahad a high ability to resist cold due to the reduction of membrane lipid peroxidation and the enhancement of biological membrane permeability. By examining the effects of endophytic fungus onPyrolaat different low temperatures, the results suggested that the fungus would improve the capacity ofPyrolato resist the cold stress of low temperature and inoculated endophytic fungus exhibited synergistic conditions. Hartig nets, which primarily existed in epidermis and cortex parenchyma cells, played a vital role inPyrola's ability to resist the cold stress.
 Journal of Northeast Agricultural University(English Edition)2021年1期
Journal of Northeast Agricultural University(English Edition)2021年1期
- Journal of Northeast Agricultural University(English Edition)的其它文章
- Identification of Co-dominant SSR Markers Associated with Genes Controlling α′- and α-subunit-null β-conglycinin Phenotypes in Soybean (Glycine max (L.) Merr.)
- Study on Marker-assisted Breeding of Soybean Vitamin E
- Effect of Drought Stress on Growth and Water Physiological Characteristics of Poa sibirica
- Biotransformation of Flavor Compositions During Fermentation of Litchi (Litchi Chinensis Sonn.) Fruits into Wine
- Uterine Expression of WNT7A and β-catenin After Induction of Oestrus in Sheep
- Multi-objective Function Optimization for Environmental Control of a Greenhouse Based on a RBF and NSGA-II
