CAN催化的一锅法合成螺[吲哚-吡唑并[3,4-b]吡啶]类化合物
丰诚杰 赵尖斌 何 潇 吴春雷
(1.绍兴文理学院 化学化工学院,浙江 绍兴 312000;2.新和成控股集团有限公司,浙江 绍兴 312500;3.嵊州市环境保护监测站,浙江 绍兴 312400)
螺环化合物因其独特的空间立体结构,展现出了广泛的生物活性.尤其是螺环吲哚酮类化合物,在吲哚3号位引入其他的结构后,在很多方面都表现出了极高的生物活性,如抗杜氏利什曼原虫[1],抗乳腺癌[2],抑制肿瘤生长[3],抗病毒[4],HDM2拮抗[5],抗菌[6]等.吡唑也是很多药物的核心结构,如塞来昔布、西地那非和羟布宗等.此外,吡唑并嘧啶类化合物由于其独特的活性,也是最近研究的热门,在PI3Kδ受体抑制[7],抑制柯萨奇病毒活性[8],抗血小板聚集[9],抗焦虑[10]等方面有着很多报道.
基于以上原因,将螺环吲哚和吡唑并吡啶结构相结合,所得的物质也能展现出独特的活性.已经有报道以靛红、5-氨基吡唑和1,3-二羰基化合物为原料,合成螺环吲哚-吡唑并吡啶类化合物,所用到的催化剂主要有[NMP]H2PO4[11]、InCl3[12]、PTSA[13-14]等.硝酸铈铵(CAN)作为一种氧化剂,同时也是一种Lewis酸,经常被应用于各种有机反应[15-17],本文拟以硝酸铈铵为催化剂,取代靛红、5-氨基吡唑和苯甲酰腈三组分一锅法合成了系列螺[吲哚-3,4’-吡唑并[3,4-b]吡啶]类化合物(见图1).

图1 螺[吲哚-3,4’-吡唑并[3,4-b]吡啶]类化合物的合成
1 实验部分
1.1 试剂和仪器
所有试剂均为市售,无需进一步纯化即可使用.NMR在Bruker AVANCE DMX III400 M 上测定;熔点在BUCHI M-560 熔点仪测得.
1.2 螺[吲哚-3,4’-吡唑并[3,4-b]吡啶]类化合物合成的一般步骤
往50 mL三口烧瓶中加入5 mL无水乙醇,再加入靛红(2 mmol)、5-氨基吡唑(2 mmol)和对氯苯甲酰腈(2 mmol),催化剂硝酸铈铵(0.16g, 0.3 mmol),80 ℃下搅拌6-8小时.TLC(乙酸乙酯:石油醚=1∶4)跟踪反应完毕,冷却至室温,加入乙酸乙酯萃取三次(3×5mL),合并有机层,旋蒸除去乙酸乙酯得到粗产物,再用乙醇重结晶,即可得到目标产物.所得产物的核磁图谱表征如下:
6’-(4-氯苯基)-3’-甲基-2-氧代-1’-苯基-1’,7’-二氢螺[吲哚-3,4’-吡唑并[3,4-b]吡啶]-5’-腈(4a)∶淡黄色晶体;m.p.∶272-273 ℃(271-274 ℃)[18];1H NMR (400 MHz,DMSO)∶δ=1.54(s,3H,CH3),6.95-6.97(m,H,ArH),7.04-7.08(m,H,ArH),7.26-7.31(m,2H,ArH),7.36-7.40(m,H,ArH),7.49-7.54(m,2H,ArH),7.57-7.59(m,4H,ArH),7.63-7.65(m,2H,ArH),10.25(s,H,NH),10.70(s,H,NH);13C NMR(100 MHz,DMSO)∶δ=11.8,39.4,40.6,51.4,82.4,99.2,110.2,118.8,123.2,123.9,125.8,127.7,128.9,129.8,131.3,132.7,134.8,135.6,138.0,138.3,141.5,144.9,151.5,178.1.
6’-(4-氯苯基)-1’,3’-二甲基-5-氟-2-氧代-1’,7’-二氢螺[吲哚-3,4’-吡唑并[3,4-b]吡啶]-5’-腈(4b)(new)∶灰白色固体;m.p.∶>300 ℃(318-320 ℃)[18];1H NMR(400 MHz,DMSO)∶δ=1.47(s,3H,CH3),3.68(s,3H,CH3),6.89-6.93(m,1H,ArH),7.08-7.16(m,2H,ArH),7.64(s,4H,ArH),10.42(s,1H,NH),10.66(s,1H,NH);13C NMR(100 MHz,DMSO)∶δ=11.6,35.5,52.1,80.8,96.9,111.0,111.1,113.3,113.5,115.9,116.2,119.1,129.1,131.2,132.6,135.9,136.5,136.6,137.5,138.4,142.4,151.2,158.0,160.4,178.5.
5-溴-6’-(4-氯苯基)-1’,3’-二甲基-2-氧代-1’,7’-二氢螺[吲哚-3,4’-吡唑并[3,4-b]吡啶]-5’-腈(4c)(new)∶灰白色固体;m.p.∶316-317 ℃(317-320 ℃)[18],1H NMR(400 MHz,DMSO)∶δ=1.47(s,3H,CH3),3.68(s,3H,CH3),6.89(d,J=8.0Hz,1H,ArH),7.40(s,1H,ArH),7.67(s,4H,ArH),10.46(s,1H,NH),10.79(s,1H,NH);13C NMR(100 MHz,DMSO)∶δ=11.6,19.0,35.5,51.7,56.5,80.6,96.8,112.2,114.8,119.1,128.5,129.1,131.3,132.4,132.5,135.8,137.2,138.4,140.6,142.3,151.2,177.9.
6-氯-6’-(4-氯苯基)-1’,3’-二甲基-2-氧代-1’,7’-二氢螺[吲哚-3,4’-吡唑并[3,4-b]吡啶]-5’-腈(4d)(new)∶灰白色固体;m.p.∶321-322 ℃(322-323 ℃)[18];1H NMR(400 MHz,DMSO)∶ δ=1.47(s,3H,CH3),3.69(s,3H,CH3),6.95(s,1H,ArH),7.09(s,1H,ArH),7.22(s,1H,ArH),7.64(s,4H,ArH),10.44(s,1H,NH),10.81(s,1H,NH);13C NMR(100 MHz,DMSO)∶δ=11.7,35.5,51.2,80.7,96.8,110.3,119.0,122.9,127.2,129.2,131.2,132.5,133.8,135.9,138.4,142.3,142.9,151.1,178.3.
6-溴-6’-(4-氯苯基)-1’,3’-二甲基-2-氧代-1’,7’-二氢螺[吲哚-3,4’-吡唑并[3,4-b]吡啶]-5’-腈(4e)(new)∶灰白色固体;m.p.∶295-297 ℃(295-296 ℃)[18];1H NMR(400MHz,DMSO)∶δ=1.46(s,3H,CH3),3.68(s,3H,CH3),7.07(s,1H,ArH),7.16-7.24(m,2H,ArH),7.63(s,4H,ArH),10.44(s,1H,NH),10.80(s,1H,NH);13C NMR(100MHz, DMSO)∶δ=11.7,35.5,51.3,80.7,96.7,113.1,122.2,125.8,127.5,129.2,131.2,132.5,134.2,135.9,138.4,142.4,143.0,151.1,178.2.
6’-(4-氯苯基)-1’,3’,5-三甲基-2-氧代-1’,7’-二氢螺[吲哚-3,4’-吡唑并[3,4-b]吡啶]-5’-腈(4f)(new)∶白色粉末;m.p.∶315-317 ℃(315-316 ℃)[18];1H NMR(400 MHz,DMSO)∶δ=1.44(s,3H,CH3),2.25(s,3H,CH3),3.68(s,3H,CH3),6.80(d,J=7.6Hz,1H,ArH),7.00(d,J=7.6Hz,1H,ArH),7.05(d,J=8.0Hz,1H,ArH),7.64(s,4H,ArH),10.33(s,1H,NH),10.52(s,1H,NH);13C NMR(100 MHz,DMSO)∶δ=11.58,19.01,21.08,35.40,51.53,56.49,81.51,97.46,109.79,119.18,126.07,129.13,129.78,131.24,131.98,132.68,135.15,135.69,138.27,138.88,142.36,150.76,178.28.
6’-(4-氯苯基)-1’,3’-二甲基-2-氧代-1-苯基-1’,7’-二氢螺[吲哚-3,4’-吡唑并[3,4-b]吡啶]-5’-腈(4g)∶白色粉末;m.p.∶296-297 ℃(296-298 ℃)[18];1H NMR(400 MHz,DMSO)∶δ=1.56(s,3H,CH3),3.72(s,3H,CH3),6.79(m,1H,ArH),6.81(m,1H,ArH),7.20-7.30(m,4H,ArH),7.50(s,1H,ArH),7.60-7.70(m,6H,ArH),10.54(s,1H,NH);13C NMR(100 MHz,DMSO)∶δ=11.8,35.5,51.3,81.1,97.0,109.5,119.0,124.5,126.0,127.0,129.0,129.2,129.8,130.4,131.3,132.5,133.9,134.5,135.9,138.4,142.4,150.9,176.2.
6’-(4-氯苯基)-1’,3’-二甲基-2-氧代-1’,7’-二氢螺[吲哚-3,4’-吡唑并[3,4-b]吡啶]-5’-腈(4h)∶白色粉末;m.p.∶271 ℃(270-272 ℃)[18];1H NMR(400 MHz,DMSO)∶δ=1.43(s,3H,CH3),3.68(s,3H,CH3),6.91(d,J=7.6Hz,1H,ArH),7.03(d,J=7.6Hz,1H,ArH),7.18(d,J=5.6Hz,1H,ArH),7.24-7.26(m,1H,ArH),7.64(s,4H,ArH),10.35(s,1H,NH),10.62(s,1H,NH);13C NMR(100 MHz,DMSO)∶δ=11.6,35.4,51.5,81.4,98.3,110.0,119.1,123.1,125.6,129.1,129.5,131.2,132.7,135.0,135.7,138.3,141.4,142.3,150.8.
6’-(4-氯苯基)-5-氟-3’-甲基-2-氧代-1’-苯基-1’,7’-二氢螺[吲哚-3,4’-吡唑[3,4-b]吡啶]-5’-腈(4i)∶浅黄色晶体;m.p.∶294-295 ℃(295-297 ℃)[18];1H NMR(400 MHz,DMSO)∶δ=1.59(s,3H,CH3),6.94-6.97(m,H,ArH),7.12-7.20(m,H,ArH),7.25-7.27(m,H,ArH),7.37-7.41(m,H,ArH),7.50-7.53(m,2H,ArH),7.57-7.60(m,4H,ArH),7.64-7.66(m,2H,ArH),10.38(s,H,NH),10.78(s,H,NH);13C NMR(100 MHz,DMSO)∶δ=11.7,51.9,81.8,98.7,111.1~111.2,113.4-113.7,116.2-116.4,118.8,124.1,127.8,128.9,129.8,131.4,132.7,135.7,136.2~136.3,137.6,138.1,138.2,144.8,151.8,158.0,160.4,178.2.
6’-(4-氯苯基)-3’,5-二甲基-2-氧代-1’-苯基-1’,7’-二氢螺[吲哚-3,4’-吡唑并[3,4-b]吡啶]-5’-腈(4j)∶浅黄色晶体;m.p.∶268-269 ℃(268-270 ℃)[18];1H NMR(400 MHz,DMSO)∶δ=1.56(s,3H,CH3),2.28(s,3H,CH3),6.83-6.85(m,H,ArH),7.09-7.11(m,2H,ArH),7.39-7.40(m,H,ArH),7.49-7.53(m,2H,ArH),7.58-7.60(m,4H,ArH),7.64-7.67(m,2H,ArH),10.29(s,H,NH),10.65(s,H,NH);13C NMR(100 MHz,DMSO)∶δ=11.7,21.1,39.3~40.6,51.5,82.6,99.3,110.0,119.0,123.9,126.2,127.7,128.9,129.8,130.0,131.4,132.2,132.8,134.8,135.6,138.0,139.0,145.0,151.4,178.1.
6’-(4-氯苯基)-6-氯-3’-甲基-2-氧代-1’-苯基-1’,7’-二氢螺[吲哚-3,4’-吡唑并[3,4-b]吡啶]-5’-腈(4k)∶浅黄色晶体;m.p.∶329-330 ℃(328-330 ℃)[18];1H NMR(400 MHz,DMSO)∶δ=1.58(s,3H,CH3),6.98-6.99(m,H,ArH),7.12-7.14(m,H,ArH),7.31-7.33(m,H,ArH),7.37-7.41(m,H,ArH),7.49-7.53(m,2H,ArH),7.58-7.60(m,4H,ArH),7.63-7.66(m,2H,ArH),10.40(s,H,NH),10.94(s,H,NH);13C NMR(100 MHz,DMSO)∶δ=11.8,39.3,40.6,51.2,81.8,98.6,110.4,118.8,123.0,124.0,127.4,127.8,129.0,129.8,131.4,132.6,133.4,134.0,135.7,138.1,138.2,142.9,144.8,151.7,178.0.
6’-(4-氯苯基)-1,1’-二苯基-3’-甲基-2-氧代-1’,7’-二氢螺[吲哚-3,4’-吡唑并[3,4-b]吡啶]-5’-腈(4l)∶浅黄色晶体;m.p.∶266-267 ℃(265-267 ℃)[18];1H NMR(400 MHz,DMSO)∶δ=1.68(s,3H,CH3),7.21-7.25(m,H,ArH),7.34-7.47(m,5H,ArH),7.51-7.55(m,4H,ArH),7.58-7.70(m,8H,ArH),10.50(s,H,NH);13C NMR(100 MHz,DMSO)∶δ=12.0,15.5,18.4,19.3-40.6,51.3,82.2,98.8,109.6,118.8,121.4,124.1,124.7,126.1,127.0,127.8,129.0,129.1,129.8,130.0,130.5,131.4,131.5,132.5,133.6,134.4,135.8,138.2,138.3,142.4,144.9,151.6,176.0.
6’-(4-溴苯基)-5-氟-3’-甲基-2-氧代-1’-苯基-1’,7’-二氢螺[吲哚-3,4’-吡唑并[3,4-b]吡啶]-5’-腈(4m)∶白色粉末;m.p.∶329-330 ℃(330-332 ℃)[18];1H NMR(400 MHz,DMSO)∶δ=1.58(s,3H,CH3),6.96(s,1H,ArH),7.12-7.16(s,1H,ArH),7.25(d,J=6.8Hz,1H,ArH),7.40(s,1H,ArH),7.50-7.58(s,6H,ArH),7.72(d,J=8.0Hz,1H,ArH),10.37(s,1H,NH),10.78(s,1H,NH);13C NMR(100 MHz,DMSO)∶δ=11.7,51.9,81.8,98.7,111.1,111.2,113.4,113.7,116.2,116.4,118.8,124.1,124.4,127.8,129.8,131.6,131.8,133.0,136.2,136.2,137.6,138.1,138.2,144.8,151.8,158.0,160.4,178.2.
6’-(4-溴苯基)-3’,5-二甲基-2-氧代-1’-苯基-1’,7’-二氢螺[吲哚-3,4’-吡唑并[3,4-b]吡啶]-5’-腈(4n)∶白色粉末;m.p.∶335-336 ℃(335-337 ℃)[18];1H NMR(400 MHz,DMSO)∶δ=1.56(s,3H,CH3),2.28(s,3H,CH3),6.84(d,J=8.4Hz,1H,ArH),7.10(d,J=6.8Hz,2H,ArH),7.39(t,J=7.2Hz,1H,ArH),7.49-7.59(m,6H,ArH),7.72(d,J=8.4Hz,2H,ArH),10.29(s,1H,NH),10.64(s,1H,NH);13C NMR(100 MHz,DMSO)∶δ11.7,21.1,51.5,82.5,99.3,109.9,118.9,123.9,124.3,126.2,127.7,129.8,130.1,131.6,131.9,132.2,133.1,134.8,138.0,138.3,138.9,144.9,151.5,178.1.
6-溴-6’-(4-溴苯基)-1’,3’-二甲基-2-氧代-1’,7’-二氢螺[吲哚-3,4’-吡唑并[3,4-b]吡啶]-5’-腈(4o)∶灰白色固体;m.p.∶296-297 ℃(295-296 ℃)[18];1H NMR (400MHz,DMSO)∶δ=1.46(s,3H,CH3),3.68(s,3H,CH3),7.07(s,1H,ArH),7.16-7.24(m,2H,ArH),7.63(s,4H,ArH),10.44(s,1H,NH),10.80(s,1H,NH);13C NMR(100 MHz,DMSO)∶δ=11.7,35.5,51.3,80.7,96.7,113.1,122.2,125.8,127.5,129.2,131.2,132.5,134.2,135.9,138.4,142.4,143.0,151.1,178.2.
2 结果与讨论
首先,选取靛红、3-甲基-1-苯基-吡唑5-氨和对氯苯甲酰腈三组分反应为模型,对影响反应的催化剂、溶剂、反应时间及温度做了优化选择(表1).当反应不加催化剂时,反应时间即使延长到24 h,反应仍很难进行(投料1).然后分别选取了ZnCl2,FeCl3和硝酸铈铵(CAN)作催化剂,做了对比,在其他条件一样时,CAN表现出了较高的催化活性(投料2,3,4).接着,对反应溶剂做了筛选,从表1可看出乙醇适合该反应(投料4,5,6,7).最后对催化剂用量作考察,发现15%mol量的CAN就足够促使反应顺利完成(投料9).
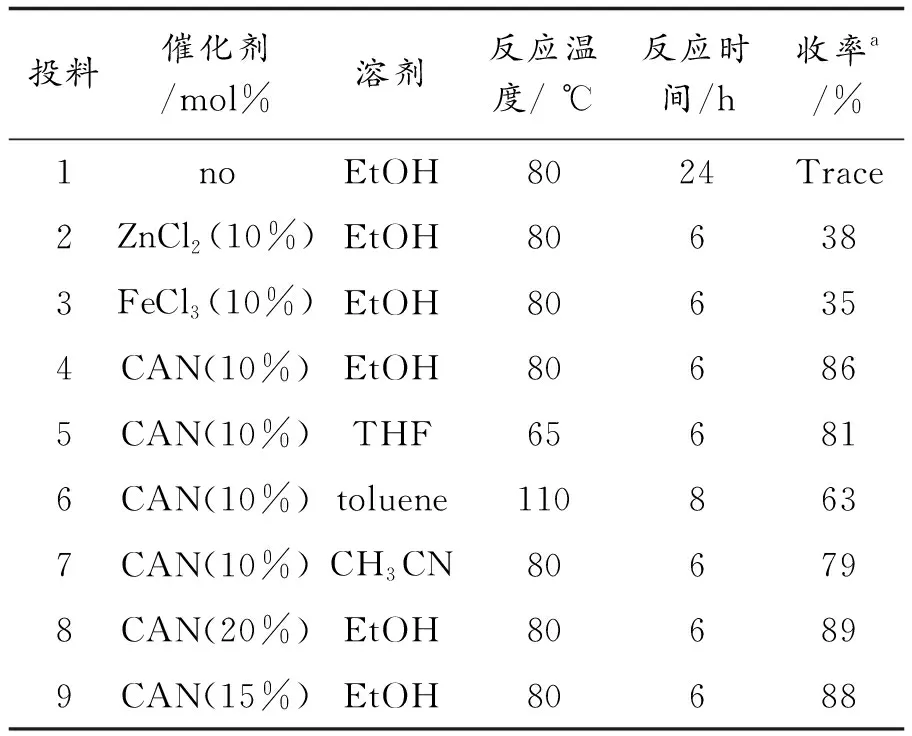
表1 不同反应条件对合成螺[吲哚-3,4’-吡唑并[3,4-b]吡啶]类化合物的影响
在上述筛选得出较佳反应条件后,利用不同取代基的三个反应底物组合合成得到了15个螺[吲哚-3,4’-吡唑并[3,4-b]吡啶]类化合物,结果见表2.反应过程中,利用TLC跟踪至反应结束,将反应液冷却至室温,过滤可得粗产物,再经无水乙醇重结晶提高纯度.从表2中可看出,靛红上的取代基,不管是吸电子还是供电子,均能使反应顺利进行.

表2 螺[吲哚-3,4’-吡唑并[3,4-b]吡啶]类化合物的合成
此外,我们对该反应可能的机理做了推导(图2),在催化剂CAN作用下,苯甲酰腈先和靛红发生了Knovenagel反应得到缩合产物5,随后中间体5和5-氨基吡唑再进行了Michel加成反应得到过渡态6,最后脱水得到目标产物4.

图2 螺[吲哚-3,4’-吡唑并[3,4-b]吡啶]合成的可能反应机理
3 结论
综上所述,本文主要研究了螺[吲哚-3,4’-吡唑并[3,4-b]吡啶]类化合物的合成.通过对反应条件的优化选择得到了较佳合成工艺,即以乙醇为溶剂,15%mol量的硝酸铈铵为催化剂,回流6~8 h,靛红、5-氨基吡唑和苯甲酰腈三组分一锅法合成目标产物,通过变换底物合成了15个化合物,均通过核磁分析表征.本方法具有操作简便、条件温和以及环境友好的特点.

