Selective breaking of C-O bonds in hydrodeoxygenation of 4-methylphenol over CoMoS/ZrO2
LI Zhi-qin ,WANG Ying ,YIN Chan-juan ,REN Xiao-xiong ,QIU Ze-gang
(College of Chemistry and Chemical Engineering, Xi'an Shiyou University, Xi'an 710065, China)
Abstract: CoMoS/ZrO2 catalysts with different Co-Mo atomic ratios (0.25, 0.30, 0.35, 0.40 and 0.45) and Co-Mo loading amounts (2.35%, 4.36%, 7.48% and 10.79%) were prepared by incipient wetness impregnation.These catalysts were characterized by X-ray diffraction (XRD), temperature-programmed reduction (H2-TPR), nitrogen adsorption/desorption and X-ray photoelectron spectroscopy (XPS).4-methylphenol was used as model compound for hydrodeoxygenation reaction.The result showed that when the atomic ratio of Co-Mo (Co/Co + Mo) was 0.30 and the Mo loading amount was 4.36%, the highest hydrogenation activity was observed.The conversion of 4-methylphenol was up to 99.86% and the selectivity of main product toluene reached to 87.85%.The formation of CoMoO4 was unfavourable to the formation of toluene.An appropriate interaction between Co-Mo and ZrO2 was required.
Key words: hydrodeoxygenation;phenols;4-methylphenol;transition metal sulfides;zirconia
Catalytic hydrodeoxygenation (HDO) of phenols is a crucial process in the conversion of the ligninderived bio-oils[1-3]and coal-derived oils (such as coal tar)[4-7].In China, hydrogenation of coal tar is becoming a crucial process in the utilization of the low rank coal through low temperature pyrolysis[4,7].The content of oxygen in the coal tar could reach as high as 8% and the oxygen mostly presented in the forms of phenols[8-11].The removal of oxygen by HDO without destroying the aromatic ring phenols molecules is becoming increasingly important in the hydrotreatment of coal tar.Obviously, catalysts play a key role in HDO process of phenols.Various catalysts, mostly concerning about the HDO of the lignin-derived bio-oils, including transition metal sulfides, reduced metal catalysts, metal phosphides, carbides and nitrides had been widely scrutinized[1,6].Among these catalysts, the transition metal sulfides[12-18], especially supported CoMoS catalysts[15-18], had long been investigated for their low cost, mature manufacturing technology and longtime application in the industrial scale.However, the sulfided catalysts owned a significant difference for dealing with the bio-oils and coal tar.The sulfided catalysts might introduce sulfur into the bio-oils,leading to a gloomy application prospect in the HDO for low or none-sulfur contented bio-oils.But for highsulfur contented coal tar, the sulfided catalyst is a perfect catalytic system from an industrial point of view.
A variety of supports, such as Al2O3, SiO2, ZrO2,TiO2, SBA-15 and Hβ, etc., had been used for CoMoS catalysts[1,6,15].It should be noted that there were two common reaction pathways in HDO of phenols.One was hydrogenation of aromatic rings first, followed by hydrodeoxygenation (HYD), and the other was direct hydrodeoxygenation (DDO).Taking phenol as an example, these two reaction pathways were shown in Figure 1.In the DDO route, whether the saturation reaction of the aromatic ring continued depended on the catalyst and reaction conditions[1].
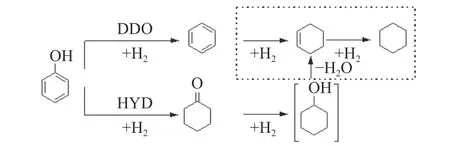
Figure 1 Two reaction pathways in the HDO reaction of phenol
Apparently, in order to obtain aromatics products,the reaction must be carried out through DDO route.It was worth noting that the hydrogen consumption in DDO route was lower than that in HYD.Although high selectivity of DDO products were observed on the unsupported Co doped MoS2catalysts[14,15], it could not be achieved by most supported CoMoS catalysts such as the typical CoMoS/Al2O3[16-18].Bui et al[16]investigated the hydrodeoxygenation of guaiacol on ZrO2, TiO2and γ-Al2O3supported CoMoS catalysts, and appeared that CoMoS/ZrO2was very selective towards DDO(producing phenol) pathway.The selectivity of phenol was 60%, while the conversion of guaiacol was close to 100%.ZrO2seems to be crucial for DDO pathways in the hydrodeoxygenation of guaiacol.On the other hand, NiMo/ZrO2[19], Ni/ZrO2[20], and Rh/ZrO2[21,22]were all inclined to favor the HYD pathway.Such results showed that CoMoS/ZrO2was a special catalytic system for HDO of phenols towards DDO pathway.However, the effect of CoMoS/ZrO2composition on its catalytic performance for the HDO of phenols(especially alkyl phenols) are still left unknown.In this paper, the influence of Co-Mo atomic ratios, Co and Mo loading amounts of CoMoS/ZrO2catalyst on the HDO of 4-methylphenol was thoroughly investigated.
1 Experimental
1.1 Catalyst preparation and pretreatment
All chemicals were purchased from Tianjin Kemiou Chemical Reagent Co., Ltd.and Shanghai Macklin Biochemical Technology Co., Ltd.Catalysts were prepared by incipient wetness impregnation method.The surface area, pore volume and pore diameter of ZrO2supports were 8.26 m2/g, 0.14 cm3/g and 21.1 nm, respectively.ZrO2supports were impregnated by an aqueous solution of Co(NO3)3and((NH4)6Mo7O24·4H2O).After impregnation, the catalysts were dried at room temperature for 12 h and then at 100 ℃ for 10 h.Subsequently, the catalysts were calcined in air at 500 ℃ for 3 h.Following such procedure, a series of CoMo/ZrO2catalysts with different Co-Mo atomic ratios (Co/Co + Mo) were prepared and marked as C1.15M5.54-0.25, C1.15M4.36-0.30,C1.15M3.50-0.35, C1.15M2.85-0.40 and C1.15M2.35-0.45(Table 1).Also, a series of CoMo/ZrO2catalysts with different loading of Co and Mo were prepared and marked as C0.62M2.37-0.30, C1.15M4.36-0.30, C1.97M7.47-0.30 and C2.84M10.79-0.30 (Table 2).
Catalysts were ex-situ pre-sulfided as follows: an aqueous solution of ammonium thiosulfate was added slowly to the catalyst under vigorous stirring for 1 h.The resulting mixture was left stand at room temperature for 4 h and then dried at 100 ℃ for 4 h,obtaining pre-sulfided catalyst.Before reaction, the pre-sulfided catalysts were pretreated by H2at 300 ℃and 3 MPa for 50 min in a 100 mL high-pressure stainless steel batch reactor.

Table 1 CoMo/ZrO2 catalysts with different Co-Mo atomic ratios
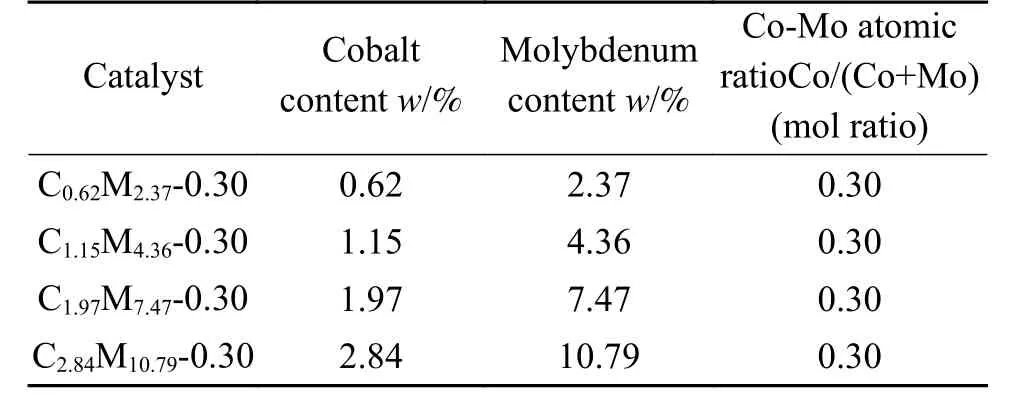
Table 2 CoMo/ZrO2 catalysts with different Co-Mo loading
1.2 Catalyst activity evaluation
The HDO reaction of 4-methyl phenol over CoMoS/ZrO2catalysts was evaluated in a 100 mL high-pressure stainless steel batch reactor.1.0 g pretreated catalyst, 1.0 g 4-methyl phenol and 25 mLnhexadecane were added into the reactor.Then the air in the reactor was replaced by nitrogen.The mixture was heated in N2while the temperature was rising until the reaction temperature (raised at a rate of 10 ℃/min) was reached.Then N2was evacuated, and H2was introduced in the reactor to begin HDO reaction.The products were analyzed by a Fuli GC9790Plus gas chromatography usingn-decane as internal standard.A hydrogen flame ionization detector (FID) and a DB-5 50 m capillary column were used.The column temperature was maintained at 50 ℃ for 6 min, then raised to 150 ℃ at 5 ℃/min for 2 min, and finally raised to 250 ℃ at 20 ℃/min for 2 min.
1.3 Catalyst characterization
XRD analysis was performed on the Shimazu XRD6000 X-ray diffractometer of Japan with a CuKα radiation (λ= 0.154 nm), a tube current of 30 mA, a tube voltage of 40 kV, a scanning range of 10°-80° (2 theta), a scanning speed of 10(°)/min, and a scanning step of 0.02(°).
The XPS analysis was performed on a PHI 5000 Versaprobe III spectrometer (Ulac-Phi, Japan) with a AlKα (hλ= 1486.6 eV) X-ray source.The vacuum degree of the main chamber is about 10-8kPa.The scanning mode is point, surface and line scanning, and the lens mode is energy transfer lens and electrostatic transfer lens.
H2-TPR was carried out with a ChemiSorb type 2750 (Micromeritics, USA) characterization equipment.Catalyst samples (60 mg) were flushed with He(99.999%), heated from room temperature to 150 ℃ and held for 1 h.Then the samples were cooled to 100 ℃.Subsequently, the samples were heated from 100 to 950 ℃ at a rate of 10 ℃/min under a flow (40 mL/min)of 20% H2(99.999%) in Ar (99.999%).The consumption of H2was monitored by a thermal conductivity detector(TCD).
2 Results and discussion
2.1 The HDO reaction of 4-methyl phenol on CoMoS/ZrO2 catalysts
Catalytic performance of CoMoS/ZrO2catalysts with different Co-Mo atomic ratios for HDO of 4-methyl phenol was shown in Figure 2.The main products were toluene and methyl cyclohexane.The conversion of 4-methyl phenol over all catalysts was close to 100%, however, the selectivity of toluene was disparate and followed the order of C1.15M4.36-0.30(87.85%) > C1.15M3.50-0.35 (69.56%) > C1.15M5.54-0.25(68.99%) > C1.15M2.85-0.40 (68.01%) > C1.15M2.35-0.45(65.24%).As it was shown, the selectivity of toluene first increased with the increase of Co-Mo atomic ratio and then dropped after the Co-Mo atomic ratio higher than 0.30.The highest selectivity of toluene was obtained over the catalyst with a Co-Mo atomic ratio of 0.30.

Figure 2 Catalytic performance of CoMoS/ZrO2 catalysts with different Co-Mo atomic ratios for HDO of 4-methyl phenol(3 MPa, 300 ℃ and 8 h)
Catalytic performance of CoMoS/ZrO2catalysts with different Co-Mo loading amounts for HDO of 4-methyl phenol was given in Figure 3.The conversion of 4-methyl phenol and the selectivity of toluene were both raised at the beginning and then dropped apparently, obtaining the highest value of 99.86% and 87.85%, respectively.The CoMoS/ZrO2catalyst with Co loading of 1.15% and Mo loading of 4.36% was found to be the most effective one in this series.
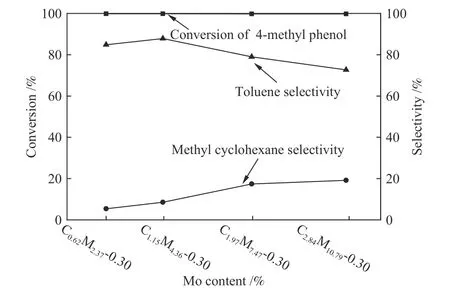
Figure 3 Catalytic performance of CoMoS/ZrO2 catalysts with different Co-Mo loading amounts for HDO of 4-methyl phenol(3 MPa, 300 ℃ and 8 h)
2.2 Characterization of catalyst
XRD patterns of CoMo/ZrO2with varying Co-Mo atomic ratios (Co/Co+Mo) were shown in Figure 4(a).ZrO2(24.3°, 28.2° and 31.4°) support existed in monoclinic phase (m-ZrO2)[23]and CoMoO4(2θat 23.4°, 26.5°, 33.6°) was detected in all catalysts[24].A very low crystallinity of CoMoO4was observed at the Co-Mo atomic ratio of 0.45.With the decrease of Co-Mo atomic ratio, the strength of characteristic peaks of CoMoO4increased, indicating that CoMoO4was enriched on the surface of the ZrO2and formed larger particles.However, no significant characteristic diffraction peaks of MoO3and CoO were detected[25],indicati ng that MoO3and CoO was in a state of very low crystallinity and evenly dispersed on the surface of ZrO2[26].
Figure 4(b) showed the XRD patterns of CoMoS/ZrO2(pre-sulfided CoMo/ZrO2by ammonium thiosulfate) with varying Co-Mo atomic ratios (Co/Co +Mo).The characteristic peaks of m-ZrO2crystal were detected at 24.3°, 28.2° and 31.4°.The characteristic peaks of CoMoO4disappeared, indicating that the CoMoO4might have been sulfided.No obvious characteristic diffraction peaks of MoS2or CoS2were detected, probably because the particle size of MoS2and CoS2were beyond the detection limit[26]or MoS2and CoS2were not formed.
XRD patterns of CoMo/ZrO2with varying loading amounts were presented in Figure 5.Just as shown in Figure 4(a),m-ZrO2(2θat 24.3°, 28.2° and 31.4°) and CoMoO4(2θat 23.4°, 26.5° and 33.6°) were detected.A very low crystallinity of CoMoO4was observed at the Mo loading of 2.37%.No significant characteristic diffraction peaks of MoO3and CoO were detected.
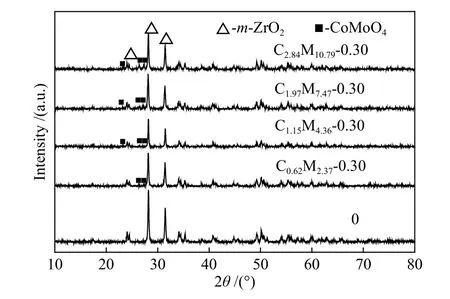
Figure 5 XRD patterns of CoMo/ZrO2 catalysts with different Co-Mo loading amounts
H2-TPR profiles of CoMo/ZrO2catalysts with different Co-Mo atomic ratios were presented in Figure 6.The reduction peak of MoO3appeared at 450-700 ℃,and the form of MoO3was mostly amorphous, highly defective and multi-layered, which were important for alkyl phenol hydrodeoxygenation[25].In addition, the intensity of reduction peak decreased with the increase of Co-Mo atomic ratio, indicating that a better reductive state could be achieved on catalyst with a lower Co-Mo atomic ratio.All catalysts showed CoO reduction peaks at 700-850 ℃.
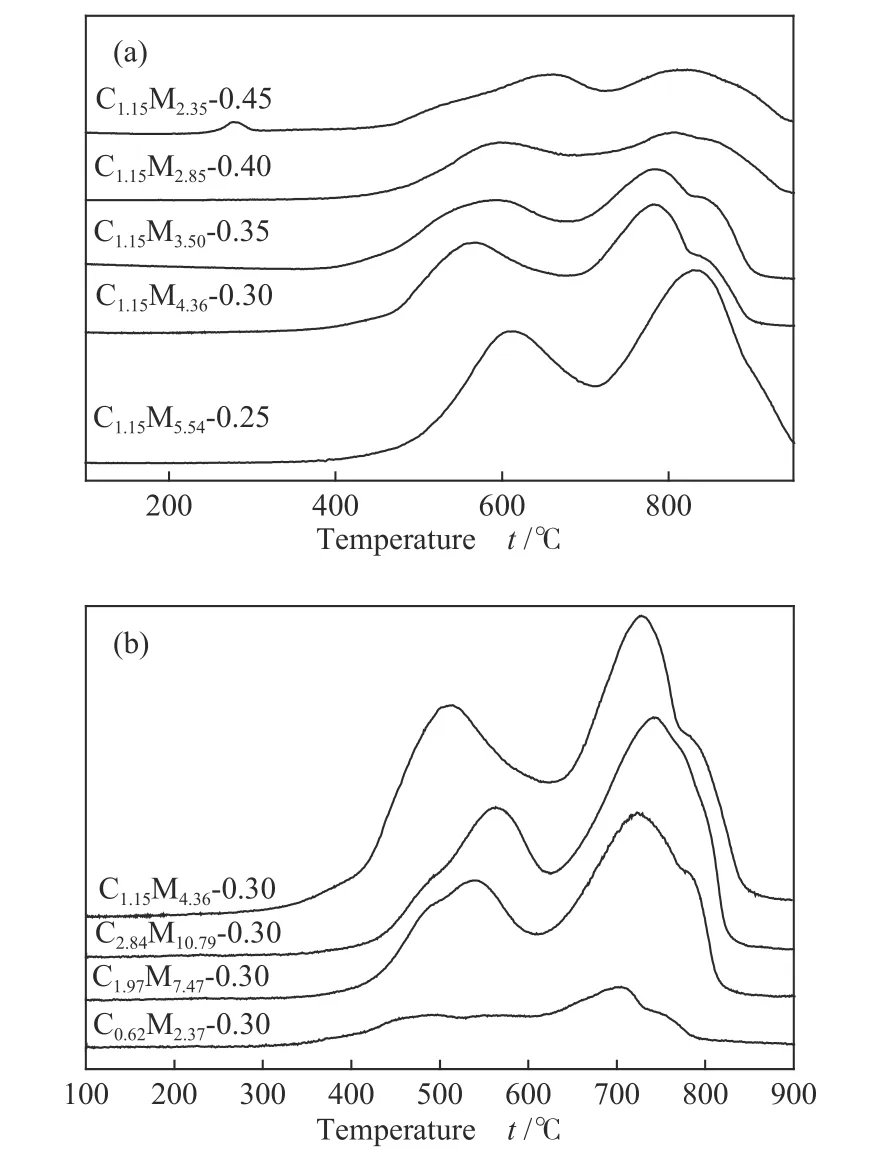
Figure 6 H2-TPR profiles of CoMo/ZrO2(a): catalysts with different Co-Mo atomic ratios;(b): catalysts with different Co-Mo loading
With the raising of the Co-Mo atomic ratio and Co-Mo loading amount, the reduction peaks of MoO3and CoO first shifted to left and then shifted to right,indicating that the interaction between CoO and ZrO2support was first weakened and then enhanced.The lowest reductive temperature was found on the C1.15M4.36-0.30 catalyst, which showed the highest selectivity of toluene.
Figure 7(a), (b) showed the XPS spectra (Mo 3dand Co 2p) of CoMo/ZrO2catalysts with different Co-Mo atomic ratios, and Figure 7(c), (d) was the Mo 3dand Co 2pdecomposition spectra of catalyst C1.15M4.36-0.30.Mo 3dhad a strong double peak at 235.9 and 231.7 eV (the characteristic peak of Mo6+), indicating the formation of MoO3species[26].It can be seen from Figure 7(d) that Co 2p3/2had a main peak at 779.05 eV and Co 2p1/2had a main peak at 797.3 eV, indicating the formation of the CoMoO4species.[26]The characteristic peak of Co oxide appeared at 785.65 eV revealing that Co was not fully sulphided and partially existed in the form of Co oxide.The binding energy of Mo 3dfist slightly shifted to left and then shifted to right.The lowest binding energy of Mo 3dwas observed on C1.15M4.36-0.30 catalyst, which was consistent with the results of H2-TPR.
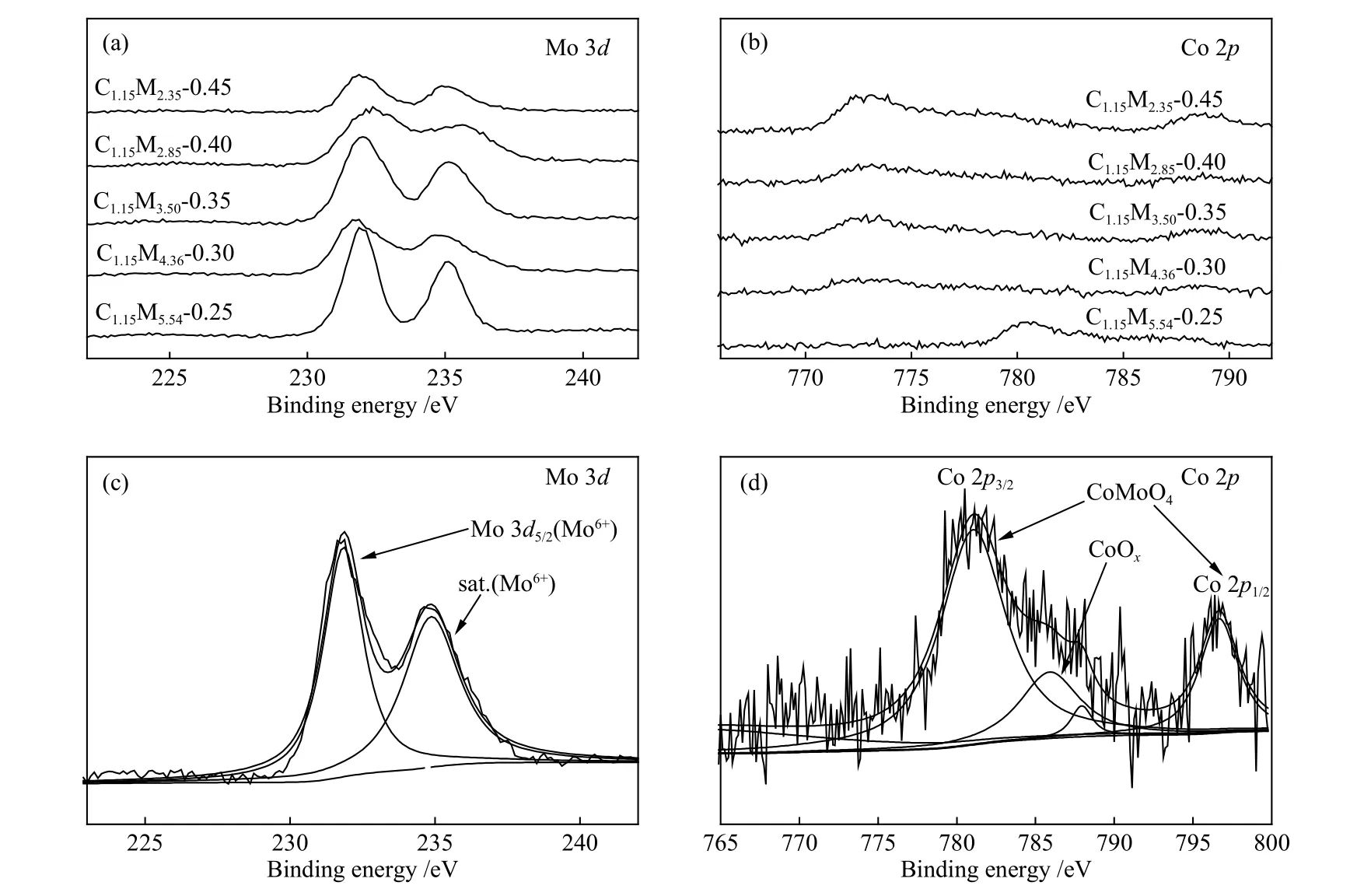
Figure 7 XPS spectra of CoMo/ZrO2 catalysts with different Co-Mo atomic ratios
Figure 8 presented the XPS spectra of CoMoS/ZrO2(pre-sulfided by ammonium thiosulfate).Figure 8(a),(b) gave the XPS spectra of Mo 3dand Co 2p, and Figure 8(c), (d) were the decomposition spectra of Mo 3dand Co 2pof the pre-sulfided C1.15M4.36-0.30 catalyst.Strong bimodal peaks (characteristic peaks of Mo4+) of Mo 3dat 228.1 and 232.4 eV were observed, indicating that Mo was not completely sulfided.According to Figure 8(d), a main peak of Co 2p3/2at 779.32 eV was detected, demonstrating the formation of CoMoS phase[26].The peak at 798.5 eV indicated the existence of Co9S8.
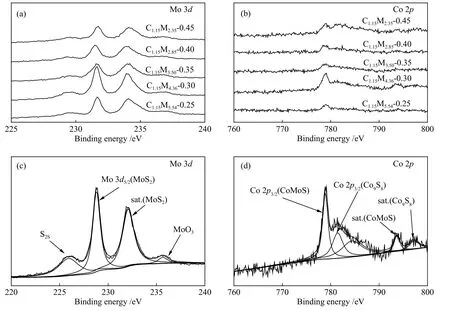
Figure 8 XPS spectra of pre-sulfided CoMoS/ZrO2 catalysts with different Co-Mo atomic ratios
XPS spectra of CoMoS/ZrO2catalysts with different Co-Mo loading were given in Figure 9.According to Figure 9(a), strong bimodal peaks at 228.1 and 232.4 eV (characteristic peaks of Mo4+)appeared on all the catalysts, demonstrating the formation of MoS2species.As shown in Figure 9(b),all catalysts presented a main peak of Co 2p3/2at 779.05 eV, which was corresponding to the Co species in the active Co-Mo-S phase.The signals of MoS2and Co were amplified with the increase of the Co-Mo loading.Also, the lowest binding energy of Mo 3dwas observed on C1.15M4.36-0.30 catalyst with the highest selectivity of toluene.
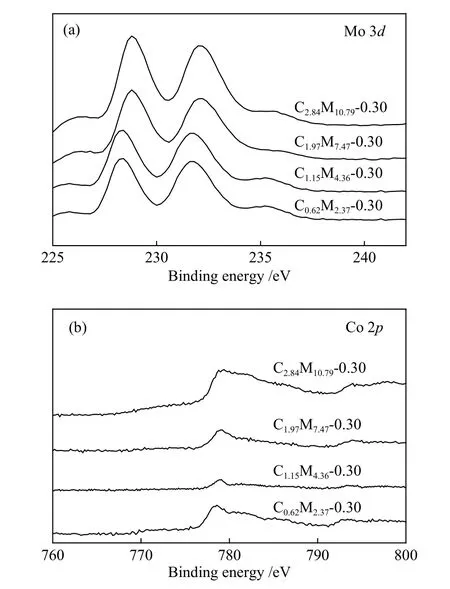
Figure 9 XPS spectra of CoMoS/ZrO2 catalysts with different Co-Mo loading
2.3 Discussion
The high selectivity of CoMoS catalysts for the DDO routes of phenols has attracted more and more attention.It was reported that high selectivity in the DDO routes could be achieved on unsupported CoMoS catalysts[13-15], indicating that CoMoS itself in some cases had specific active phase that favored the formation of DDO product.However, it turned to be more complicated when supports were used, and high selectivity to product could not be obtained on most supports.Therefore, it could be inferred that the interaction between active components and support was an important factor.
Combining the catalytic performance and characterization of CoMo/ZrO2as aforementioned, the active phase favoring the DDO route was indeed formed on the ZrO2.According to the XRD, the formation of CoMoO4seemed to be unfavourable to the selectivity of toluene (product of DDO route),suggesting that CoMoO4was not the precursor of active phase favoring the DDO route.As to the H2-TPR and XPS, an appropriate interaction between Co-Mo and ZrO2was required.
3 Conclusions
CoMoS/ZrO2catalysts with different Co-Mo atomic ratios and different loading amount were prepared.The reaction products were mainly toluene and methyl cyclohexane.The Co-Mo atomic ratio and Co-Mo loading amount had very little effect on the conversion of 4-methyl phenol, but affected the selectivity of toluene remarkably.The conversion of 4-methyl phenol was up to about 100% and the highest selectivity of toluene reached to 87.85%, indicating that a high selective breaking of C-O bonds in 4-methyl phenol was achieved.The formation of CoMoO4was unfavourable to the selectivity of toluene (product of DDO route).An appropriate interaction between Co-Mo and ZrO2was required.
- 燃料化学学报的其它文章
- 多级孔ZSM-5 分子筛对低阶煤流化床快速热解产物分布的影响
- 胜利褐煤及盐酸洗煤热解过程中微结构演变特性研究
- 淖毛湖次烟煤的热溶及热溶物的催化加氢转化
- Hydroliquefaction kinetics of coal-derived preasphaltenes catalyzed by FeS and S
- Effects of iron catalyst and atmosphere on sulfur transformation during pressurized low-temperature pyrolysis of Baishihu coal
- 溶剂溶胀对鄂尔多斯褐煤结构及热解产物分布的影响

