Effects of iron catalyst and atmosphere on sulfur transformation during pressurized low-temperature pyrolysis of Baishihu coal
LIU Min ,WANG Yong-gang ,CHEN Gui-feng ,SHI Quan ,HU Yong-feng ,ZHAO Peng
(1.China University of Mining and Technology, Beijing 100083, China;2.State Key Laboratory of Coal Mining and Clean Utilization, China Coal Research Institute, Beijing 100013, China;3.State Key Laboratory of Heavy Oil Processing, China University of Petroleum, Beijing 102249, China;4.Canadian Light Source, Saskatoon, SK S7N 2V3, Canada)
Abstract: A vitrinite-rich low rank coal, Baishihu (BSH) coal with moderate sulfur content was treated by dehydration and crushing.The treated samples were pyrolyzed in an alloy tubular reactor under 2 MPa.Influence of iron catalyst and atmosphere on sulfur transformation during pressurized low-temperature coal pyrolysis was investigated.Molecular composition of sulfur compounds in tar was characterized by gas chromatography with sulfur chemiluminescence detector(GC-SCD) combined with Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR MS).Sulfur K-edge XANES was used to study sulfur molecular structure after pyrolysis.Sulfur compounds in BSH coal are predominantly S1 class species in branch chain of the coal.Elemental sulfur in catalyst enters the tar and forms mercaptan or thioether compounds during pyrolysis.Iron catalyst promotes activation of hydrogen atoms in coal and contributes to hydrogenation saturation and cracking of aromatic sulfide in tar.The catalyst preferentially captures H2S to increase content of pyrite in char and inhibits formation of sulfate.Under H2 atmosphere, significant decrease of thiophene compounds is observed with catalyst coupled with decrease of sulfoxide compounds.
Key words: sulfur transformation;FT-ICR MS;XANES;coal pyrolysis;iron catalyst
Coal is the backbone of energy supply in China,meeting 70% of its energy needs.Low-rank coal accounts for ~40% of known total coal reserves and the percentage is increasing with exploration developments.Efficient and clean utilization of rich low-rank coal resources has been a focus for many researchers[1,2].
Hydrogenation of coal into fuels and commodity chemicals is an important path towards utilization of low-rank coal resources.Pyrolysis is an important intermediate step in hydrogenation of coal and compositional understanding of the process is vital in controlling the step.Considering activity and cost of catalyst, high dispersive iron catalyst is the best choice.In catalytic process of hydrogenation of coal, sulfur as one of the primary heteroatom in coal transforms to gas, liquid and solid products mainly in the forms of hydrogen sulfite (H2S), mercaptan, thioether and thiophene.Sulfur transformation pathway during coal pyrolysis is closely related to the initial forms of sulfur in coal and pyrolysis conditions[3,4].Due to diversity of sulfur-containing functional groups and complexity of coal structure, it is difficult to accurately track the transformation of sulfur-containing compounds in coal[5-8].Instead, pyrolysis products have been monitored by GC, MS, and XPS in literatures[9-13].By studying types of sulfur-containing compounds from pyrolysis conducted under different atmospheres, it is feasible to identify the different decomposition mechanisms and transformation behaviors during pyrolysis and sheds light on types of sulfur-containing compounds in original coal samples.Concurrently, catalysts including potassium carbonate, pyrite and potassium hydroxide have shown impacts on molecular composition of sulfur in coal pyrolysis products.
X-ray absorption spectroscopy (XAS) is a synchrotron radiation-based technique for direct and nondestructive determination of all major sulfur forms in coal[14-16].Positive and negative ion electrospray coupled with Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR MS) is an effective means to analyze nonvolatile high-boiling compounds.FT-ICR MS has been applied to characterize coal-based oil.The ultrahigh resolution and mass accuracy of FT-ICR MS enables unique elemental composition assignment to each peak in the mass spectrum of an oil sample[17-21].Along with GC-SCD, these methods were used to characterize sulfur transformation during pressurized low-temperature pyrolysis of BSH coal under H2and Ar atmospheres.A systematic understanding of sulfur transformation behavior is obtained under different atmospheres, which is conducive to a deeper understanding of the catalytic mechanism and process.This provides a theoretical basis for efficient and clean utilization of low-rank coal.
1 Experiment
1.1 Sample
Baishihu (BSH) coal with high volatility and high vitrinite was selected for this study.After crushing,drying, fine crushing and screening, the final coal sample has a water content of less than 3% with a particle size ranging in 0.03-0.075 mm.The ultimate and proximate analyses listed in Table 1 are measured as follows: refer to GB/T212-2008 for industrial analysis of coal samples, and adopt Vario MACRO Cube instrument for element analysis.Analysis of sulfur morphology analysis is shown in Table 2,indicating a high organic sulfur content of 90.66% and low pyrite and sulfate content.Figure 1 is thermogravimetric analysis of the coal.The iron catalyst used in this experiment was developed by CCRI, with an iron content of 3.82%.The catalyst was uniformly loaded in the form of iron oxide hydrate.The catalyst loading is 1% of coal.The added amount of elemental sulfur met a S/Fe atomic ratio of 2 in the catalyst.
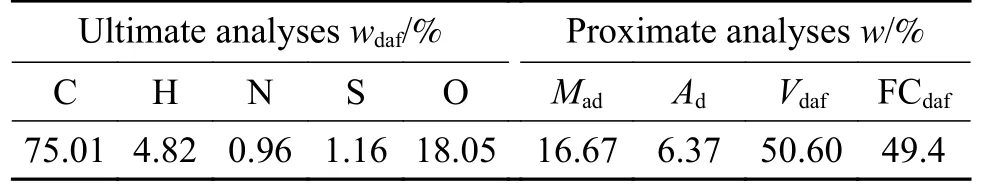
Table 1 Proximate and ultimate analyses of coal samples
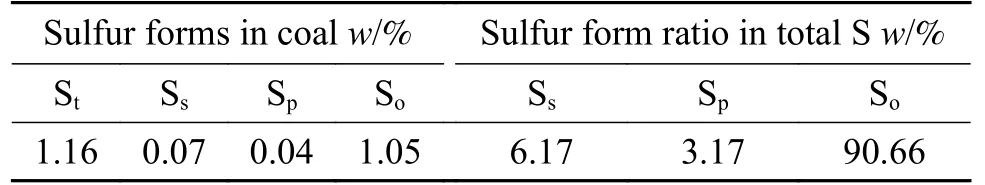
Table 2 Sulfur forms analysis of coal samples
1.2 Pyrolysis condition
The initial pressure is 2 MPa.15 g coal samples,with and without catalyst loading, were independently subject to pyrolysis under pure Ar or H2from room temperature to 420 ℃ with a heating rate of 10 ℃/min in an alloy tubular reactor[22,23].Each pyrolysis reaction ran for 60 min at constant temperature.The char was extracted with tetrahydrofuran (THF) and the sulfur content of raw coal and char was determined by vario MACRO cube.
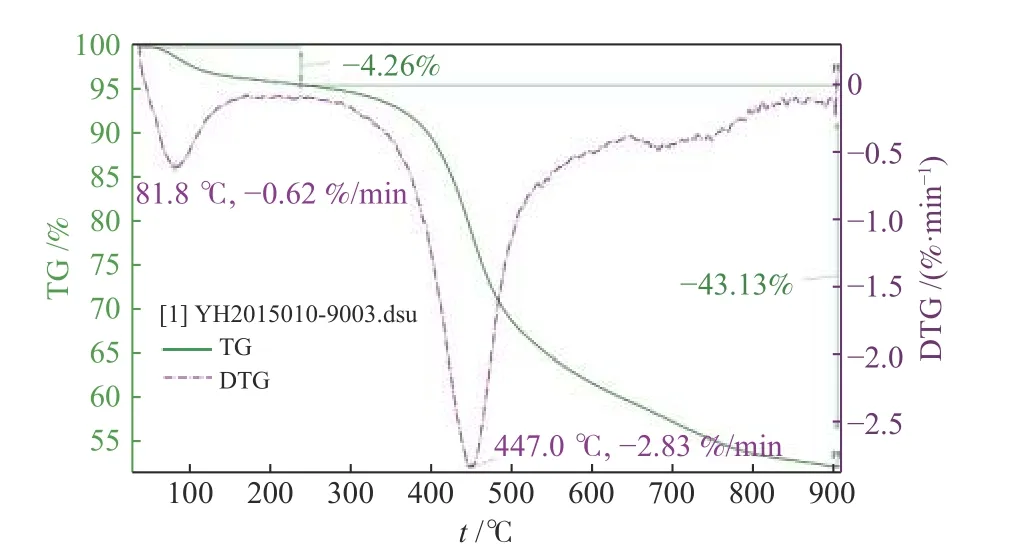
Figure 1 TGA analysis of coal
1.3 Calculation of sulfur in different phase
1.3.1 Coal without catalyst
Content of sulfur in the char is calculated by the following formula:

WhereWs,cis sulfur weight of the raw coal,Cs,charis sulfur content in the char,mcharis weight of the char.
The content of sulfur in tar is calculated as follows:

WhereCs,taris sulfur content in the tar,mtaris weight of the tar.
Content of sulfur in gas phase is calculated by:
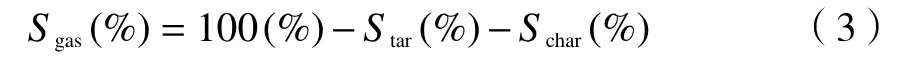
1.3.2 Coal with catalyst
Content of sulfur in the char is calculated by:

Ws,cis sulfur weight of the raw coal,Wsis sulfur weight of the catalyst,Cs,charis sulfur content in the char,mcharis weight of the char.
Content of sulfur in tar is calculated by:

Cs,taris sulfur content in the tar,Wsis sulfur weight of the catalyst,mtaris weight of the tar.
Content of sulfur in gas is calculated by:
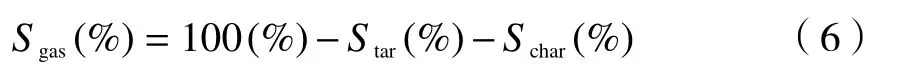
1.4 GC-SCD analysis
Agilent 7890A gas chromatography with sulfur chemiluminescence detector (GC-SCD) was used to analyze the tar.Specific operating conditions are as follows: HP-5 capillary column, initial temperature 40 ℃, heating rate 10 ℃/min, final temperature 300 ℃,20 min run time, pure N2as carrier gas with flow rate of 0.8 mL/min, vaporizing chamber temperature 280 ℃.SCD burner temperature: 800 ℃, and N2flow: 5 mL/min.
1.5 Fourier transform ion cyclotron resonance mass spectrometry
A Bruker Apex-ultra FT-ICR MS was used to analyze the tar.Sulfide is converted to methyl sulfonium salt by methylation to improve polarity and allow mass detection without affecting the signal response of other compounds[24,25].The ESI source was operated in the positive mode.The emitter voltage was 3.6 kV.The capillary column front end voltage was 4.0 kV.The injection velocity was 5 mL/min and timeof-flight window was 1 ms.Mass calibration and data processing of FT-ICR MS have been described in the literature[19,21].
1.6 X-ray absorption spectroscopy analysis
X-ray absorption spectroscopy (XAS) was used to characterize sulfur forms in raw coal and char at the Canadian Light Source using the Soft X-ray Micro characterization Beam line (SXRMB).The sample was pasted on a double-sided conductive carbon band, and then loaded into a vacuum chamber.By recording leakage current of the sample, the SK-edge XAS spectrum was measured simultaneously in the surface sensitive mode, that is, the total electron yield (TEY).Si (Li) drift detector was used in fluorescence yield(FY) to calibrate beam line energy at peak of sulfuric acid at 2481.6 eV, and the energy accuracy was 0.2 eV[14-16].With reference to the previous selection of possible sulfur-containing compounds in coal, the peaks at 2470.1, 2471.4, 2477.7 and 2481.6 eV should be attributed to FeS, FeS2, CaS, sulfate, respectively.The peaks at 2473.1, 2475.4, 2479.88 and 2480.78 eV should be assigned to thiophene, sulfoxide, sulfone,and sulfonate, respectively.
2 Results and discussion
The yield of pressurized low-temperature pyrolysis products of BSH coal with or without catalyst loading under different atmospheres are shown in Table 3.
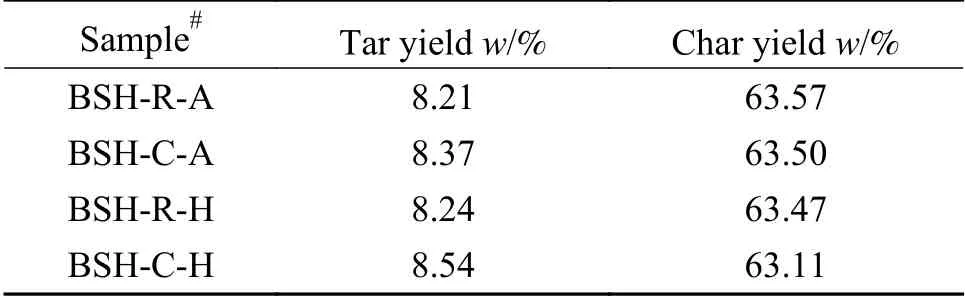
Table 3 Effect of iron catalysts and atmosphere on pyrolysis
As evidenced by Table 3, iron catalysts have effect on coal pyrolysis and slightly decrease char yield.Hydrogen atmosphere is conducive to improvement of tar yield.
2.1 Effect of catalysts and atmosphere on sulfur distribution in different phases during pyrolysis
According to equations (1)-(6), sulfur distributions in different phases after pyrolysis are shown in Table 4.In the absence of iron catalyst, a low proportion of sulfur, 5.22% and 5.51% under H2and Ar atmosphere respectively, transforms to gas products during pyrolysis.The source of gaseous sulfur compounds is mainly decomposition of mercaptan or aliphatic sulfide with poor thermal stability in raw coal.By contrast,sulfur transforms into a relatively high proportion of tar at 57.97% and 63.85% under H2and Ar atmosphere,respectively.This suggests that sulfur compounds in raw coal mainly exist in side chain of the coal.As most of side chains cracked below 420 ℃, sulfur compounds entered the tar during pyrolysis.
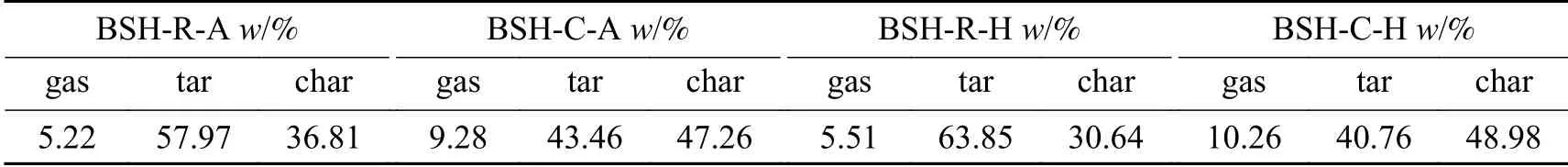
Table 4 Sulfur distribution in different phases
The addition of iron catalyst influences sulfur distributions in different phases during pyrolysis, and elemental sulfur in catalyst also transforms to different phases at the same time.Sulfur content in gas and char increased greatly, suggesting that elemental sulfur in the catalyst mainly transforms into gaseous products and char.To shed light on effect of catalyst on sulfur transformation into the tar, samples were further investigated by GC-SCD and FT-ICR MS.
2.2 Effect of catalysts and atmosphere on molecular composition of sulfur compounds in tar during pyrolysis
2.2.1 GC-SCD analysis of tar
Table 5 summarizes GC-SCD analysis results.BSH coal is a relatively young low-rank coal, and hence sulfur compounds are abundant in tar.Mercaptan and thioether accounted for more than 40% sulfur compounds in tar, followed by thiophene and benzothiophene compounds with dibenzothiophene compounds taking the last.
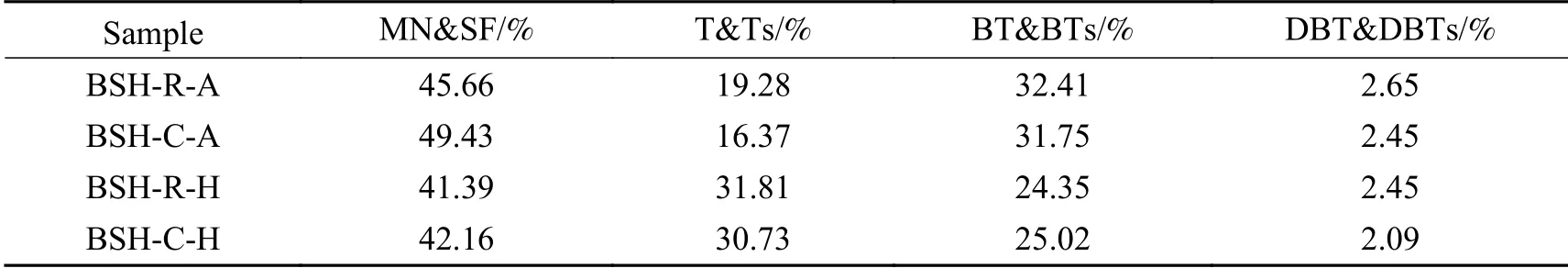
Table 5 GC-SCD analysis result of tar by pyrolysis of (a)BSH-R-A, (b)BSH-C-A, (c)BSH-R-H, (d)BSH-C-H
It has been reported that iron catalysts contribute to production of reactive hydrogen atoms and promote saturation and cleavage of aromatics[26-28].Table 5 and Figure 2 show that addition of iron catalyst promotes hydrogenation saturation of benzene ring in the tar,especially in H2atmosphere, while proportion of thiophene compounds increase significantly.At the same time, mercaptan and thioether in the tar increase slightly under this condition.This observation may be indicative of elemental sulfur in catalyst reacting with coal pyrolysis free radicals and entering light oil fraction of the tar.
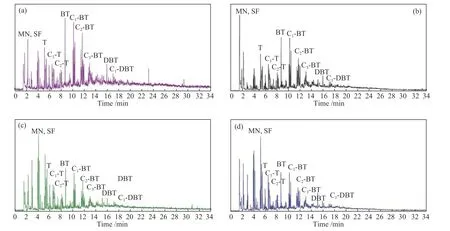
Figure 2 GC-SCD analysis of tar by pyrolysis of (a)BSH-R-A, (b)BSH-C-A, (c)BSH-R-H, (d)BSH-C-H sulfur-containing compounds are denoted by the following letters: mercaptan (MN), sulfoether (SF), thiophene (T),thiophene derived compounds (Ts), benzothiophene (BT), benzothiophene derived compounds (BTs),dibenzothiophene (DBT), dibenzothiophene derived compounds (DBTs)
2.2.2 Molecular characterization of sulfur compounds in tar by positive-ion ESI FT-ICR MS
Figure 3 shows molecular classification analysis of heteroatom content in methylated tar samples,analyzed by positive-ion ESI FT-ICR MS.Relative abundance is defined as magnitude of each peak divided by sum of all identified peaks (excluding the isotopic peaks) in each mass spectrum.
Tar from four pyrolysis conditions had the highest relative abundance of S1class species, followed by,O1S1, O2S1, N1S1and S2.S1class species were predominant and accounted for more than 60% of the total relative abundance of sulfur species.
Detailed molecular composition analyses of the class species in tar were carried out by plotting the relative abundance maps of double bond equivalence(DBE) as a function of carbon number.
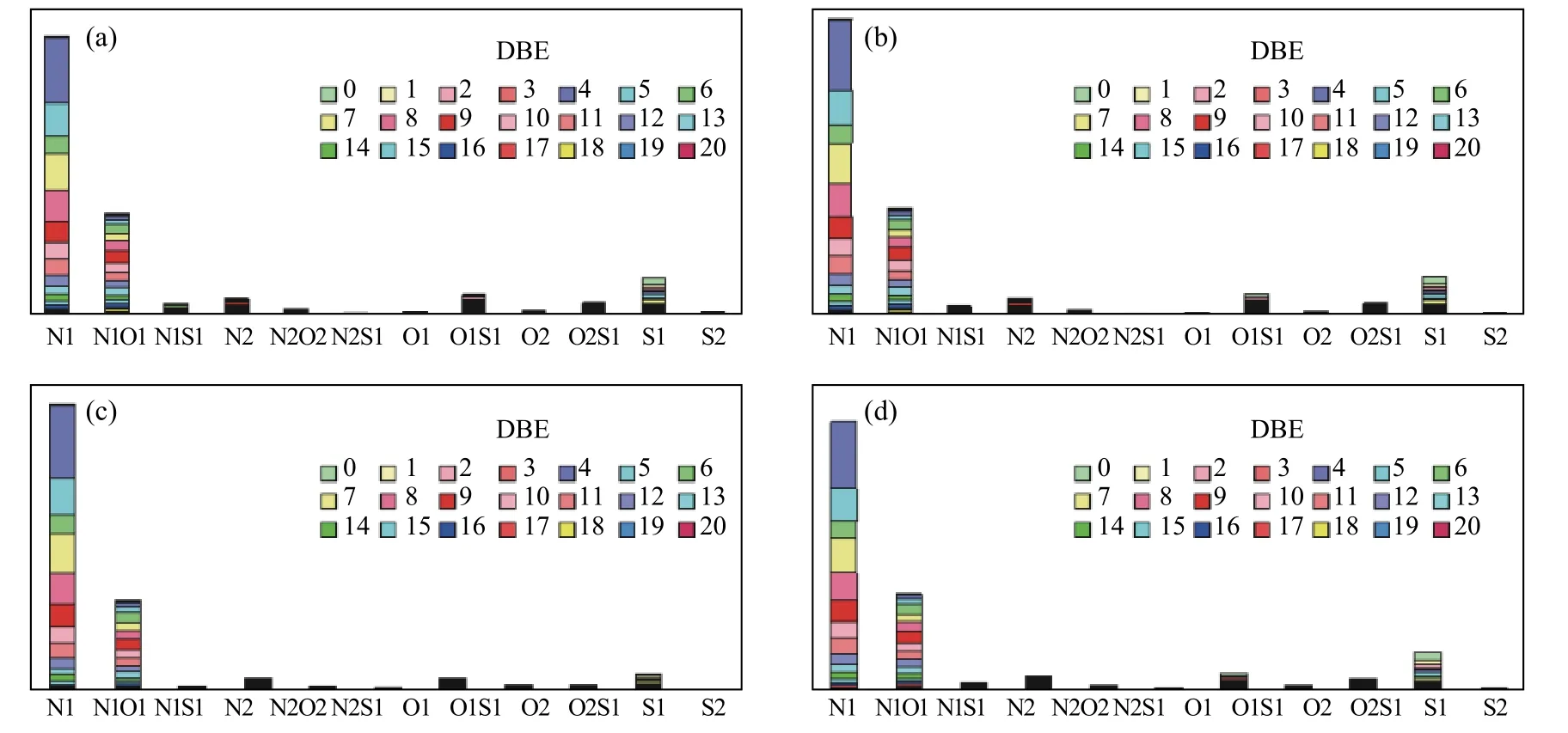
Figure 3 Relative abundance of heteroatom classes for the methylated tar by pyrolysis of (a) BSH-R-A, (b) BSH-C-A,(c) BSH-R-H, (d) BSH-C-H ESI FT-ICR mass spectrum
Figure 4 shows relative abundance maps of DBE as a function of carbon number for the S1class species in methylated tar.Under Ar atmosphere without catalyst, the S1class species in tar had DBE values of 0-23 and carbon numbers of 10-40.S1class species with high abundancies exhibit 7-15 DBE and 20-30 carbon numbers.The most abundant S1class species had 15 DBE, indicating a tetrabenzothiophene core with alkyl side chains, i.e., class Ⅴ sulfur compounds in Figure 3.Under Ar atmosphere with catalyst,similarly, the S1class species in tar had DBE values of 0-23 and carbon numbers of 10-40.However, the abundant S1class species cluster had around 0-5 DBE and 10-25 carbon numbers.The most abundant S1class species had 5 DBE, indicating thiophene compound produced by hydrogenation of benzothiophenol and dibenzothiophene, and 0 DBE, indicating mercaptan or thioether sulfur compounds.
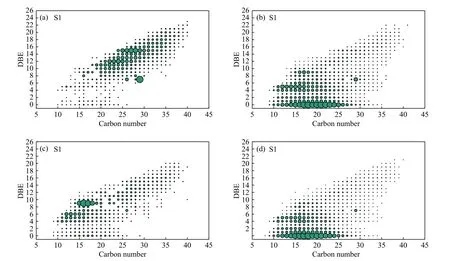
Figure 4 Plots of DBE versus the carbon number for S1 class species in methylated tar by pyrolysis of (a) BSH-R-A,(b) BSH-C-A, (c) BSH-R-H, (d) BSH-C-H ESI FT-ICR mass spectrum the largest dot corresponds to the most abundant monosulfur species in the sample
Under H2atmosphere, the S1class species in tar had DBE values of 0-23 and carbon numbers of 10-40.The abundant S1class species had 4-9 DBE and 10-20 carbon numbers.The most abundant S1class species had 9 DBE, indicating a dibenzothiophene core with alkyl side chains, namely, class Ⅲ sulfur compounds in Figure 5.Under H2atmosphere with catalyst, the molecular characterization of sulfur compounds in tar are similar to those under Ar atmosphere with catalyst.

Figure 5 Main sulfur compounds in tar
These observations may indicate that in the absence of iron catalyst for pyrolysis, H2can enter the tar, hydrogenate saturated aromatic ring, and stabilize free radicals from coal pyrolysis.As such,repolymerization initiated by coal pyrolysis free radicals can be prevented.Regardless of H2or Ar atmosphere, the iron catalyst can promote both hydrogenation saturation and cracking of aromatic rings.Concurrently, the elemental sulfur in catalyst can enter into the tar and successively form mercaptan or sulfur ether compounds with coal pyrolysis free radicals, which account a high content in tar.
2.3 Effect of atmospheres and catalyst on sulfur transformation during pyrolysis
For a comprehensive understanding of sulfur transformation during pyrolysis, sulfurK-edge XANES was used to analyze sulfur molecular structure and their concentration in raw coal and BSH-R-A, BSH-C-A,BSH-R-H, BSH-C-H chars.As shown in Figure 6, the peak value of H2and Ar atmosphere at 2473.1 eV is close to that of raw coal in the absence of iron catalyst for pyrolysis, indicating that thiophene compounds in raw coal have little change after pyrolysis.The use of catalyst effectively promotes removal of thiophene compounds, especially under H2atmosphere, and the peak value of 2473.1 eV in the spectrum is significantly reduced.
Pyrite content in char visibly increased compared with raw coal, especially with adding catalyst, as the peak value of 2470.1 eV became more intense compared with raw coal.Pyrite has catalytic activity for hydrogenation of coal; defects in crystal structure are often the active center of hydrogenation of coal[28,29].For pyrolysis in the absence of iron catalyst, sulfate peak around 2481.6 eV is from SO2absorption of alkaline minerals.However, this peak showed no significant change using catalyst, indicating that the iron catalyst may inhibit formation of sulfate in char.
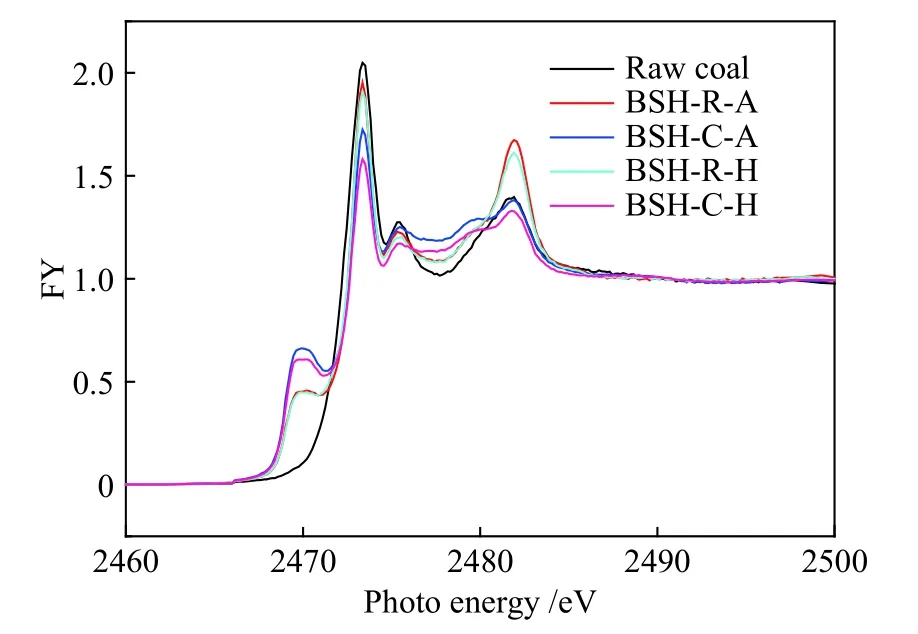
Figure 6 Sulfur K-edge XANES spectra of BSH coal and its chars under different atmospheres
Sulfoxide content in char increased significantly,especially under Ar atmosphere with catalyst.The peak value of 2479.88 eV became more pronounced compared with that of raw coal.Formation of sulfoxide in char may be repolymerization of thiophene compounds in coal with oxygen-containing macromolecules in the absence of active hydrogen after pyrolysis.
3 Conclusions
This study presents effects of iron catalyst on sulfur transformation during pressurized lowtemperature pyrolysis of coal through GC-SCD and FT-ICR MS investigation, combined with sulfurKedge XANES.The iron catalyst has limited effect on pyrolysis behavior of coal, but has apparent influence on sulfur transformation pathways during pyrolysis.Under H2and Ar atmospheres with Fe catalyst, some stable thiophene compounds decompose, while pyrite and sulfoxide increase significantly.Under H2and Ar atmospheres without the catalyst, however, thiophene compounds in char have little change after pyrolysis,while pyrite and sulfate increase significantly.Additionally, the catalyst inhibits formation of sulfate in the char.Elemental sulfur in catalyst enters the tar and forms mercaptan or sulfur ether compounds with free radicals from coal pyrolysis.
This study also provides illuminating results on sulfur composition in BSH coal.Sulfur compounds of BSH coal mainly exist in side chain structures of coal,most of which crack below 420 ℃ and sulfur compounds enter into the tar.Independent of the presence of iron catalyst, hydrogen enters tar, saturates aromatic ring,stabilizes free radicals, and prevents repolymerization of free radicals from coal pyrolysis.The DBE value of sulfur compounds in tar also increases.Notably, the catalyst promotes activation of hydrogen atoms in coal,and thereby boosts hydrogenation saturation and cracking of aromatic rings in sulfur compounds in the tar.
Acknowledgements
We thank all staffs at China State Key Laboratory of Heavy Oil Processing for the technical support for the ESI FT-ICR MS analysis and Canadian Light Source (CLS) for the technical support for the SKedge XANES measurement.

