A Study of Normal Epidermal Melanocyte Distribution
Kai-Lv Sun, Wan Liu, Xiao-Man Gao, Min Yang, Jian-Min Chang∗
Department of Dermatology, Beijing Hospital, National Center of Gerontology; Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing 100730, China.
Abstract
Objective: To elucidate the epidermal melanocyte distribution according to sex, age, and body part with the goal of providing benchmark data for the diagnosis and therapeutic effect evaluation of pigmentary skin diseases.
Methods: Epidermal melanocytes and keratinocytes were assessed using direct immunofluorescence staining, and the melanocyte density and epidermal thickness were calculated. The obtained data were statistically analyzed using SPSS Version 20.0 software. An independent-samples t test was used to compare the data between two groups, while data among three or more groups were compared by one-way analysis of variance. Data correlations were evaluated using Pearson correlation analysis.
Results: Melanocytes were uniformly distributed among the keratinocytes in the basal layer, and the average ratio of melanocytes to keratinocytes was 1:7. Among them, the ratio in males was 1:6.5 and that in females was 1:7.4, with no significant difference (P=0.127). The melanocyte density gradually declined as age increased; the ratio was 1:5.8 before 50 years of age without an obvious downtrend. The average melanocyte density was 1:7.9 within 51 to 65 years of age and 1:8.5 at >65 years of age, and the difference was statistically significant (P<0.01). Obvious differences were found in the melanocyte density among different body parts; in descending order, these densities were as follows: face(1:4.0) >neck (1:5.1) >hip (1:5.7) >upper limb (1:7.4) >lower limb (1:8.3) >lower back (1:9.2) >thorax and abdomen(1:9.9). The melanocyte density was not related to the epidermal thickness.
Conclusion: The melanocyte density showed a declining trend with age and significantly changed after 50 years of age. The melanocyte density was associated with body part; specifically, the density in the face, neck, and hip was higher than that in the limbs and torso. However, the melanocyte density was not associated with sex or epidermal thickness.
Keywords: distribution, immunofluorescence, melanocytes
Introduction
In the epidermal basal layer of adults, the average melanocyte density is about 1,500 cells/mm2. In vertical sections, the ratio of melanocytes to keratinocytes in the basal layer varies from 1:4 in the cheek to 1:10 in the limbs.1Research has shown no obvious differences in melanocyte density between the sexes or among people of different colors or races, but the melanocyte density can differ according to age, body part, and UV irradiation.2–5The differences in melanocyte density among different ages and body parts are not consistent in the literature. Many pigmentary skin diseases, such as vitiligo, piebaldism, caféau-lait spots, and progressive acromelanosis, are correlated with abnormalities in melanocyte density. However,increases and decreases in melanocytes are relative, and benchmark data are needed for reference; only in this way can data be clinically meaningful.
Few Chinese studies have focused on the quantitative distribution of normal epidermal melanocytes. We retrieved only two such publications,6-7both of which were written years ago and had problems such as a small sample size (36 and 53 cases, respectively) and manual counting errors. Grouping by age and body parts was inadequate, and the study results differed from those of research performed outside China. Therefore, improvements in such studies are needed.
In the present study, the direct immunofluorescence staining method was used to calculate the ratio of melanocytes to keratinocytes in the basal layer and elucidate the relationships of this ratio with sex, age,body part, and epithermal thickness. The aim of this study was to reveal the distribution of normal epidermal melanocytes among the Chinese Han population and provide benchmark data for the diagnosis and therapeutic effect evaluation of pigmentary skin diseases.
Material and methods
Source of cells
Skin samples were obtained from the outpatient operating room of our hospital. No samples contained peripheral tumor tissue, inflammation, or pigmentary abnormalities, and all were confirmed to represent normal skin through hematoxylin–eosin staining by at least two dermatopathologists. The tip tissue of the fusiform incision was taken from the samples of patients undergoing skin benign tumor resection, and the skin sample collected was the routine removal of excess skin during the resection. Patients with malignant tumors, autoimmune diseases, endocrine diseases such as pituitary and adrenal glands, systemic hormones or immunosuppressants, and recent local phototherapy were excluded.
Melanocytes were labeled with dopachrome tautomerase (DCT), keratinocytes were labeled with keratin 14(Krt14), and cell nuclei were labeled with 4′,6-diamidino-2-phenylindole (DAPI) staining solution.
Detection assays
Direct immunofluorescence staining was used to examine frozen skin tissue sections. The primary antibodies used in the experiment were anti-DCT (rabbit source, selfproduced by National Institute of Biological Sciences,Beijing, China) and anti-Krt14 (rat source, self-produced by National Institute of Biological Sciences). The secondary antibodies were Alexa Fluor 488-labeled donkey antirabbit IgG (ThermoFisher Scientific, Waltham, MA, USA)and Alexa Fluor 546-labeled donkey anti-rat IgG(ThermoFisher Scientific). Three sections were taken from each sample for staining, and three fields of each section were randomly shot. DAPI was used to label the cell nucleus with blue fluorescence, Krt14 was used to label keratinocytes with red fluorescence, and DCT was used to label melanocytes with green fluorescence (Fig. 1).
Imaris7.4 software (Bitplane, Zürich, Switzerland) was used for auxiliary calculation of the epidermal melanocyte density and epidermal thickness. The epidermal thickness at the skin projection and that at the dermal papilla were substantially different; therefore, they were separately measured and recorded as thickness A and thickness B, respectively. The average value of multiple sections from each sample was calculated for analysis.All skin samples were categorized according to sex, age(<35, 35–50, 50–65, and >65 years), and body part(face, neck, thorax, and abdomen, back, hip, upper limbs, and lower limbs).
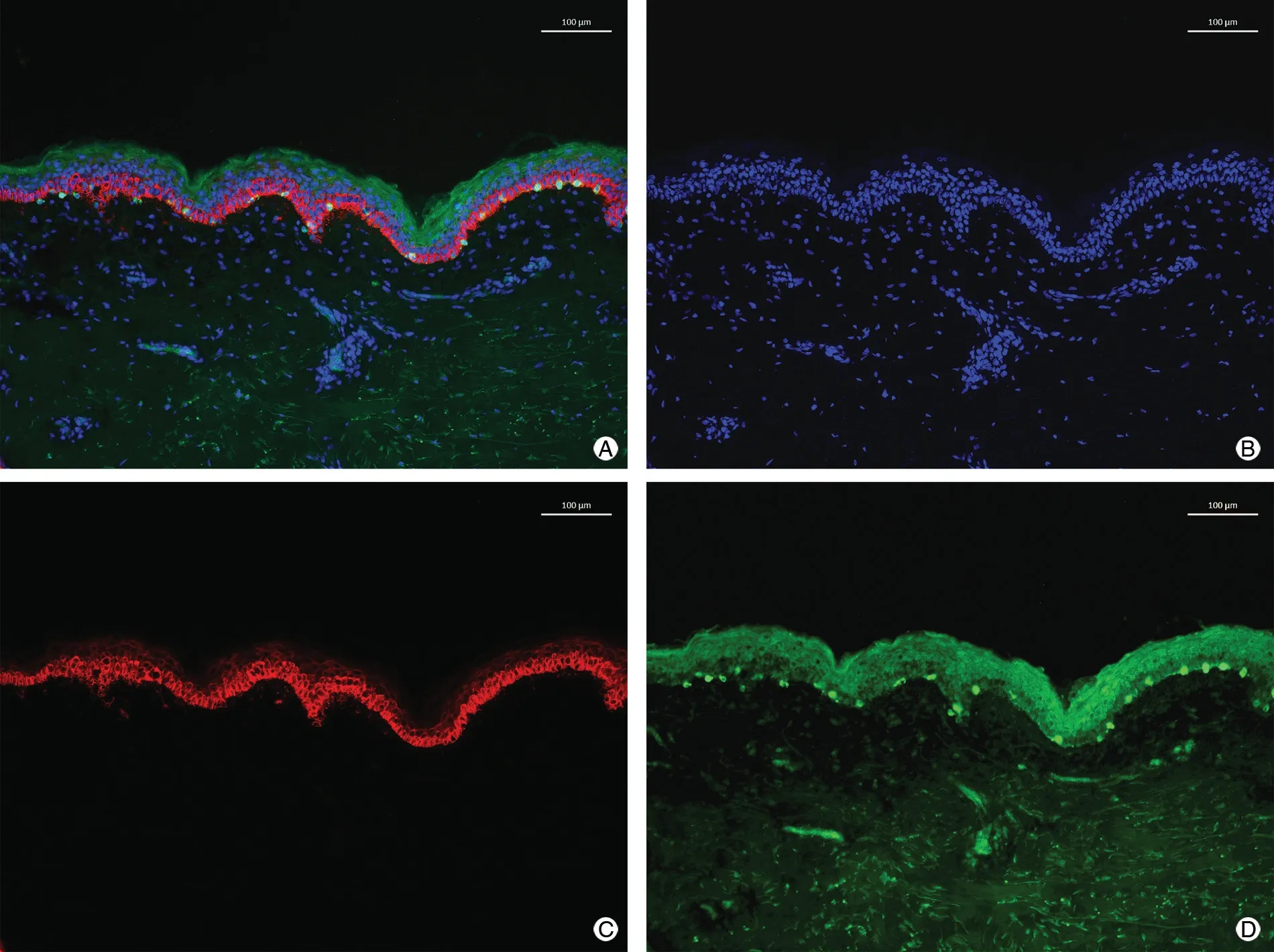
Figure 1. Normal skin immunofluorescence staining. A: DAPI + Krt14 + DCT staining. B: DAPI staining. C: Krt14 staining. D: DCT staining. DAPI:4′,6-diamidino-2-phenylindole; DCT: dopachrome tautomerase; Krt14: keratinocytes via Keratin 14.
Statistical analyses
SPSS 20.0 software (IBM Corp., Armonk, NY, USA) was utilized for statistical analysis of the obtained data.Normally distributed data are presented as mean ±standard deviation. After performing a normality test and homogeneity test of variance, an independent-samples t test was used to compare the data between two groups,while data among three or more groups were compared by one-way analysis of variance. Data correlations were evaluated using Pearson correlation analysis. A P-value of<0.05 was considered statistically significant. Multiple linear regression analysis was used to screen out indexes influencing melanocyte density.
Results
In total, 144 normal skin samples were collected from patients aged 16 to 87 years (51.58±18.14, years). Of the 144 samples, 57 were obtained from male patients and 87 were obtained from female patients. The specimens covered seven major body parts: face (n=16),neck (n=15), thorax and abdomen (n=34), lower back(n=20), hip (n=8), upper limb (n=26), and lower limb(n=25).
Analysis of the melanocyte density between the two sexes showed that melanocytes were uniformly distributed between keratinocytes at the basal layer, with an average ratio of melanocytes to keratinocytes of 1:7. This ratio was 1:6.5 in males and 1:7.4 in females, with no significant difference between the sexes (t=1.537, P=0.127).
The correlation analysis of the melanocyte density among different age groups showed that the melanocyte density gradually declined as age increased (r=-0.331,P<0.001). The ratio was 1:5.8 among patients aged <50 years without an obvious downtrend, 1:7.9 among patients aged 51 to 65 years, and 1:8.5 among patients aged>65 years. The difference among the three age groups was statistically significant (F=6.233, P<0.01).
An obvious difference in the melanocyte density was observed among different body parts. In descending order,the density was as follows: face (1:4.0) >neck (1:5.1) >hip(1:5.7) >upper limb (1:7.4) >lower limb (1:8.3) >back(1:9.2) >thorax and abdomen (1:9.9).
The correlation analysis between the melanocyte density and epidermal thickness showed that the melanocyte density had no correlation with either thickness A (r=0.106, P=0.204) or thickness B (r=-0.018, P=0.826).
In the analysis of the factors influencingmelanocyte density screened out via multiple linear regression analysis, the melanocyte densitywas taken as the dependent variable, and sex, age, body part, thickness A, and thickness B were taken as the independent variables; all were included in themultiple linear regression model. The results indicated that age, body part, and basal length were significant. The formula was as follows: melanocyte density=-0.002×age - 0.016×body part (P<0.05) (Table 1).
Discussion
More than 1,000 direct immunofluorescence-stained pictures of normal skin samples were obtained in this study. These images showed that melanocytes with green fluorescence were uniformly distributed between keratinocytes with red fluorescence in the epidermal basal layer.The ratio of melanocytes to keratinocytes varied substantially among individual patients, mostly ranging from 1:4 to 1:15 (average, 1:7); in some samples, however, the ratio was as low as 1:2.6 or as high as 1:19. The reasons for these individual differences remains unclear, but congenital factors and postnatal environmental factors might be among them. In general, the proportions of melanocytes in different body parts approximated those found in studies outside China,1indicating that the melanocyte density in Chinese people has no race specificity.
The melanocyte density in male patients was slightly higher than that in female patients, but the difference was not statistically significant; that is, the melanocyte density was not influenced by sex.
Several scholars previously examined the correlation between age and melanocyte density, but conflicting results were obtained. Gilchrest4 and Mitchell5 used the 3,4-dihydroxy-L-phenylalanine (DOPA) staining method to examine 8 and 15 skin samples, respectively, and found that the melanocyte density declined by about 6% to 8% with every 10-year increase in age. Likewise, Whiteman et al.8used tyrosine-related protease-1 and S100 as labels to perform immunohistochemical staining of 123 skin samples on the back of patients’ left hand and found that the melanocyte density decreased as age increased. However,Staricco9 and two domestic research groups6-7found that the melanocyte density did not progressively decrease with age. The present study showed that the melanocyte density had a negative correlation with age. Specifically, as age increased, the overall melanocyte density tended to decline,but the change was not significant before 50 years of age;however, the melanocyte density significantly decreased in the 51- to 65-year age group and the >65-year age group.Because of the limited sample size and sample type, most studies that grouped by age did not exclude interference from confounding factors with respect to body part; this may explain the differences in the research results. Notably,the effects of age on the melanocyte density indeed differ among different groups of people.
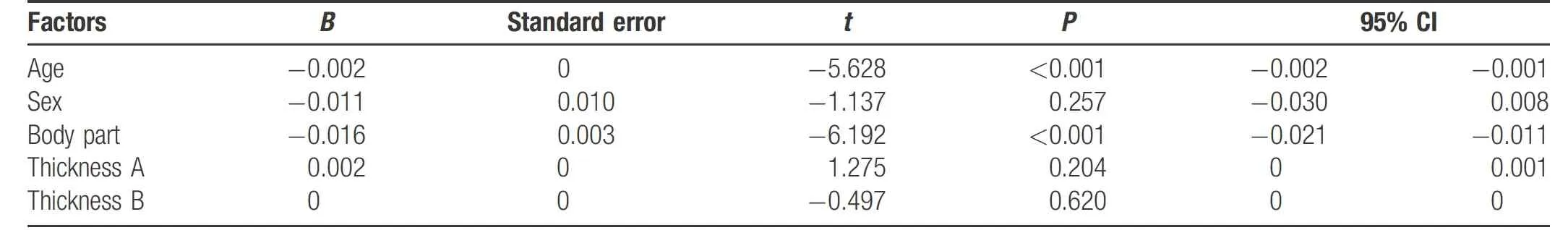
Table 1 Factors influencing melanocyte density.
The present study also showeddifferences in the density of epidermal melanocytes among different body parts. In descending order, these densities were as follows: face >neck > hip > upper limb > lower limb > lower back >thorax and abdomen. The melanocyte densities in the exposed parts of the body, were obviously higher than those in unexposed parts. These results were identical to the intensity of UV irradiation received by various body parts under normal circumstances; thus, the melanocyte number is correlated with the UV irradiation dose, which has been verified in many studies. This might be explained by the fact that UV irradiation promotes melanocyte division or activates melanocyte precursors.10-11However, the hip was an exception as an unexposed body part. The average ratio of melanocytes to keratinocytes in this part was 1:5.7,which was higher than the ratio in other body parts and even exceeded that in the upper limb. We speculate that these differences might be related to friction or hormone concentrations. In addition, the melanocyte density in the hip was generally high, but individual differences were great(1:12 in some patients).
As the epidermal thickness affected the dose of UV irradiation received by the epidermal basal layer, so it was initially speculated that the epidermal thickness might influence the melanocyte density, but this has not been reported in any related literature. Additionally, the melanocyte density was not associated with the epidermal thickness.Common methods used to label melanocytes include the Masson-Fontana argentaffin method, DOPA staining method, and other immunohistochemical staining methods.The Masson-Fontana argentaffin method is with poor specificity because of both melanocytes and keratinocytes positively stainded, and a relatively complicated operation.DOPA staining has high specificity with melanocytes being of only DOPA-positive. DOPA staining can cause melanocyte plasma to be chocolate brown, which can clearly display the dendrite structure and is conducive to the observation of melanocyte morphology. Moreover, the color can reflect the intracellular melanin change. However,keratinocytes in the DOPA-stained section are not colored,so it goes against keratinocyte counting if DOPA staining is used. Commonly used labels for immunohistochemical staining of melanocytes include S100, Melan-A and human menopausal gonadotropin-45, cytokines such as microphthalmia- associated transcription factor and Sry-related HMGbox-10, and critical enzymes in the melanin synthesis process such as TYR, tyrosine-related protease-1, andDCT.These diversified labels differ in sensitivity and specificity.Immunohistochemical staining is mostly performed in paraffin sections because it can preserve the histiocyte structure; however, it can also destroy antigens to a great degree. Therefore, direct immunofluorescence staining of frozen sections was used in the present study. This method guarantees favorable fidelity of antigens, high sensitivity,and an uncomplicated operation. The primary antibody used to label melanocytes wasanti-DCT, a critical enzymein the melanin synthesis process that can catalyze dopachrome to generate 5,6-dihydroxyindole-2-carboxylic acid, which is then transformed into eumelanin with high staining sensitivity and specificity. Moreover, multiple antibodies can be selected to label different cells (eg, Krt14 is used to label keratinocytes and DAPI is used to stain genetic materials). The images observed under different fluorescence conditions are clearer and more intuitive. Notably,tissue sections are not repeatedly frozen in the operation process to prevent destruction of histiocyte morphology and alterations of the experimental result.
In summary, this study showed that melanocytes were uniformly distributed between keratinocytes in the basal layer, and the average ratio of melanocytes to keratinocytes was 1:7. The melanocyte density tends to decline with age and changes significantly after 50 years of age. The melanocyte density is related to the body part; specifically,the density in the face, neck, and hip are higher than that in the limbs and torso. However, the melanocyte density is not associated with sex or epidermal thickness. The present study has provided basic data of normal epidermal melanocytes, which will be helpful for the diagnosis and therapeutic effect evaluation of pigmentary skin diseases.
- 国际皮肤性病学杂志的其它文章
- Circulating MicroRNAs as Potential Biomarkers in the Diagnosis of Neurosyphilis: A Case Control Study
- NCSTN Gene Silencing Inhibits the Retinoic Acid Signaling Pathway in Human Immortalized Keratinocytes
- A Clinical Retrospective Analysis of 340 Inpatients With Malignant Skin Tumors in Western Inner Mongolia
- Subepidermal Autoimmune Bullous Disease Associated With Monoclonal Gammopathy of Unknown Significance and Unexpected Positive Direct Nikolsky Phenomenon: A Case Report
- Nicolau Syndrome Following Metamizole Injection: A Case Report
- Erosive Pustular Dermatosis of the Scalp After Excision and Skin Grafting of Scalp Squamous Cell Carcinoma

