Single or combined effects of dietary arabinoxylanoligosaccharide and inulin on growth performance, gut microbiota, and immune response in Pacific white shrimp Litopenaeus vannamei*
Yun LI , Wei YUAN, Yi ZHANG, Hong LIU ,**, Xilin DAI
1 International Research Center for Marine Biosciences at Shanghai Ocean University, Ministry of Science and Technology,Shanghai 201306, China
2 Key Laboratory of Exploration and Utilization of Aquatic Genetic Resources (Shanghai Ocean University), Ministry of Education, Shanghai 201306, China
3 National Demonstration Center for Experimental Fisheries Science Education (Shanghai Ocean University), Shanghai 201306,China
Abstract The single and synergistic eff ects of dietary arabinoxylan-oligosaccharide (AXOS) and inulin at diff erent doses (2, 4, and 8 mg/g diet) on survival, growth performance, gut microbiota, and immune response in Pacific white shrimp Litopenaeus vannamei were assessed. Singular application of either inulin or AXOS at doses of 4 mg/g diet showed the most stimulatory eff ects on the growth rate and gene expression levels of chitinase, cathepsin L, chymotrypsin, and extracellular signal-regulated kinase(ERK) in each single prebiotic feeding trial. Compared with single prebiotic treatments, simultaneous application of AXOS and inulin at 4 mg/g diet enhanced remarkably the growth parameters of shrimp and the expression of related genes (chitinase, cathepsin L, chymotrypsin, ERK, myeloid diff erentiation factor 88, and phenoloxidase) after 8-week feeding ( P <0.05). Additionally, gut microbiota analysis indicated the dietary supplementation with combined prebiotics increased significantly the bacterial community richness and relative abundance of Bacillus, Pseudomonas, Bacteriovorax, and Lactobacillus, and reduced the abundance of Vibrio, Rhodococcus, and Photobacterium in the digestive tract ofl. vannamei. Compared with the single prebiotic treatment and the control, combined prebiotics supplementation boosted notably the survival rate and expression levels ofimmune-related genes in shrimp infected with Vibrio alginolyticus or white spot syndrome virus. Therefore, simultaneous application of AXOS and inulin shall have a great potential of dietary supplement in the culture ofl. vannamei.
Keyword: prebiotic; growth; intestinal microflora; immunity; survival; Litopenaeus vannamei
1 INTRODUCTION
Pacific white shrimp ( Litopenaeus vannamei)originates in South America, and has become the most important penaeid shrimp cultured in the world.However, various diseases and stresses induced by environmental pollution and high cultural density have become the major obstacles to the industry of shrimp culture (Walker and Mohan, 2009). Therefore,it is urgent to search for natural dietary supplements to improve the growth performance and disease resistance of cultured shrimp.
Having merits of natural, safe, and reliable characteristics, prebiotics have recently attracted extensive attention as good potential supplements in aquaculture (Ringø et al., 2010). Prebiotics are defined as non-digestible food ingredients, providing advantageous eff ects for the host by improving growth as well as the activity of the intestinal beneficial bacteria (Gibson, 1999). Gut microbiota constitutes a highly complex and eff ective microecosystem in the host gastrointestinal tract and plays a crucial role in regulating nutrition,metabolism, and immunity of animal (Wardwell et al., 2011). Thus, certain groups ofintestinal bacteria,including Bacillales and lactic acid bacteria, could enhance their relative populations in the gut flora by utilizing the fermentation of prebiotics that might not be hydrolyzed in the digestive tract of the host(Pool-Zobel et al., 2002; Flickinger et al., 2003).Additionally, prebiotic-stimulated beneficial bacteria have been shown to improve the host growth performance by increasing the expression and activity ofintestinal digestive enzymes in aquatic animals (Daniels et al., 2010; Akhter et al., 2015).Carbohydrases (cellulase, amylase, and chitinase),lipases, and proteases (trypsin and chymotrypsin)constitute the major digestive enzymes in crustacean gastrointestinal tract, and the expression levels as well as activities of these enzymes are believed to be the important indicators of digestive function and growth status of the animal. Moreover, dietary prebiotics have also been demonstrated to improve the disease resistance and innate immune response in aquatic species (Dong and Wang, 2013; Zhang et al., 2014).
Prebiotics can be divided generally into two main groups: oligosaccharides and polysaccharides,according to the chain length of carbohydrate.Oligosaccharides, such as fructooligosaccharides(FOS), galactooligosaccharides (GOS), mannan oligosaccharides (MOS), xylooligosaccharides(XOS), and arabinoxylan-oligosaccharides (AXOS),have been extensively applied as growth or immunity stimulants in fish and shellfish (Ringø et al., 2010;Ganguly et al., 2013). Arabinoxylan-oligosaccharide is a short-chain hydrolysis product of arabinoxylan,and naturally present in outer cell wall of various cereal grains. As a component of dietary fiber, AXOS has been utilized by the intestinal flora, which induces positive impacts on many physiological activities of organism (Ringø et al., 2010). Using AXOS and other hydrolysis products of arabinoxylan as natural dietary supplementations has been proved beneficial to growth performance, feed utilization, and innate immunity in many animals. AXOS supplemented diet showed obviously stimulatory eff ects on growth performance, intestinal microbiota, and immune responses in Siberian sturgeon ( Acipenser baerii)(Geraylou et al., 2013). Application of dietary xylooligosaccharides remarkably elevated the activities of peroxidase and lysozyme, survival, and resistance against bacterial infection in Nile tilapia( Oreochromis niloticus) (Van Doan et al., 2018).Furthermore, polysaccharides are polymeric carbohydrates composed of a quantity of monosaccharides bound by glycosidic linkages, and generally obtained from natural biomaterials (Chen and Huang, 2018). Diets supplemented with polysaccharides, such as glucan, pectin, inulin or chitin, have also shown a great potential for growth promotion and immune regulation in aquatic species(Roberfroid, 2005; Ringø et al., 2010; Ganguly et al.,2013). Administration of dietary β-glucan exhibited significant improvements in the growth rate, survival and immune response in Labeo rohita fingerlings(Misra et al., 2006) and large yellow croaker( Pseudosciaena crocea) (Ai et al., 2007).Supplementation with inulin caused positive eff ects on growth performance and gut microbiota in Asian seabass ( Lates calcarifer) (Syed Raffi c Ali et al.,2016). Inulin-fed shrimp gained obvious improvements of phenoloxidase activity and pathogen resistance, compared with the control (Luna-González et al., 2012).
A growing number of reports recently indicates that combined application of diff erent prebiotics has shown more eff ective promotion of growth performance and immune response in aquaculture. A previous study showed the synergistic eff ects of MOS and FOS in narrow-clawed crayfish ( Astacus leptodactylus) with significant enhancement in growth rate, survival, and stress resistance compared with the individual treatment and control (Safari et al., 2014). In addition, applying β-glucan combined with MOS obviously promoted the resistance against bacterial infection and increased specific growth rate of sea cucumber ( Apostichopus japonicus) (Gu et al.,2011), and immune response in pacu ( Piaractus mesopotamicus) (Soares et al., 2018).
However, research on the eff ects of dietary AXOS combined with inulin on the growth performance and immune responses in crustaceans, especially in L. vannamei, is still limited. Therefore, the simultaneous eff ects of the prebiotic combination,AXOS, and inulin on gut microbiota, growth performance, and immune response in Pacific white shrimp were evaluated, and a potential dietary supplement in shrimp culture was explored.
2 MATERIAL AND METHOD
2.1 Culture system and shrimp
Litopenaeus vannamei in similar size were obtained from Pudong aquatic products market (Shanghai,China), and reared at the Aquaculture Breeding Center of Shanghai Ocean University (Shanghai, China).Random PCR detection was performed to assure the experimental shrimp to be pathogens free. The culture system consisted of 36 indoor fiberglass tanks (high:1.2 m; diameter: 1.9 m; volume: 2 000 L) with continuous aeration and recirculating seawater supply at a flow rate of 20 L/min per tank.Healthy shrimp were randomly distributed into 36 tanks in the initial population density of 220 shrimp per tank, and fed with untreated formulated diet for 14 days to acclimate to the culture conditions. Uneaten food and feces were siphoned out every day. After acclimation, the experimental diets containing prebiotics were provided at 5%-8% of body weight daily and fed to shrimp at 7:00, 11:00, 16:00, and 20:00. Shrimp in the control were fed with basal diet with no prebiotics. The feeding rate was regulated according to the feed consumption. Salinity,temperature, pH, and total ammonia nitrogen of water were monitored daily and kept at 31±0.5, 28±1 °C,8.1±0.05, and 0.06±0.005 mg/L, respectively.
2.2 Preparation of experimental diets
All the experimental diets were manufactured at Aquaculture Nutrition and Feed Laboratory of Shanghai Ocean University. The basal formulated diets, designed according to the nutrition requirement of Pacific white shrimp, were supplemented with inulin (90% purity) (Sigma-Aldrich, Saint Louis,Missouri, USA) and arabinoxylan-oligosaccharide(94% purity) (Yinuo Biotech Co. Ltd., Ningbo,Zhejiang, China) at diff erent designed concentrations.Mixture of prebiotic and other ingredients were pelletized into uniform pellets (diameter of 1.2 mm),and dried in oven in 40 °C for 18 h. All the diets were kept at -20 °C before being used. Following the accredited methods (AOAC, 2000), the experimental diets were analyzed to contain averagely 45.87%crude protein, 7.88% crude lipid, 10.2% ash, and 9.27% moisture, and the ingredient contained soybean meal, fish meal and squid muscle meal as protein sources, and fish oil as lipid source. The formulation of the diets applied in feeding experiments is shown in Table 1.
2.3 Feeding trial
Single-prebiotic feeding trials were conducted respectively to evaluate the stimulatory eff ects of two diff erent prebiotics (AXOS or inulin). Single prebiotic feeding trial consisted of four experimental groups,each of which was further divided into three replicates.Two hundred acclimated shrimp in similar size(8.08±0.46 g) were kept in each replicative tank and fed with experimental diet for 8 weeks. Prebiotictreated groups were fed with AXOS or inulin at three supplemented levels: 2, 4, and 8 mg/g diet. Shrimp of the control group were fed with zero prebiotic basal diet.
On the basis of the results of single feeding trials, a 56-day combined feeding experiment was performed subsequently to assess the synergistic eff ects of dietary supplementation of combined prebiotics as compared to the single treatments. Similar to the previous single feeding trial, the combined feeding trial was composed of four diet groups, giving three replicates per diet. The four groups were fed diets with AXOS only, inulin only, combination of AXOS and inulin, and control, respectively. The dose of prebiotic used in diff erent supplemented diets was 4 mg/g.
2.4 Pathogen challenge trial
Pathogen challenge tests were performed to assess the eff ects of prebiotics on the immune response of shrimp against two major pathogens, Vibrio alginolyticus and white spot syndrome virus (WSSV).On the termination of combined feeding trial, 100 shrimp from each replicate were equally distributed into two groups for WSSV and V. alginolyticus challenge, respectively. The WSSV stock and bacterial strain were provided by National Pathogen Collection Center for Aquatic Animals (Shanghai, China). The purified WSSV inoculant was prepared as the method described previously (Xie et al., 2005) and quantified by the real-time PCR using specific primers of WSSV(Table 2). Then the WSSV inoculum was completely diluted into sterile phosphate buff ered saline (PBS)solution to the designed concentration (108copies/mL). In addition, after an incubation on the tryptic soy agar overnight at 28 °C, V. alginolyticus used in bacteria challenge were cultured in tryptic soy broth at 28 °C for 24 h, and then the bacterial suspension was centrifuged at 8 000× g for 15 min and diluted with PBS to the designed concentration of 107 CFU/mL.
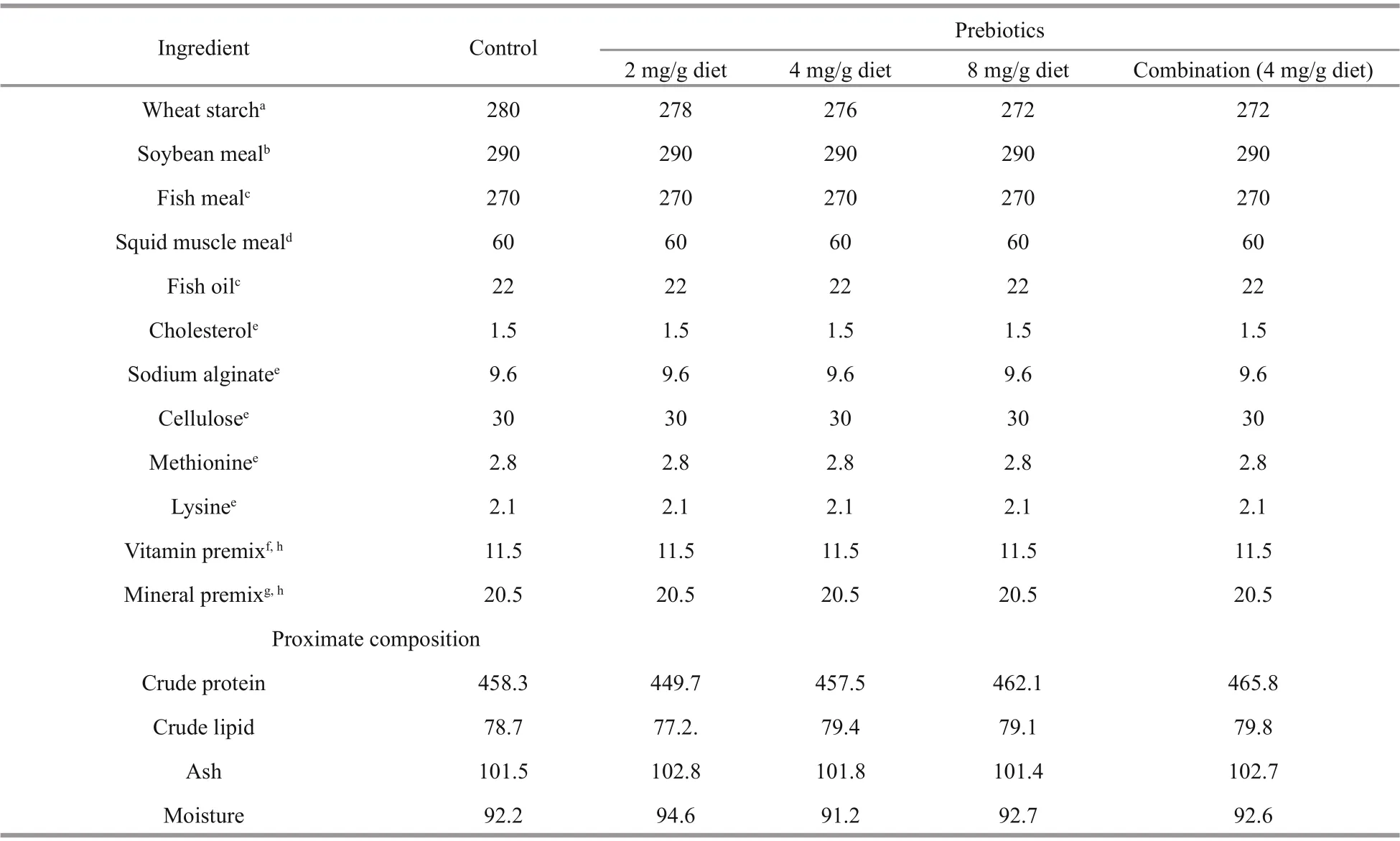
Table 1 Formulation and proximate chemical composition (g/kg dry weight) of the experimental diets

Table 2 Nucleotide sequences of the primers used in this study
Every experimental shrimp received an injection into the ventral sinus of cephalothorax with 200-μL virus inoculum containing 2×107WSSV copies for virus challenge or 200-μL V. alginolyticus suspension for bacteria challenge. In addition, 50 untreated shrimp injected with 200 μL/shrimp PBS were used as the negative control. The challenge trial lasted for five days, and all the experimental shrimp were also fed with the same diets applied in the previous feeding trial. During the challenge period, the infected shrimp in diff erent experimental groups were monitored for the cumulative mortality daily.
2.5 Sampling and calculation
Biometric indexes of experimental shrimp under diff erent treatments were measured and recorded on the termination of feeding experiment. All the experimental shrimp were fasted on the sampling days.All the animal experiments were conducted in accordance with the guidelines and approval of Animal Ethics Committee of Shanghai Ocean University. After anesthesia in ice bath, hepatopancreas, intestine,hemolymph, and gill tissues of 15 shrimp from each experimental group were collected on Days 28 and 56 of feeding trials. Gill and hemolymph from nine shrimp in each group were sampled on Day 5 post infection(dpi) in the challenge test of WSSV or V. alginolyticus,respectively. Hemolymph (approximately 200 μL) was collected from the ventral sinus cavity of each sampled animal with 1-mL syringe, and mixed with same volume of modified anticoagulant solution (Huang et al., 2010) immediately.
In this research, the related parameters were calculated as follows:
specific growth rate (SGR; %/d)=(lnFBWlnIBW)×100/d,
average daily weight gain (ADG; g/d)=(FBWIBW)/d,
feed conversion ratio (FCR)=FO/(FBW-IBW),
where FBW is the final mean body weight (g) of shrimp, IBW is initial mean body weight (g), d is the experimental duration in days, and FO is feed off ered(g).
Survival (%)=( Nf/ Ni)×100,
where Nfand Niare the final number and initial number of the experimental shrimp, respectively.
2.6 High-throughput sequencing of bacterial 16S rRNA
gDNA ofintestinal tissue was extracted using a TIANamp Marine Animals DNA Kit (Tiangen Biotech,Beijing, China) according to the manufacturer’s instructions, and the V3-V4 hypervariable region of the bacterial 16S rRNA gene was amplified with barcoded primers 341F (5′-CCTAYGGGRBGCASCAG-3′) and 806R (5′-GGACTACNNGGGTATCTAAT-3′). The PCR products were purified with a SanPrep Column PCR Product Purification Kit (Sangon Biotech, Shanghai,China) and sent to OE Biotech. Co., Ltd., Shanghai,China, for intestinal bacterial community analysis.
2.7 Relative quantitative real-time PCR analysis
Total RNA from diff erent tissue samples(Hemolymph, hepatopancreas, intestine, and gill) was isolated with a PureLink™ RNA Mini Kit (Invitrogen,Carlsbad, California, USA). Concentration and quality of extracted RNA were determined by the SP-752 spectrophotometer (Spectrum, Shanghai, China).The total RNA was treated with gDNase (Tiangen Biotech, Beijing, China) to remove the contaminated DNA. The first strand of cDNA was reversely transcribed from total RNA using SuperScript™ IV First-Strand Synthesis System (Invitrogen, Carlsbad,California, USA) following the instruction.
The relative quantitative real-time PCR analysis with gene specific primers (Table 2) were used to investigate the change in related gene transcription levels in this study. The quantitative PCR reaction was carried out with Eco Real-Time PCR System(Illumina Inc., San Diego, California, USA) in a 20-μL reaction volume containing 0.6 μmol/L of each specific primer, 10 μL RealUniversal PreMix (SYBR Green I) (Tiangen Biotech, Beijing, China), and 50-ng cDNA template. After an initial denaturation for 10 min, the PCR reaction was carried out for 40 cycles followed the cycles of 95 °C for 10 s, 60 °C for 25 s,and 72 °C for 30 s. The target gene expression was normalized with L. vannamei 18S rRNA gene and quantified with the comparative CT method (Livak and Schmittgen, 2001).
2.8 Data analysis
All the data were statistically analyzed using software SPSS 19.0 (SPSS Inc., Chicago, Illinois,USA) and presented as mean±standard deviation. The normality of data was assessed by Shapiro-Wilk’s test prior to analysis, and homogeneity of variance was assessed by Levene’s test. One-way analysis of variance (ANOVA) was used to identify the differences of related data between diff erent treatments, and two-way ANOVA was used to analyzethe synergistic eff ects between AXOS and inulin on growth parameters, survival, and the expression of related genes. The significant differences among the groups were detected with Duncan’s multiple range test. Significance was accepted at the probability level less than 0.05. In addition, Kaplan-Meier plot logrank χ2tests were performed to examine the significant differences of survivals in the challenge trials.
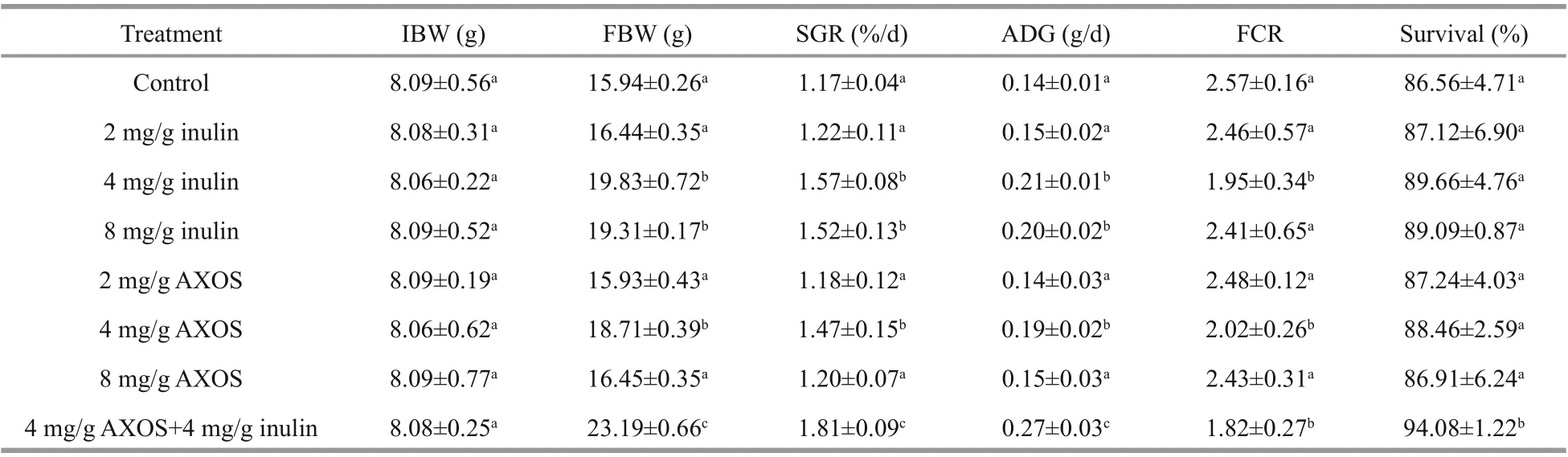
Table 3 Growth parameters and survival ofl. vannamei fed in diff erent doses of prebiotics in 56-day feeding trials
3 RESULT
3.1 Growth parameters and growth-related genes expression in AXOS feeding experiment
Compared to the control group, significantly enhanced final mean body weight (FBW)(18.71±0.39 g), average daily weight gain (ADG)(0.19±0.02 g/d) and specific growth rate (SGR)(1.47%±0.15%/d), as well as noticeably lower feed conversion ratio (FCR) (2.02±0.26) were observed in shrimp fed with 4 mg/g AXOS ( P <0.05) (Table 3).However, dietary supplementation with 2 or 8 mg/g AXOS showed no significant eff ect on FBW, ADG,SGR, and FCR compared to the control ( P >0.05).Survival ranged from 86.56% to 88.46%, and no obvious difference was observed among all the groups in AXOS-feeding experiment ( P >0.05).
Expressions of chitinase, cathepsin L, and chymotrypsin in hepatopancreas were investigated on Days 28 and 56 of the trial. Chitinase and chymotrypsin expression in shrimp from 4 or 8 mg/g AXOS treatment was clearly elevated on Day 28 in comparison to control ( P <0.05), and gradually increased to the peak on Day 56 (Fig.1). Additionally,shrimp fed with diets supplemented with various doses of AXOS (2, 4, and 8 mg/g) showed significantly boosted expressions of cathepsin L and extracellular signal-regulated kinase (ERK) on Days 28 and 56 comparing to the control, and the highest values of cathepsin L and ERK expression were detected in the 4 and 8 mg/g AXOS treatments on Day 56.
3.2 Growth parameters and growth-related genes expression in inulin feeding experiment
The result ofinulin feeding experiment revealed that the dietary supplementation with 4 or 8 mg/g inulin could significantly enhance FBW, SGR, and ADG of shrimp comparing to the control ( P <0.05),and no noticeable difference of those growth parameters mentioned above was detected between 4 and 8 mg/g inulin treatments ( P >0.05). Meanwhile,dietary supplementation with 4 mg/g inulin induced significantly lower FCR (1.95±0.34) than that of the control ( P <0.05). Similar to the result of AXOS treatments, there was no obvious difference of survival among groups by the end ofinulin feeding experiment ( P >0.05).
The diets supplemented with inulin from 2 to 8 mg/g noticeably promoted the expression of cathepsin L, chymotrypsin, and ERK on Days 28 and 56 in comparison to the control, and the highest expression levels of these gene were observed in 4 mg/g inulin treatment on Day 56 (Fig.2). Additionally,the shrimp from 2 and 4 mg/g dietary inulin groups showed no obvious difference of cathepsin L expression after 56-day feeding, and no significant difference of chymotrypsin expression between 4 and 8 mg/g inulin groups was observed ( P >0.05). However,the expression of chitinase exhibited no significant change among all the groups on Day 28, but then obviously increased on Day 56 in the inulin-treated groups (2, 4, and 8 mg/g) ( P <0.05). Furthermore, the highest chitinase expression was observed in the 4 mg/g inulin group on Day 56, followed by 8 mg/g inulin, 2 mg/g inulin, and the control.
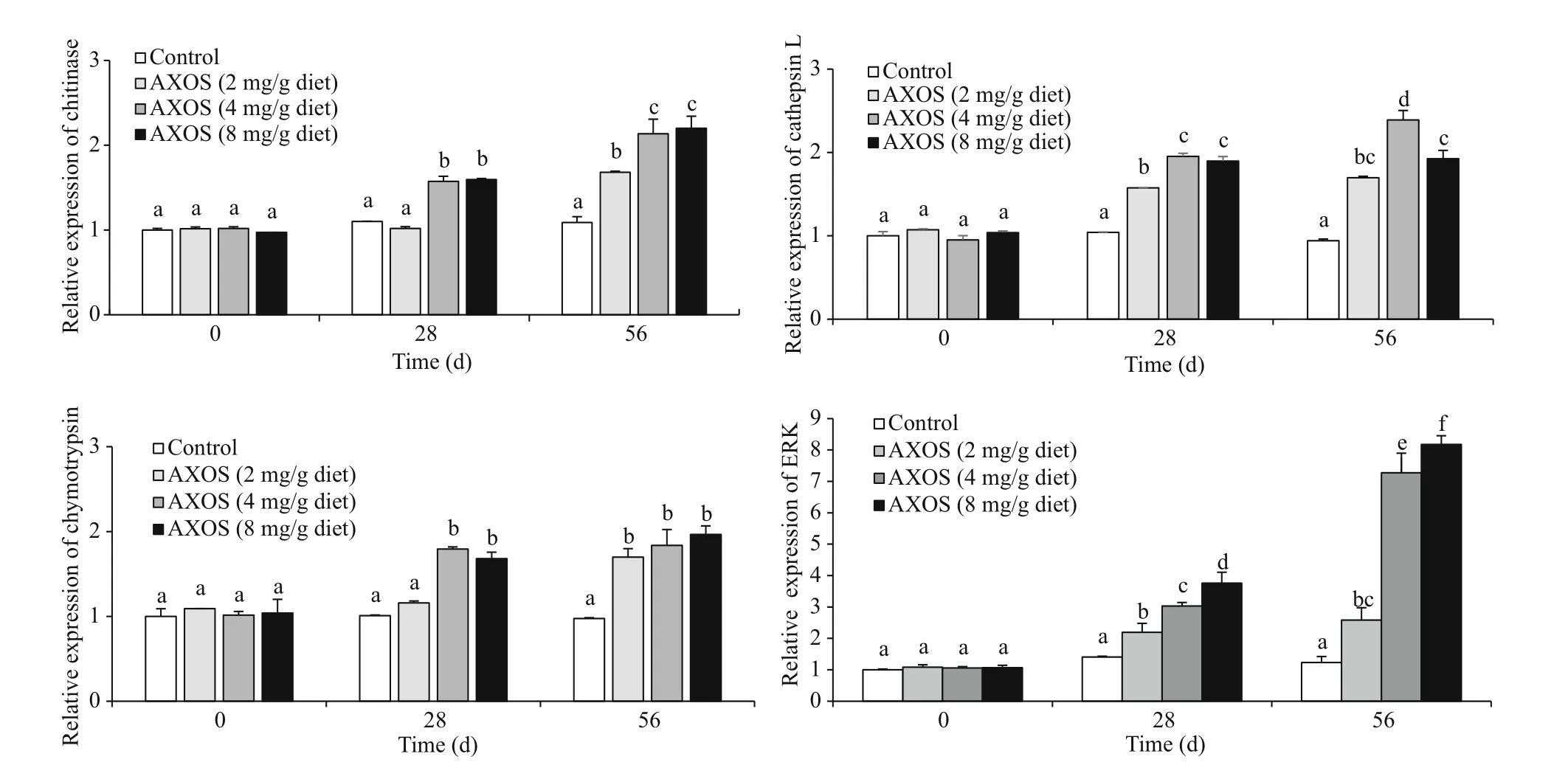
Fig.1 Eff ects of diets supplemented with AXOS at diff erent doses on the expression levels of growth-related genes in L. vannamei
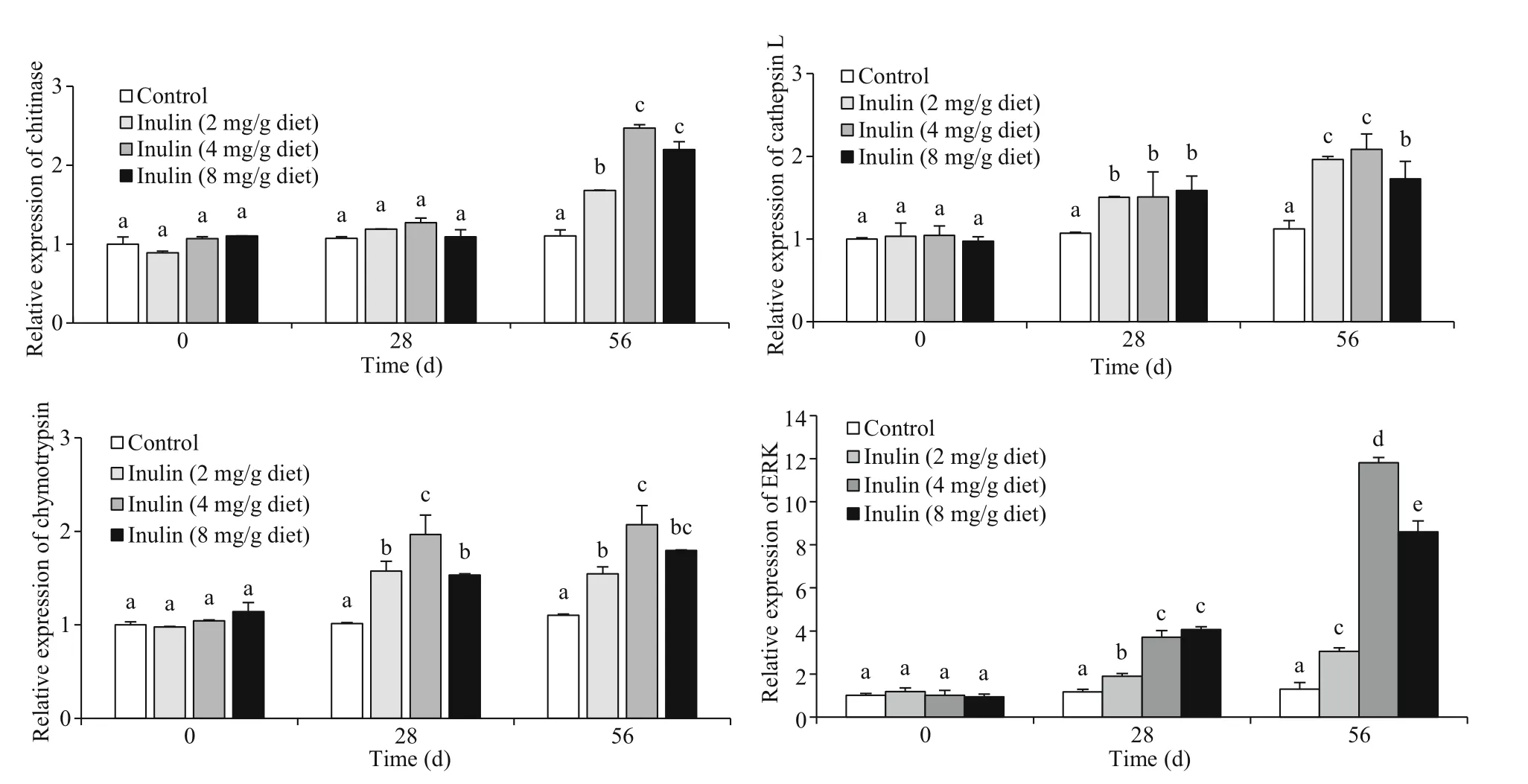
Fig.2 Eff ects of diets supplemented with inulin at diff erent doses on the expression levels of growth-related genes in L. vannamei
3.3 Growth performance and expression of growth and immune related genes in the combined prebiotics feeding experiment
Compared with single prebiotic feeding, 56-day feeding of combined prebiotics (4 mg/g AXOS and 4 mg/g inulin) induced significant improvements of FBW (23.19±0.66 g), SGR (1.81%±0.09%/d), ADG(0.27±0.03 g/d), and survival (94.08%±1.22%), as well as lower FCR (1.82±0.27) of experimental shrimp ( P <0.05) (Table 3).
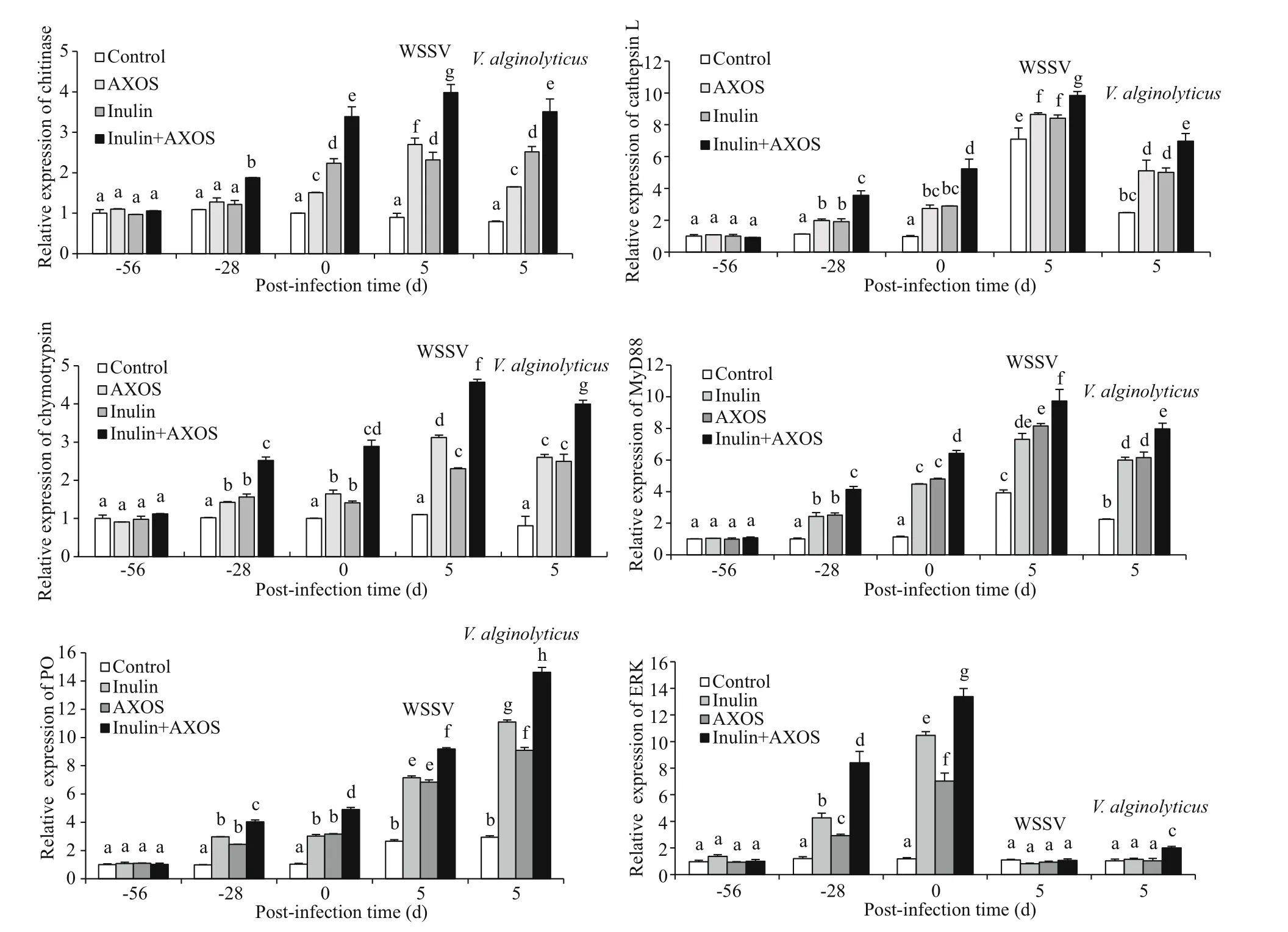
Fig.3 Eff ects of combined dietary AXOS (4 mg/g diet) and inulin (4 mg/g diet) on the expression levels of growth and immune related genes ofl. vannamei in combined feeding trial and before (0 day) or 5 days after WSSV/ V. alginolyticus challenge
Similar to the result of growth performance, the related genes expression exhibited much more improvement in the combined prebiotics feeding group. The shrimp fed with prebiotic combination showed obviously higher expression of chitinase,cathepsin L, and chymotrypsin in hepatopancreas than those treated with prebiotic separately on Days 28 and 56 ( P <0.05) (Fig.3). Furthermore, the expression levels of ERK, myeloid diff erentiation factor 88 (MyD88), and phenoloxidase (PO) of hemolymph samples were obviously elevated in simultaneous prebiotic treatment, compared to the other treatments, on the termination of the feeding trial.
3.4 Intestinal microbiota
To investigate the impacts of supplementations on the gut microflora of experimental shrimp, the intestinal samples from shrimp fed with prebiotics or basal diet were analyzed. The Alpha diversity analysis indicated that the bacterial community richness (Chao and Ace) of shrimp in separate or combined supplemented groups were remarkably higher than those in the control group ( P <0.05), but there was no significant difference among the prebiotic-treated groups ( P >0.05) (Table 4). In addition, diets supplemented with AXOS and inulin, separately or simultaneously, showed no significant eff ect on the intestinal bacterial diversity (Shannon and Simpson)of experimental shrimp, compared to the basal diet without prebiotic ( P >0.05).
The result of high-throughput sequencing revealed that the most abundant bacterial phyla in intestinal bacterial community ofl. vannamei were related to Proteobactaria, Actinobacteria, Bacteroidetes,Firmicutes, Gemmatimonadetes, and Acidobacteria(Fig.4). The Top 5 most abundant bacterial genera in the untreated shrimp were Vibrio, Rhodococcus,Photobacterium, Pseudomonas, and Acinetobacter(Fig.5). The 56-day administration of prebiotic combination remarkably increased the abundance of Bacillus, Pseudomonas, Lactobacillus, and Bacteriovorax in shrimp gastrointestinal tract in comparison to the other diets ( P <0.05) (Fig.5). In addition, the intestinal Vibrio, Rhodococcus, and Photobacterium in shrimp fed diets with supplementation, separately or simultaneously,decreased observably at the end of feeding trial( P <0.05).
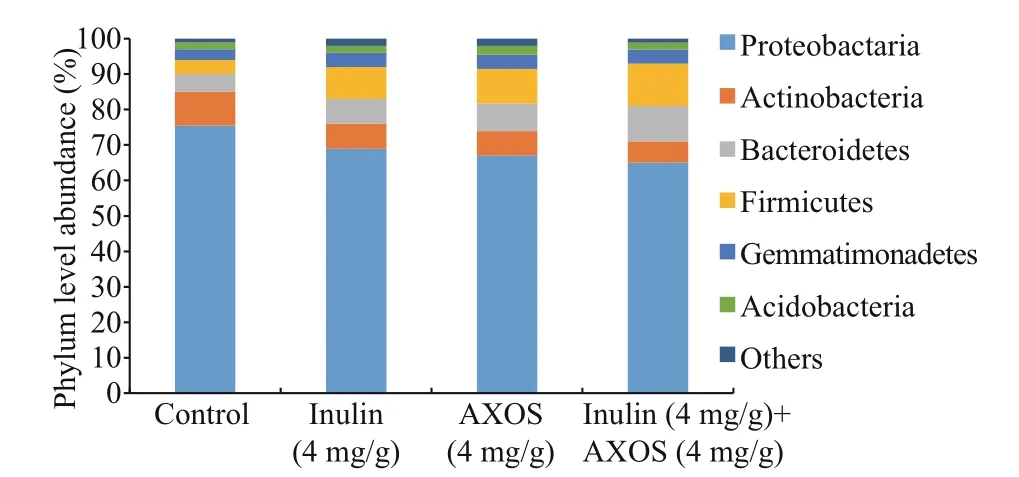
Fig.4 Intestinal bacterial community composition at phylum level in L. vannamei fed with diff erent diets in combined prebiotics feeding experiment
3.5 WSSV/ Vibrio alginolyticus challenge
Monitoring the shrimp survival during the pathogen infections demonstrated that either individual or simultaneous application of dietary AXOS as well as inulin markedly increased the survival of shrimp under the virus or bacterial infection ( P <0.05). In addition, the combined prebiotics-treated shrimp showed the maximum value of survival at the end of two challenge tests (Fig.6).
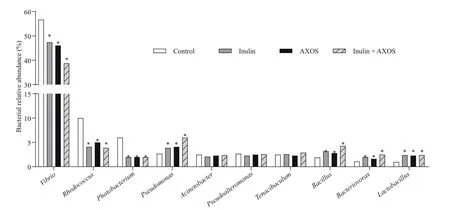
Fig.5 Top 10 most abundant bacterial genera in intestine ofl. vannamei in diff erent treatment groups
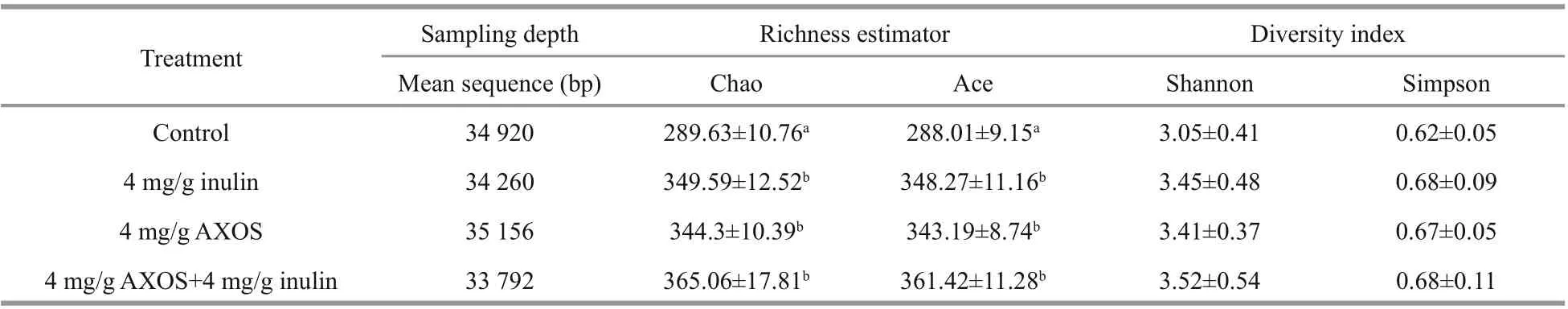
Table 4 Data from high-throughput sequencing yields bacterial diversity and richness
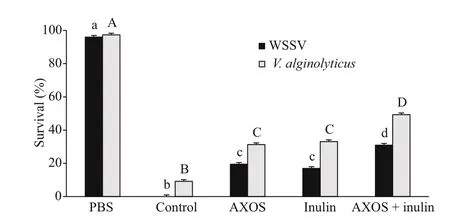
Fig.6 The survival ofl. vannamei treated with dietary AXOS and inulin, singularly and together, on Day 5 after infection of WSSV or V. alginolyticus
The diets with separate or combined supplementations obviously elevated the gene expressions of chitinase, cathepsin L, chymotrypsin,MyD88, and PO in L. vannamei on Day 5 comparing to those before WSSV infection ( P <0.05) (Fig.3).Additionally, the combined-prebiotic supplemented diet exhibited more positive eff ects to expression of those genes mentioned above in comparison to other treatments on Day 5. In contrast, the ERK expression of every dietary treatment declined markedly on Day 5 compared with the beginning of virus challenge( P <0.05), and no obvious difference of ERK expression was detected among treatments.In V. alginolyticus challenge experiment, the expressions of cathepsin L, chymotrypsin, MyD88 and PO in each prebiotic treatment were markedly promoted on the 5thday post bacteria injection (dpi)( P <0.05), and shrimp treated with AXOS and inulin together exhibited remarkably higher expression levels of these four genes on 5 dpi in comparison with shrimp fed single prebiotic or basal diet ( P <0.05)(Fig.3). However, the chitinase expression of shrimp fed diff erent diets on 5 dpi was not observably diff erent to the expression at the beginning of bacterial challenge, and the peak level of chitinase expression on 5 dpi appeared in combination group. Furthermore,the expression level of ERK in each prebiotic-fed group decreased significantly at the end of the infection test ( P <0.05), but the shrimp in combination feeding group still gained observably higher ERK expression as compared to the other experimental groups.
4 DISCUSSION
Application of dietary prebiotic as feed supplementation has been shown significant positive eff ects on growth performance and survival in fish as well as shellfish, while the eff ects seemed to be varied in diff erent species of hosts and dosages of prebiotic. For example, administration of 0.05, 0.1,and 0.2 mg/g dietary xylooligosaccharides (XOS)significantly enhanced the relative gain rate (RGR)and average daily weight gain (ADG) of allogynogenetic crucian carp ( Carassius auratus gibelio) (Xu et al., 2009). European lobster ( Homarus gammarus) larvae fed MOS showed remarkable improvement in growth parameters and survival comparing with the control (Daniels et al., 2010). On the contrary, dietary isomaltooligosaccharide (IMO)and fructooligosaccharide (FOS) at doses of 2 and 6 mg/g showed no obvious eff ect on the growth rate and FCR of hybrid tilapia ( Oreochromis sp.) (He et al., 2003). No observable impact on the feed intake,weight gain, and feed effi ciency of Atlantic salmon( S. salar) was detected after 4-month feeding with 10 g/kg MOS (Grisdale-Helland et al., 2008). In present study, diets supplemented with AXOS or inulin at diff erent doses showed significant promotion to the growth, feed conversion, and expressions of related genes in L. vannamei. The highest values of those parameters mentioned above were detected in the application of single prebiotic at dose of 4 mg/g,which was considered as the optimum dose of prebiotic for the subsequent combined feeding.Previous research indicated that the stimulatory eff ects might vary in the prebiotics of diff erent chain lengths. Comparative studies on fecal cultures revealed that the fermentations ofinulin and FOS could be utilized by diff erent beneficial bacteria in host digestive tract (Rossi et al., 2005; van de Wiele et al., 2007), and the transition time through the host gastrointestinal tract increased following the increasing chain length of prebiotic (Rumessen and Gudmand-Høyer, 1998). Thus, previous studies provided a new perspective on the application of combined prebiotics of diff erent chain lengths as a more eff ective dietary supplement, due to the potentially synergistic and adjuvant eff ects on the modulation of gut microbial community. The application of combined dietary prebiotics has already been shown to promote growth and immune responses much more effi ciently in several aquatic species (Ringø et al., 2010; Safari et al., 2014). In this research, administration of combined prebiotics at the optimum dose (4 mg/g) showed greater promotion on the expressions of related genes and growth parameters than those ofindividual treatments of basal diet.
The stimulatory eff ect of prebiotic on shrimp growth might be realized mainly through the upregulation of beneficial bacteria in host gastrointestinal tract. Several species of bacteria (generally known as“probiotic” with other beneficial microorganisms),particularly members of the genus Bacillus,Pseudomonas, and Lactobacillus have been reported as stimulants of growth performance and immune response in finfish and shellfish (Wang, 2007; Sahu et al., 2008). However, since the potential impact to the environment, food safety, and decline of bacterial activity during the feed preparation, the direct application of probiotic bacteria in aquaculture has been still limited (Ringø et al., 2010). Therefore,administration of dietary prebiotic was believed to be a safer and more eff ective approach to perform the function of beneficial bacteria in industrial aquaculture. The fermentation of prebiotics provides carbon and energy sources to certain groups of beneficial bacteria in the digestive tract of aquatic species. This study indicated that the application of dietary AXOS and inulin together significantly improved the abundance of several species of beneficial bacteria such as Bacteriovorax, Bacillus,Pseudomonas, and Lactobacillus in the shrimp gastrointestinal tract. Meanwhile, enhanced bacterial community richness was also observed within prebiotic-treated shrimp in this study. Similar findings have been reported with the dietary supplementation of MOS in European lobster ( Homarus Gammarus)(Daniels et al., 2010), as well as application of dietary inulin on hybrid surubim ( Pseudoplatystoma sp.)(Mouriño et al., 2012). The result of this study also demonstrated that AXOS and inulin showed obviously negative eff ects on the abundance ofintestinal pathogenic bacteria such as Vibrio. Previous investigations suggested that probiotic bacteria also showed the positive impacts to the immune response via production of specific antimicrobial substances and competitive exclusion of detrimental bacteria(Pandiyan et al., 2013).
Probiotic bacteria were regarded as improving the growth through stimulating the secretion of digestive enzymes (Wang, 2007; Pandiyan et al., 2013), the synthesis of essential nutrients such as vitamins,biotin and fatty acids (Newaj-Fyzul et al., 2014), and improvement to the intestinal morphology in aquatic animals (Daniels et al., 2010). The digestive enzymes play a crucial role in digestion of nutrients, molting regulation and immune response (Lovett and Felder,1990). Proteases, chitinase, and lipases constitute the major digestive enzymes in the crustacean digestive system, and the expression levels of these enzymes are believed the important indicators to evaluate the growth status of organism. Chymotrypsin and trypsin are the most abundant proteolytic enzymes in the digestive glands of Decapoda, and play a key role in protein digestion (Lemos et al., 2000). In addition,chymotrypsin also exerts great influences on immunoregulation including blood clotting,melanization, and pathogen resistance (Lai et al.,2005; Xue et al., 2013). Chitinase is another essential enzyme for crustaceans, which is involved in molting process, digestion of chitinous food and degradation of endogenous chitin (Proespraiwong et al., 2010;Zhou et al., 2017). Similar to chymotrypsin, chitinase was reported to participate in the immune response to pathogens and ammonia nitrogen stress (Zhou et al.,2017). Additionally, cathepsin L is regarded as a multifunctional lysosomal endopeptidase and involved in various physiological processes, including protein degradation, food digestion and immune regulation (Hu and Leung, 2007). Previous studies on shrimp species indicated that cathepsin functioned as a major digestive enzyme during the intracellular digestion of endocytosed nutrition in hepatopancreas(Hu and Leung, 2007; Stephens et al., 2012), and participated in the regulation ofintermolt cycle(Glenn et al., 2005). Therefore, the significant improvements of shrimp growth parameters (SGR,ADG, and FCR), with the simultaneous use of AXOS and inulin could be attributed to the remarkably enhanced abundance ofintestinal beneficial bacteria and consequent up-regulation of the expressions of various digestive enzymes in hepatopancreas.
In addition, we also demonstrated that the dietary supplementation with AXOS and inulin exerted positive eff ects on the survival and immune-related gene expression ofinfected shrimp during the pathogen challenges. Similar findings have been reported in tropical spiny lobsters ( Panulirus ornatus)and rainbow trout ( Oncorhynchus mykiss) with dietary supplementation of MOS (Sang and Fotedar,2010; Ahmadi et al., 2014), and Siberian sturgeon( A. baerii) treated with dietary AXOS (Geraylou et al., 2013). It is well known that crustaceans lack an adaptive immune system, and the innate immune system plays a vital role in the immune defense against invading pathogens. The first step of the innate immune defense is the recognition of conserved motifs in exogenous pathogens by a series of receptors in innate immune system that referred to as patternrecognition receptors (PRRs). Toll-like receptor(TLR) functions as the most important PRR in the Toll pathway, which is an important signaling pathway in crustacean innate immune system. Following the specific recognition of pathogen structurally conserved motifs, MyD88, an important adapter protein, is recruited to activate a series ofintracellular signaling cascades through specific interaction with cytoplasmic Toll/interleukin-1 receptor (TIR) domain of TLR (Li and Xiang, 2013). Recent studies show that MyD88 plays a key role to link the TLRs and a set of downstream immune factors in the shrimp immune responses against diff erent microorganisms(Li and Xiang, 2013; Wen et al., 2013). Furthermore,melanotic encapsulation is another eff ective innate immune mechanism of arthropods to resist invading pathogens. Phenoloxidase (PO) is a key enzyme in the prophenoloxidase (proPO)-activating system,which is the main enzymatic reaction mechanism to result in melanization in crustaceans. Similar to the Toll signaling pathway, the recognizing reaction between pathogen conserved motif and specific PRR led to the proteolytic cleavage of prophenoloxidase zymogen to form active PO enzyme, and the activation of PO subsequently induced the formation of the polymeric melanin (Amparyup et al., 2013).Meanwhile, dietary supplementation with prebiotic showed obvious improvement of PO in several aquatic species (Ringø et al., 2010; Amparyup et al.,2013; Dong and Wang, 2013). As a member of mitogen-activated protein kinase (MAPK)superfamily, the signaling pathway of extracellular signal-regulated kinase (ERK) played an important role in a variety of cellular activities, including cell diff erentiation, cell proliferation, and cytokine production (Li et al., 2013). In invertebrate, ERK was reported to participate in the immune responses to bacterial and viral infections (Shi et al., 2012; Li et al., 2013). In addition, previous research demonstrated that some species of viruses might utilize host ERK signaling pathway to maximize viral replication and expression of related genes (Shi et al., 2012).Therefore, our findings demonstrate that the diet supplemented with AXOS or inulin could obviously improve ERK expression of experimental shrimp,and the subsequent reduction in the gene expression of ERK during the virus challenge test should be associated with the utilization of ERK signaling pathway by white spot syndrome virus to benefit its invasion to the organism. Therefore, the dietary supplementation with AXOS and inulin could not only significantly improved the shrimp growth performance via regulating the gut microbiota and expression of related digestive enzymes, but also show a greater success in improving the immune response and pathogen resistance by up-regulating various immune-related genes.
5 CONCLUSION
We revealed that the diet supplemented of AXOS and inulin, separately or simultaneously, could clearly promote the growth performance by up-regulating expressions of the growth-related genes and modulating the gut microbiota in Pacific white shrimp.Furthermore, the prebiotic combination induced more remarkable elevation in the resistance against pathogen infection via boosting innate immune responses in shrimp. All the findings indicated the simultaneous application of AXOS and inulin could serve as a candidate dietary supplement in L. vannamei culture.
6 DATA AVAILABILITY STATEMENT
The data generated during the present study are available from the corresponding author upon reasonable request.
 Journal of Oceanology and Limnology2021年2期
Journal of Oceanology and Limnology2021年2期
- Journal of Oceanology and Limnology的其它文章
- Predicting sediment flux from continental shelfislands,southeastern China*
- Laboratory simulation of dissolved oxygen reduction and ammonia nitrogen generation in the decay stage of harmful algae bloom*
- Development of high-resolution chloroplast markers for intraspecific phylogeographic studies of Phaeocystis globosa*
- Effects ofiron and humic acid on competition between Microcystis aeruginosa and Scenedesmus obliquus revealed by HPLC analysis of pigments*
- Effect of river plume on phytoplankton community structure in Zhujiang River estuary*
- Exploring the sublethal genotoxic effects of class II organophosphorus insecticide quinalphos on freshwater fish Cyprinus carpio
